Biomaterial-Assisted Self-Healing for Crack Reduction in High-Performance Centrifugal Concrete Piles
Abstract
:1. Introduction
2. Materials and Methods
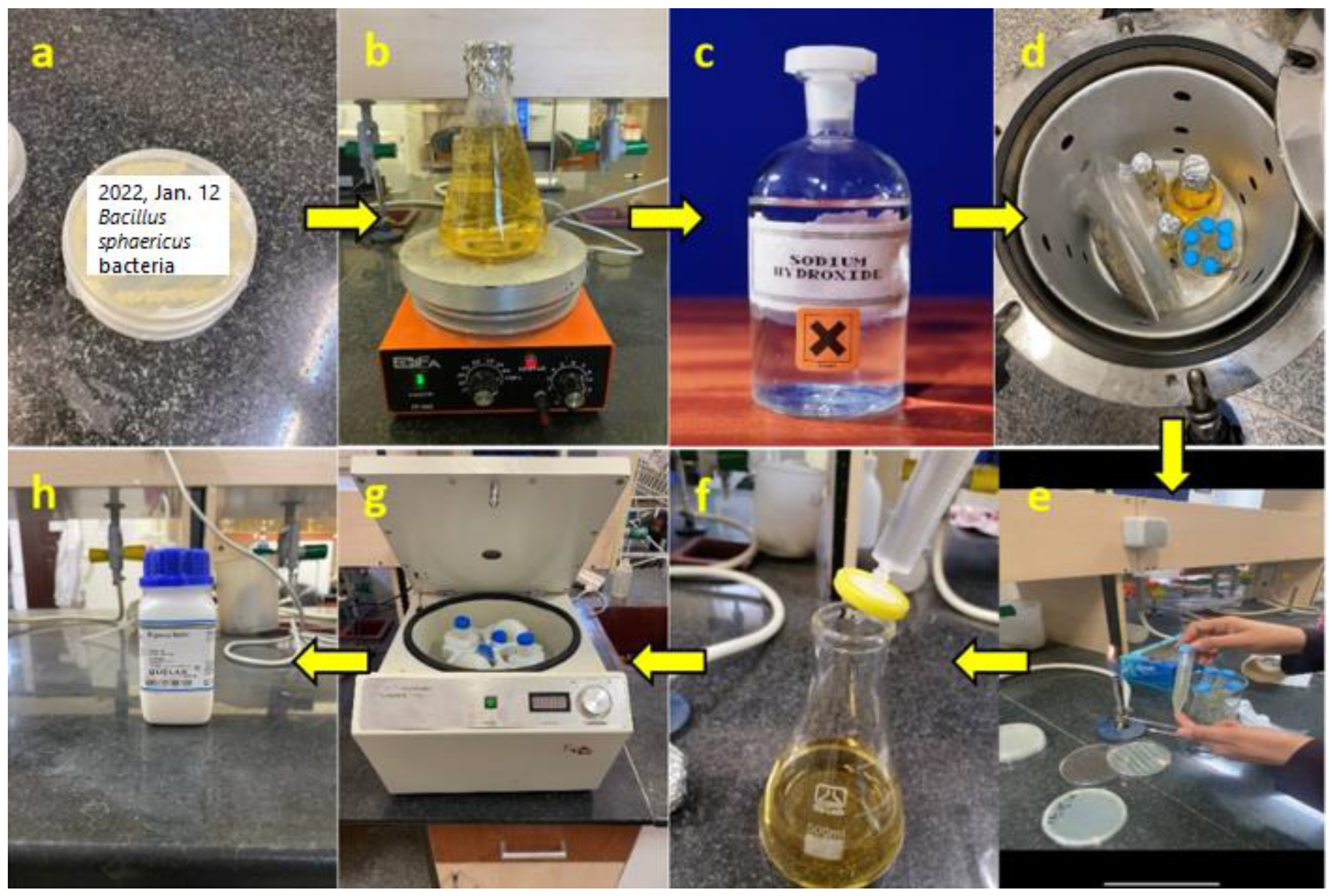
2.1. Bacterial Culture
2.2. Creating Small-Scale Cylindrical Specimens of HPC Pile
2.3. Preparing Bacterial HPC Specimens
3. Tests and Analyses
3.1. Compressive Strength Test
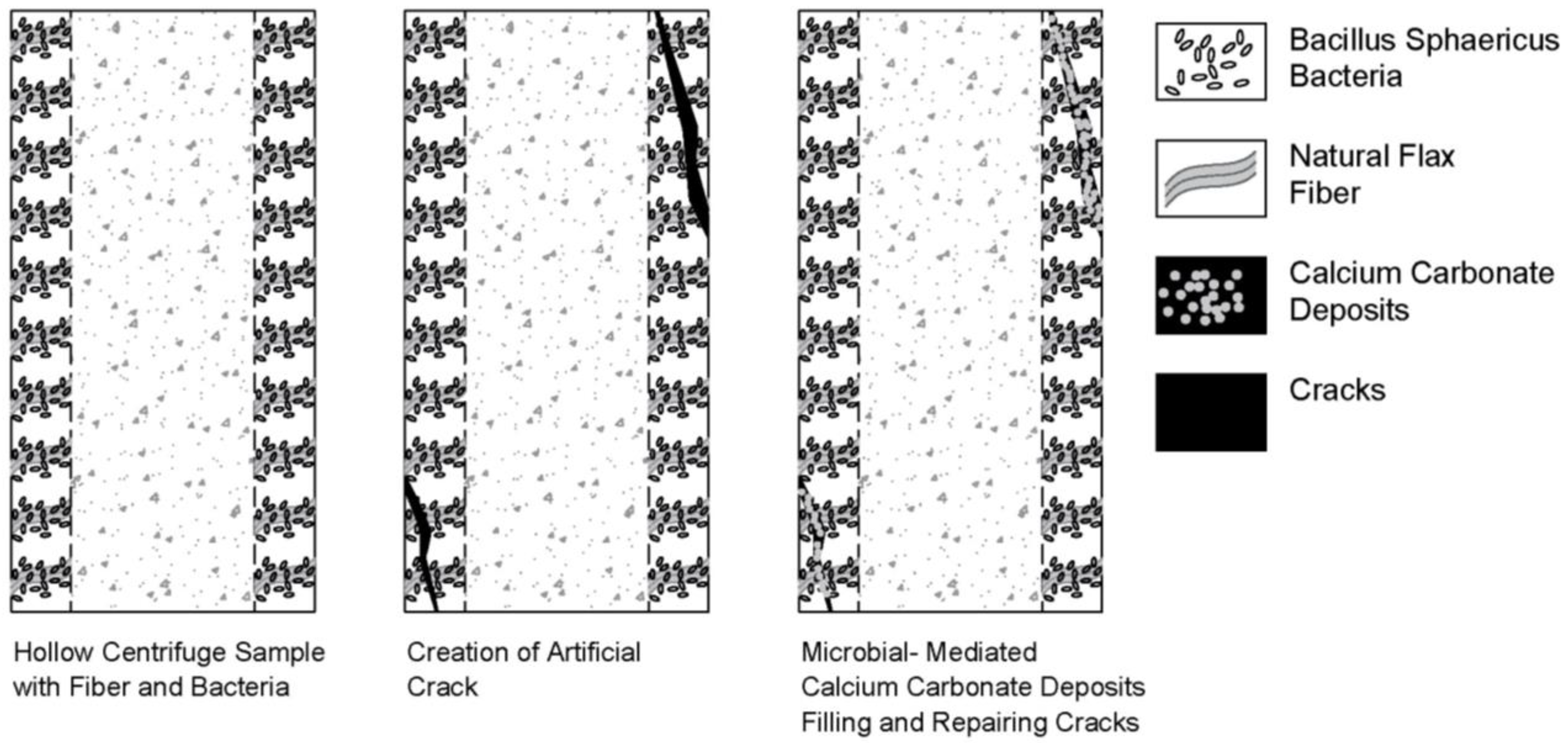
3.2. Artificial Crack Creation
3.3. 30-min Water Absorption Test
3.4. SEM/EDS Analysis of Bacteria-Induced Crack Repair
3.5. Enhancing SEM Analysis of Small-Scale Samples: EDS and MAP Techniques
3.6. Research Schematic
4. Results and Discussion
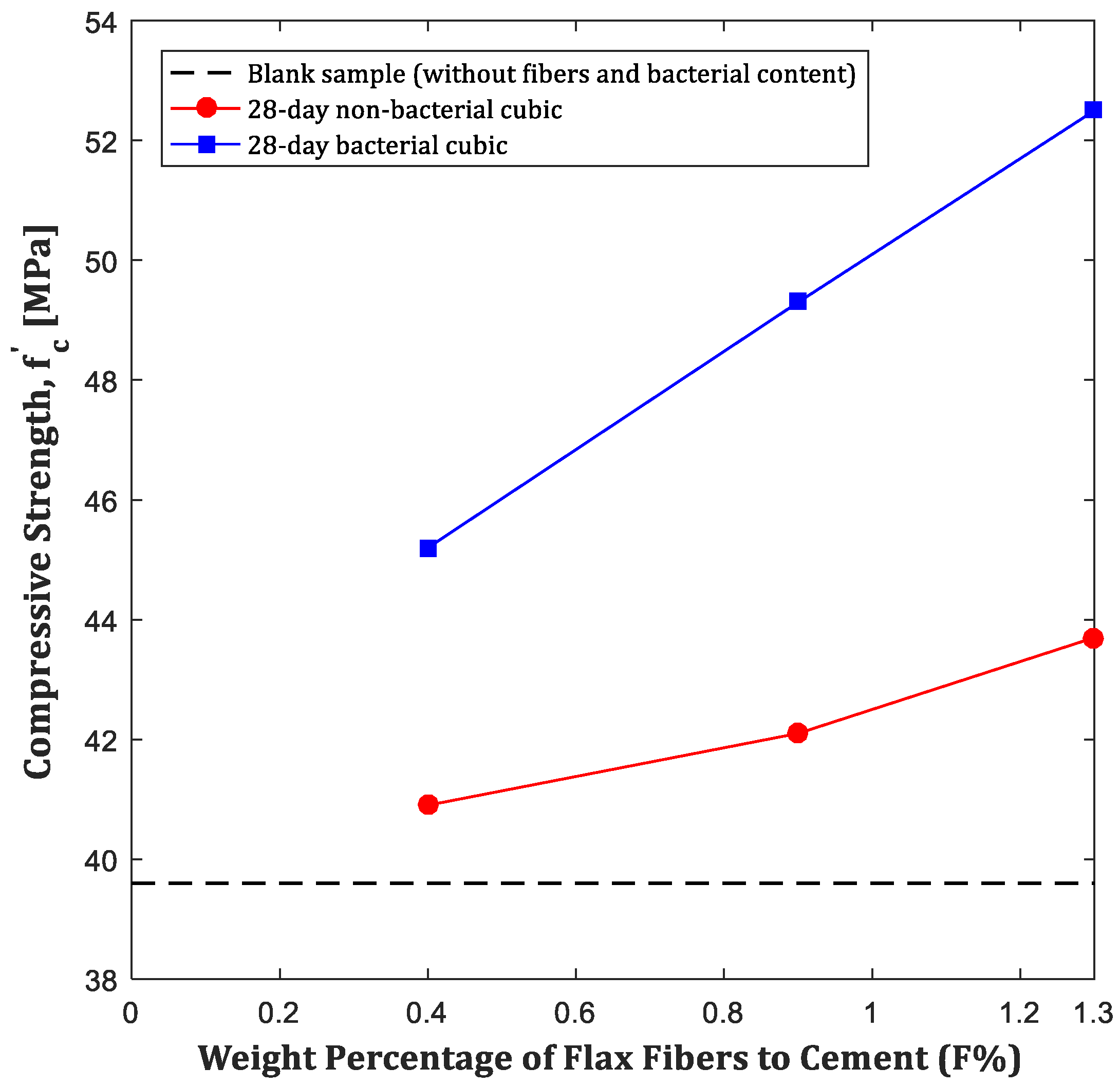
4.1. Compressive Strength Test Results
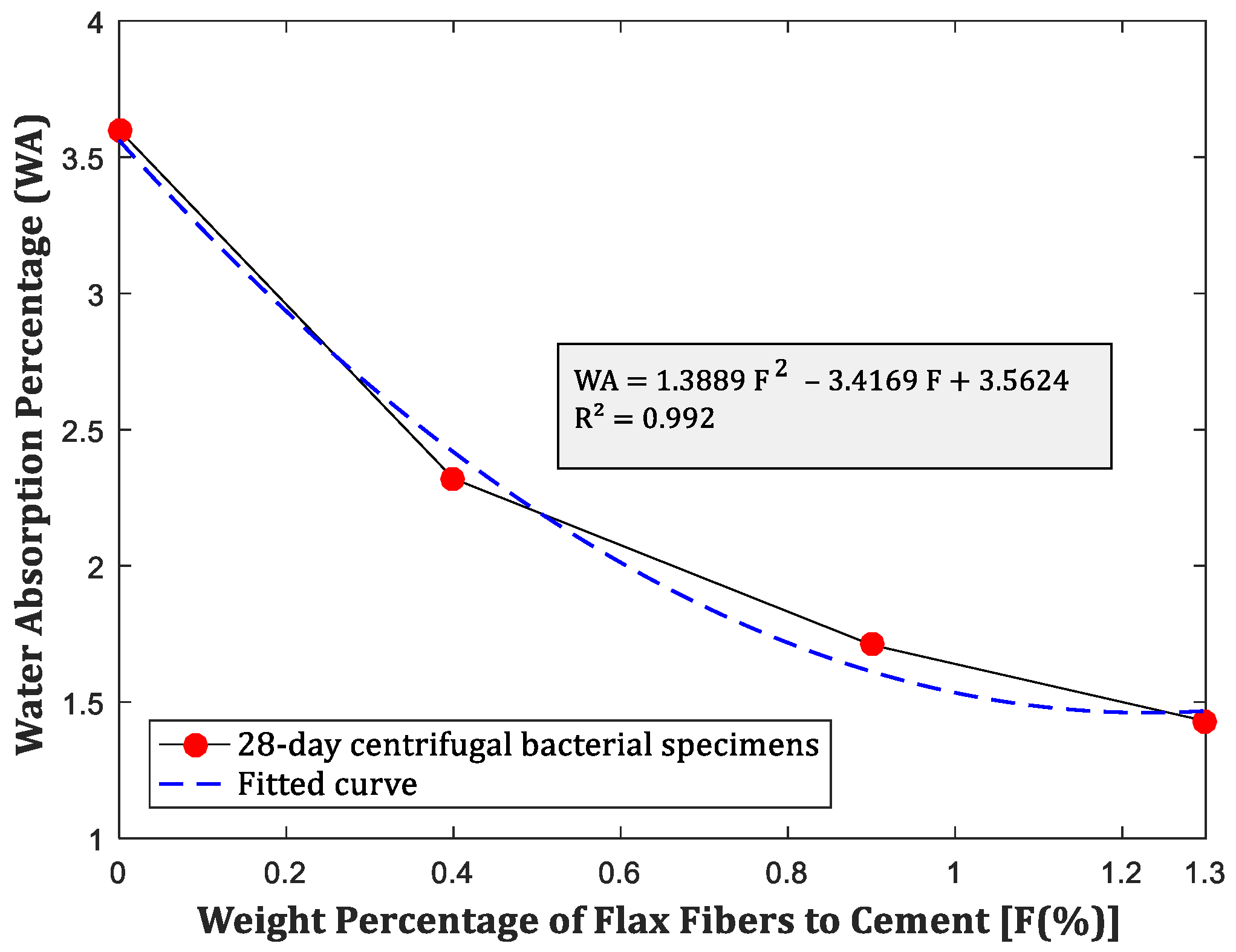
4.2. Results of 30-min Water Absorption Test
4.3. Results of SEM, EDS, and MAP Analysis of Centrifuge Bacterial Samples
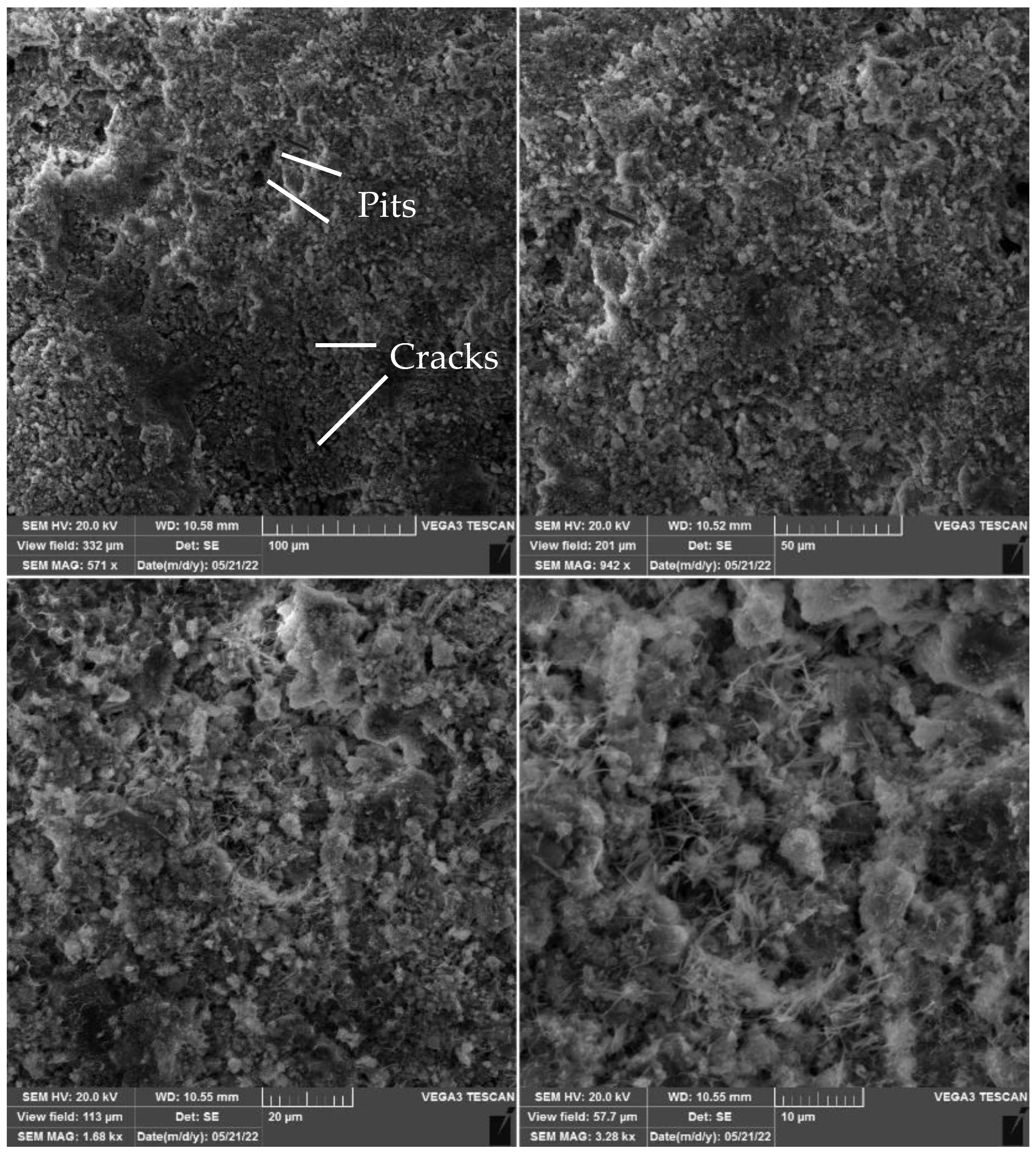
4.3.1. SEM Analysis Results
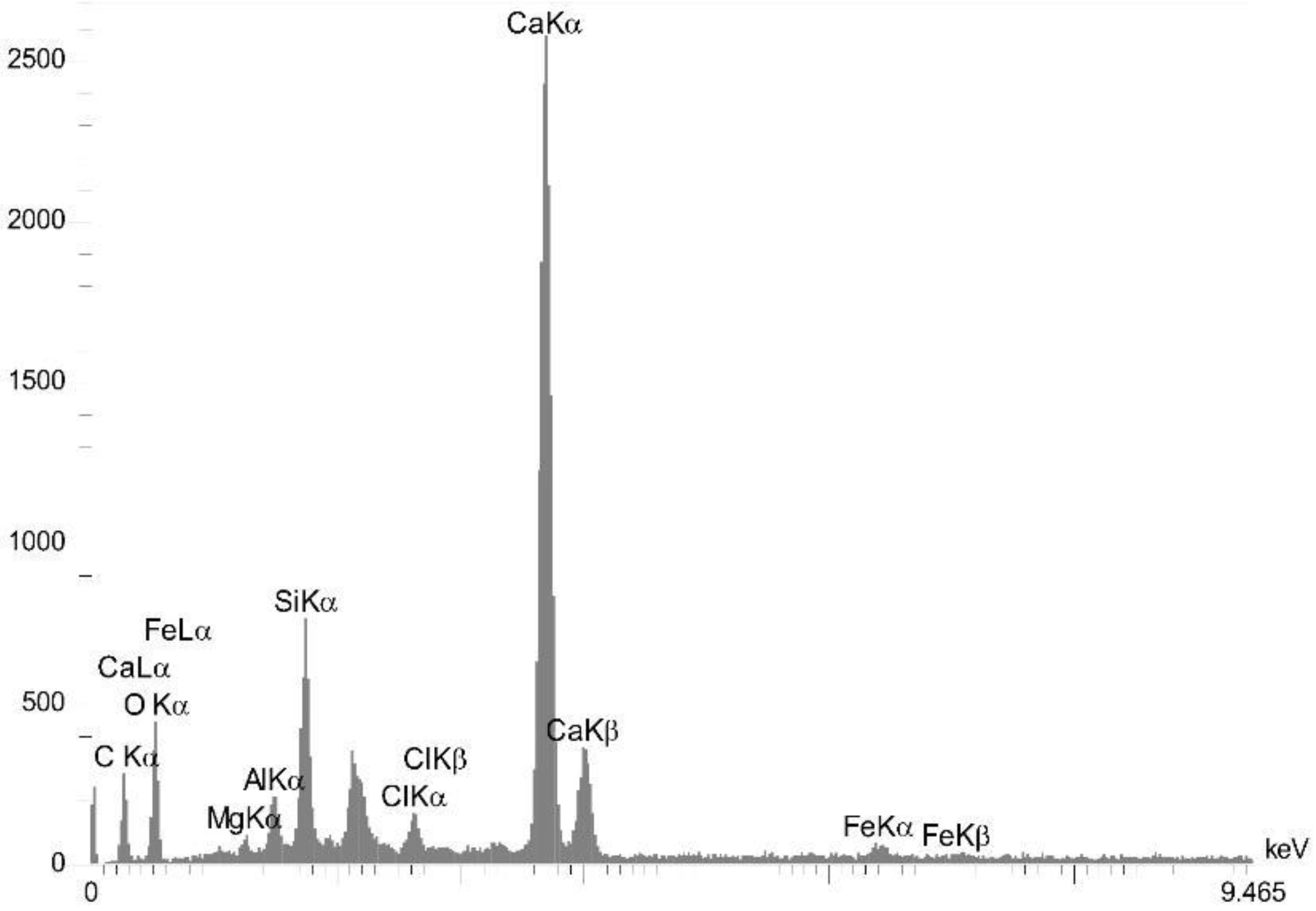
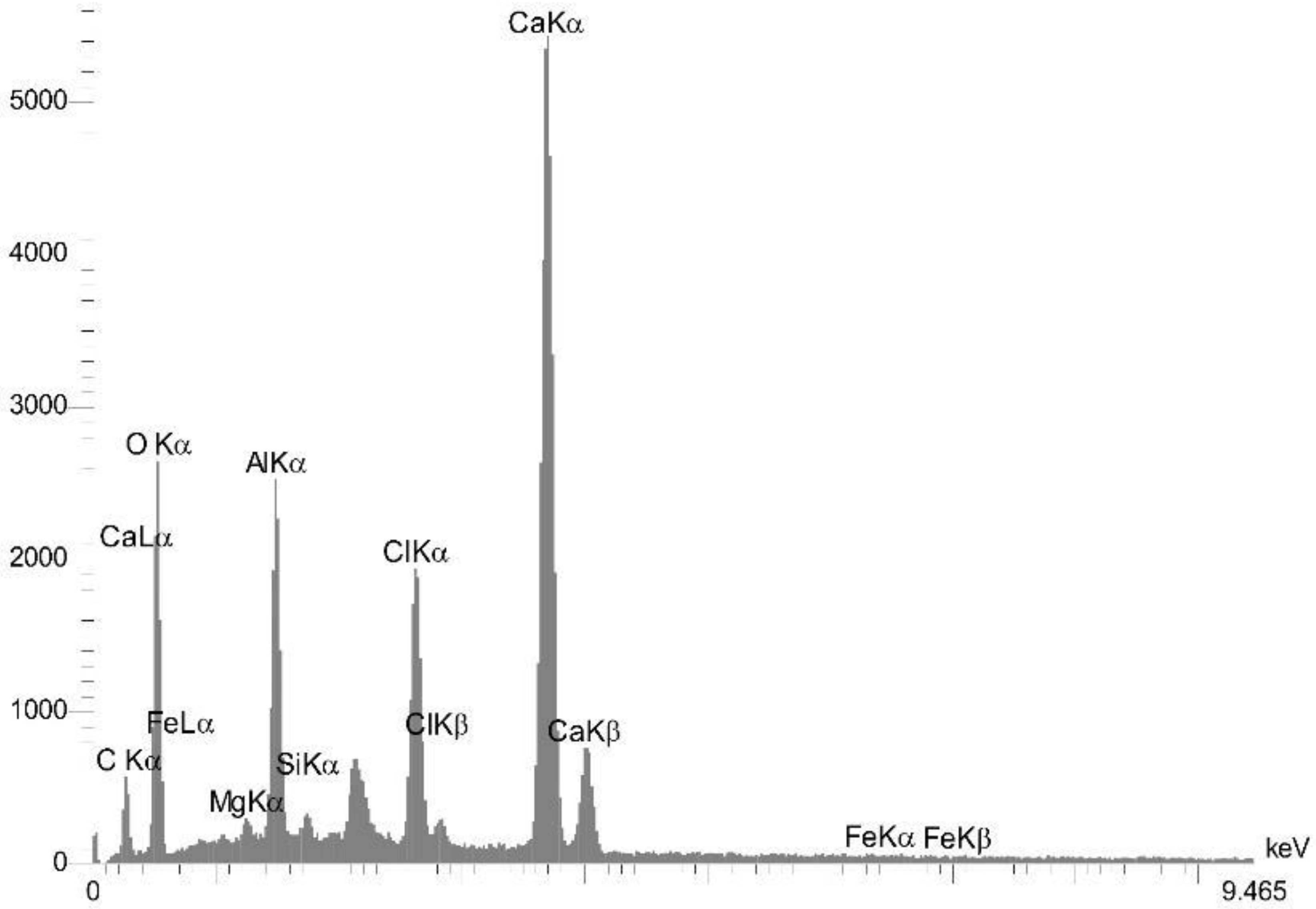
4.3.2. EDS Analysis Results
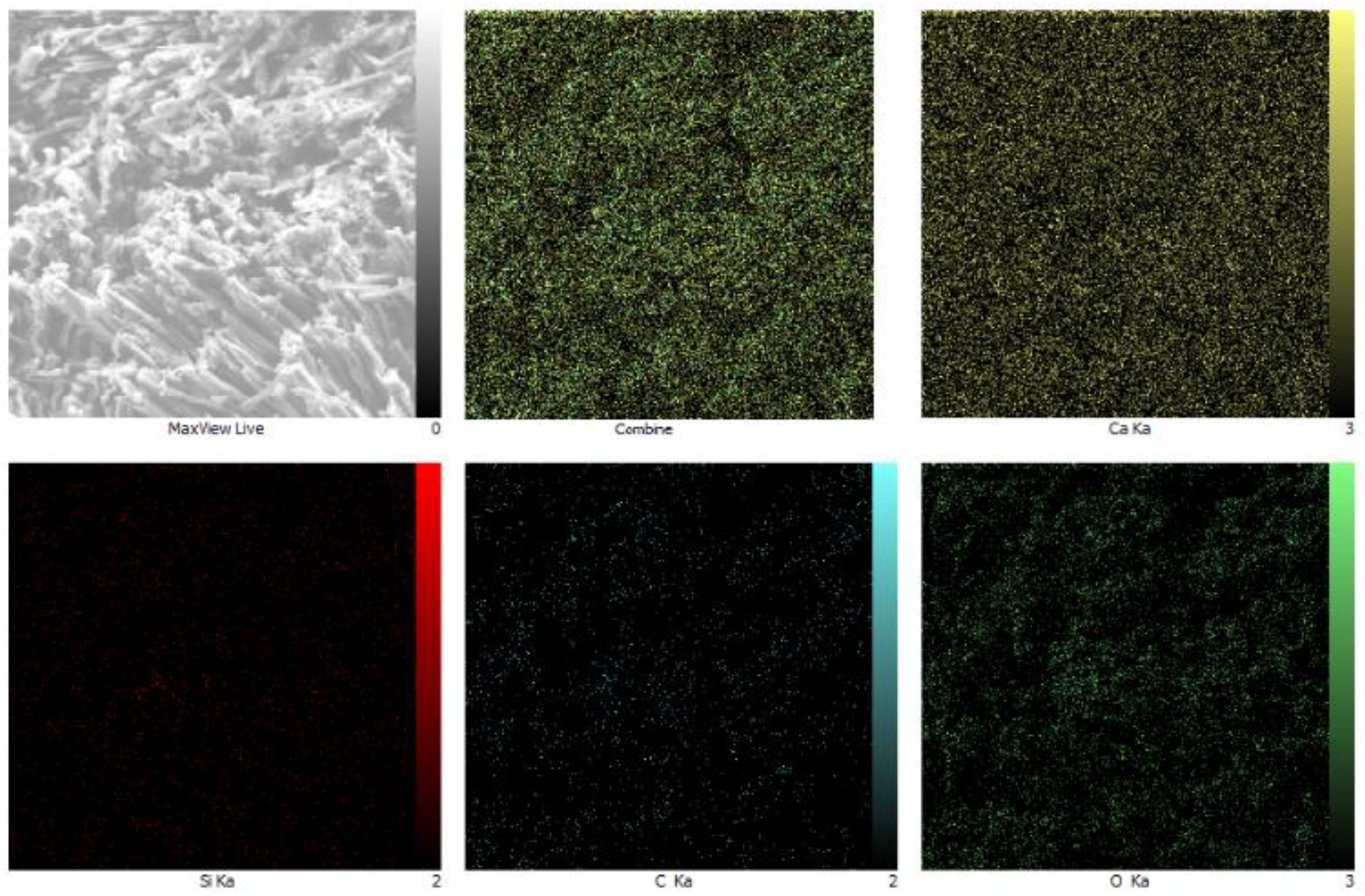
4.3.3. MAP Analysis Results
4.3.4. Examples of Bacterial Centrifuge Samples Before and After Self-Healing
5. Conclusions
- SEM images confirmed that calcium carbonate sediment is produced in calcite, aragonite, and vaterite forms within centrifugal samples.
- The addition of Bacillus sphaericus bacteria and fibers resulted in a 14.21% increase in compressive strength in centrifugal samples and up to a 20% increase in cubic samples compared to those without bacteria.
- The inclusion of fibers and bacteria led to reduced water absorption, facilitating increased calcium carbonate sediment production, further strengthening the concrete.
- Visual observations showed that most of the cracks in centrifugal pile foundations were successfully restored, improving the overall integrity of the concrete. Therefore, the self-healing concrete method can be considered as an effective solution for deep foundations, as any cracks and corrosion that may form are not visually detectable.
- The combination of reduced water absorption, increased compressive strength and crack removal has shown that the combination of bacteria and fibers can mitigate corrosion of piles.
- MAP analyses revealed that calcium carbonate deposits were distributed throughout the cracks, ensuring effective healing across the concrete structure.
- In summary, the biological self-healing method using Bacillus sphaericus bacteria and flax fibers can improve the durability and life of HPC piles. This study provides important insights into the potential applications of this method for mitigating corrosion and enhancing concrete performance in various construction projects.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qaidi, S.; Al-Kamaki, Y.S.; Al-Mahaidi, R.; Mohammed, A.S.; Ahmed, H.U.; Zaid, O.; Althoey, F.; Ahmad, J.; Isleem, H.F.; Bennetts, I. Investigation of the effectiveness of CFRP strengthening of concrete made with recycled waste PET fine plastic aggregate. PLoS ONE 2022, 17, e0269664. [Google Scholar]
- Qaidi, S.M.; Mohammed, A.S.; Ahmed, H.U.; Faraj, R.H.; Emad, W.; Tayeh, B.A.; Althoey, F.; Zaid, O.; Sor, N.H. Rubberized geopolymer composites: A comprehensive review. Ceram. Int. 2022, 48, 24234–24259. [Google Scholar] [CrossRef]
- AliPour, I.S.P. Factors Affecting the Corrosion of Concrete and Steel Reinforcement in Reinforced Concrete Structures. 2021. In Proceedings of the 4th National Conference on New Technologies in Architectural, Civil and Urban Engineering of Iran, Teheran, Iran, 26 August 2021. [Google Scholar]
- Mirsayapov, I.; Yakupov, S.; Hassoun, M. About concrete and reinforced concrete corrosion. In Proceedings of the International Scientific Conference on Socio-Technical Construction and Civil Engineering (STCCE—2020), Kazan, Russian, 29 April–15 May 2020; p. 012061. [Google Scholar]
- Zhang, W.; Zhao, C.; Zhang, C.; Guo, C. Concrete Corrosion Mechanism and Durability Design of Municipal Sewerage. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Zhuhai, China, 15–17 January 2021; p. 012021. [Google Scholar]
- Li, V.C.; Herbert, E. Robust self-healing concrete for sustainable infrastructure. J. Adv. Concr. Technol. 2012, 10, 207–218. [Google Scholar] [CrossRef]
- Reddy, S.V.; Satya, A.K.; Rao, S.M.; Azmatunnisa, M. A biological approach to enhance strength and durability in concrete structures. Int. J. Adv. Eng. Technol. 2012, 4, 392–399. [Google Scholar]
- Althoey, F.; Zaid, O.; Arbili, M.M.; Martínez-García, R.; Alhamami, A.; Shah, H.A.; Yosri, A.M. Physical, strength, durability and microstructural analysis of self-healing concrete: A systematic review. Case Stud. Constr. Mater. 2023, 18, e01730. [Google Scholar]
- Qian, C.; Wang, R.; Cheng, L.; Wang, J. Theory of microbial carbonate precipitation and its application in restoration of cement-based materials defects. Chin. J. Chem. 2010, 28, 847–857. [Google Scholar]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 2016, 5, 250. [Google Scholar]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Wang, J.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Rauf, M.; Khaliq, W.; Khushnood, R.A.; Ahmed, I. Comparative performance of different bacteria immobilized in natural fibers for self-healing in concrete. Constr. Build. Mater. 2020, 258, 119578. [Google Scholar]
- Dabbagh, H.; Ashengroph, M.; Zare, P.; Salehi, P. Investigating the Effect of Two Types of Bacteria on the Compressive Strength and Water Absorption of Lightweight Fiber-Reinforced Concrete. In Proceedings of the 11th National Congress of Civil Engineering, Shiraz, Iran, 30 April 2019. [Google Scholar]
- Xiao, Y.; Stuedlein, A.W.; Pan, Z.; Liu, H.; Matthew Evans, T.; He, X.; Lin, H.; Chu, J.; Van Paassen, L.A. Toe-bearing capacity of precast concrete piles through biogrouting improvement. J. Geotech. Geoenviron. Eng. 2020, 146, 06020026. [Google Scholar] [CrossRef]
- Yamasamit, N.; Sangkeaw, P.; Jitchaijaroen, W.; Thongchom, C.; Keawsawasvong, S.; Kamchoom, V. Effect of Bacillus subtilis on mechanical and self-healing properties in mortar with different crack widths and curing conditions. Sci. Rep. 2023, 13, 7844. [Google Scholar] [CrossRef]
- Wani, S.; Gęca, M.J.; Selvaraj, T.; Priya, T.S. Assessing the influence of Bacillus megaterium and Bacillus sphaericus in cementitious materials: Promoting sustainability towards strength, durability and crack repair. Ain Shams Eng. J. 2024, 15, 102748. [Google Scholar] [CrossRef]
- Adil, M.R.; Khatun, S.; Jamal, M.; Mondal, A. Investigation on Self-Healing Concrete Using Bacillus subtilis Bacteria. MATEC Web Conf. 2024, 400, 01002. [Google Scholar] [CrossRef]
- Vishal, A.; Chepuri, A.; Chandana, N. Assessment of bacteria-based self-healing concrete through experimental investigations—A sustainable approach. J. Mater. Sci. Mater. Eng. 2025, 20, 15. [Google Scholar] [CrossRef]
- Elshazly, M.A.; Elakhras, A.A.; Elshami, A.A.; Ahmad, S.S.; Elmahdy, M.A. Investigating the effectiveness of a bacterial self-healing mechanism for repairing cracks in sustainable cement mortar at low temperatures. Results Eng. 2025, 25, 103907. [Google Scholar] [CrossRef]
- BS 1881; Testing Concrete-Part 124: Methods for Analysis of Hardened Concrete. British Standards Institution: London, UK, 1998.
- Ahmed, I.; Yokota, A.; Yamazoe, A.; Fujiwara, T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1117–1125. [Google Scholar] [CrossRef]
- Chen, H.-J.; Peng, C.-F.; Tang, C.-W.; Chen, Y.-T. Self-healing concrete by biological substrate. Materials 2019, 12, 4099. [Google Scholar] [CrossRef]
- Konstantinou, C.; Wang, Y.; Biscontin, G.; Soga, K. The role of bacterial urease activity on the uniformity of carbonate precipitation profiles of bio-treated coarse sand specimens. Sci. Rep. 2021, 11, 6161. [Google Scholar] [CrossRef]
- ASTM C 39/C 39M; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2006.
- Luo, M.; Qian, C.-X.; Li, R.-Y. Factors affecting crack repairing capacity of bacteria-based self-healing concrete. Constr. Build. Mater. 2015, 87, 1–7. [Google Scholar] [CrossRef]
- Sabir, B.; Wild, S.; O’farrell, M. A water sorptivity test for martar and concrete. Mater. Struct. 1998, 31, 568–574. [Google Scholar]
- Kabir, H.; Wu, J.; Dahal, S.; Joo, T.; Garg, N. Automated estimation of cementitious sorptivity via computer vision. Nat. Commun. 2024, 15, 9935. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar]
- Tie, Y.; Ji, Y.; Zhang, H.; Jing, B.; Zeng, X.; Yang, P. Investigation on the mechanical properties of Bacillus subtilis self-healing concrete. Heliyon 2024, 10, e34131. [Google Scholar]












| Cycle Stages | First Stage | Second Stage | Third Stage | Fourth Stage |
|---|---|---|---|---|
| RPM (Revolutions per minute) | 75–85 | 195–205 | 420–430 | 510–520 |
| Duration (minutes) | 5 | 4 | 3 | 3 |
| Chemical Compound | Chemical Nature | Color | Physical State | pH | Specific Weight (kg/L) | Chloride (PPM) |
|---|---|---|---|---|---|---|
| Modified Poly Carboxylic Acid Polymers | Anionic | Light Brown | Liquid | 7 ± 1 | 1/1 ± 0.02 at 20 °C | 500 |
| Sample | Cement (kg/m3) | Aggregate (kg/m3) | Sand (kg/m3) | Water (kg/m3) | Water to Cement Ratio (L/m3) | Superplasticizer (L) | Fibers (kg/m3) | Urea (W/Wc) | Calcium Carbonate (W/Wc) |
|---|---|---|---|---|---|---|---|---|---|
| Fiber-Free | 500 | 1200 | 760 | 141 | 0.28 | 2.8 | - | - | - |
| F0.4% | 500 | 1200 | 760 | 141 | 0.28 | 2.8 | 2.26 | - | - |
| F0.9% | 500 | 1200 | 760 | 141 | 0.28 | 2.8 | 4.52 | - | - |
| F1.3% | 500 | 1200 | 760 | 141 | 0.28 | 2.8 | 6.79 | - | - |
| FB0.4% | 500 | 1200 | 760 | 141 | 0.28 | 2.8 | 2.26 | 2.8 | 1.2 |
| FB0.9% | 500 | 1200 | 760 | 141 | 0.28 | 2.8 | 4.52 | 2.8 | 1.2 |
| FB1.3% | 500 | 1200 | 760 | 141 | 0.28 | 2.8 | 6.79 | 2.8 | 1.2 |
| Sample Name | Al | C | Ca | Cl | Fe | Mg | O | Si |
|---|---|---|---|---|---|---|---|---|
| FB0.4% | 1.97 | 13.82 | 23.84 | 1.23 | 1.89 | 0.99 | 47.71 | 8.55 |
| FB1.3% | 0.64 | 14.68 | 33.33 | 0.26 | 0.22 | 0.49 | 49.04 | 1.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adibinia, A.; Dehghan Khalili, H.; Mohebbi, M.M.; Momeni, M.; Moradi, P.; Ghouhestani, S.; Poorkarimi, A. Biomaterial-Assisted Self-Healing for Crack Reduction in High-Performance Centrifugal Concrete Piles. Buildings 2025, 15, 1064. https://doi.org/10.3390/buildings15071064
Adibinia A, Dehghan Khalili H, Mohebbi MM, Momeni M, Moradi P, Ghouhestani S, Poorkarimi A. Biomaterial-Assisted Self-Healing for Crack Reduction in High-Performance Centrifugal Concrete Piles. Buildings. 2025; 15(7):1064. https://doi.org/10.3390/buildings15071064
Chicago/Turabian StyleAdibinia, Arian, Hesam Dehghan Khalili, Mohammad Mehdi Mohebbi, Mohammad Momeni, Pezhman Moradi, Soleiman Ghouhestani, and Ali Poorkarimi. 2025. "Biomaterial-Assisted Self-Healing for Crack Reduction in High-Performance Centrifugal Concrete Piles" Buildings 15, no. 7: 1064. https://doi.org/10.3390/buildings15071064
APA StyleAdibinia, A., Dehghan Khalili, H., Mohebbi, M. M., Momeni, M., Moradi, P., Ghouhestani, S., & Poorkarimi, A. (2025). Biomaterial-Assisted Self-Healing for Crack Reduction in High-Performance Centrifugal Concrete Piles. Buildings, 15(7), 1064. https://doi.org/10.3390/buildings15071064






