Synthesis and Property Characterization of Low-Activity Waste-Derived Quaternary Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Industrial By-Products
2.1.2. Calcium Additives
2.1.3. Other Components
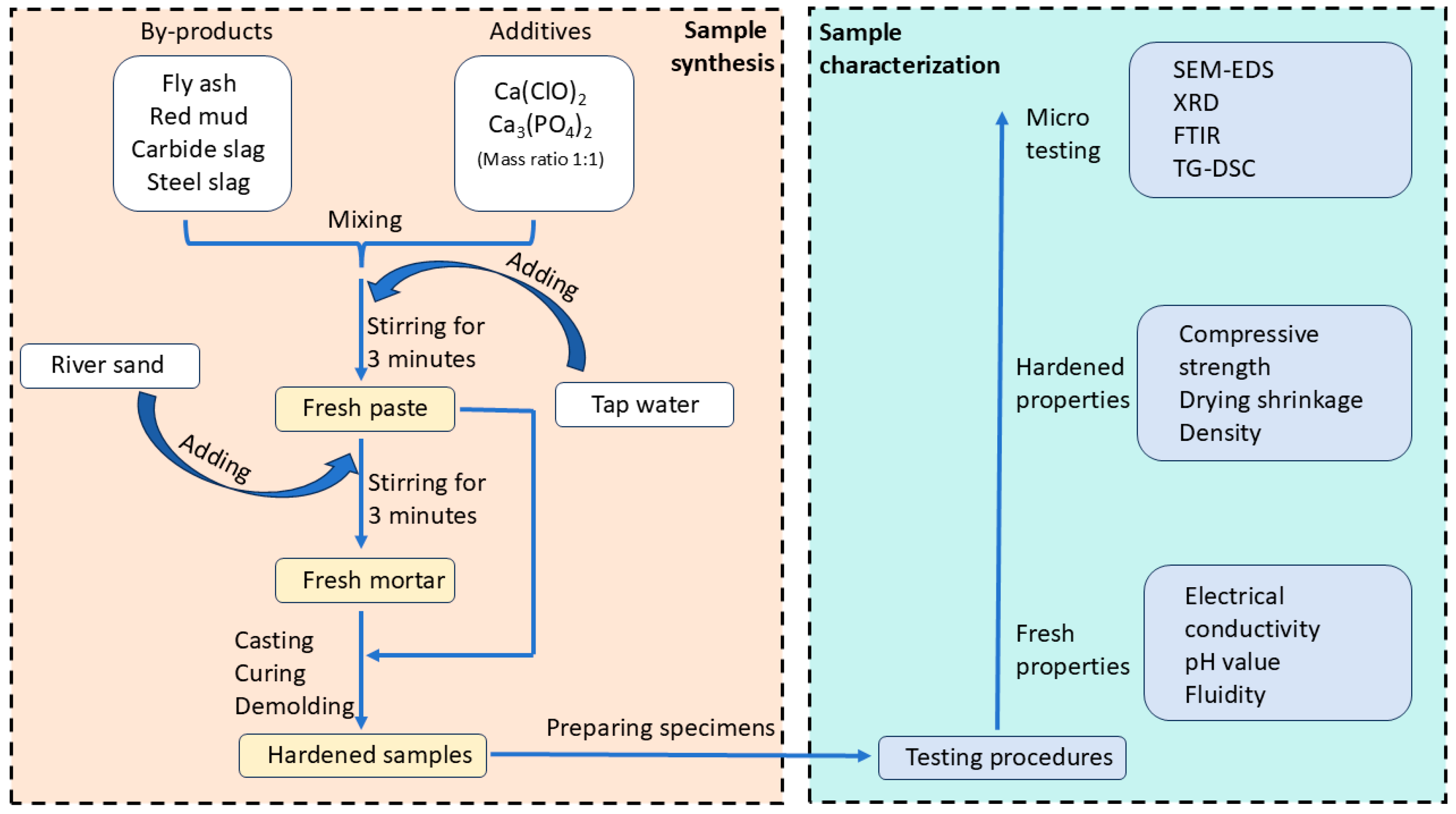
2.2. Sample Fabrication
2.3. Testing Methods
2.3.1. Determination of Fresh Properties
2.3.2. Evaluation of Hardened Properties
2.3.3. Detection of Micro-Characteristics
3. Results
3.1. Fresh Properties of All-Solid-Waste Materials
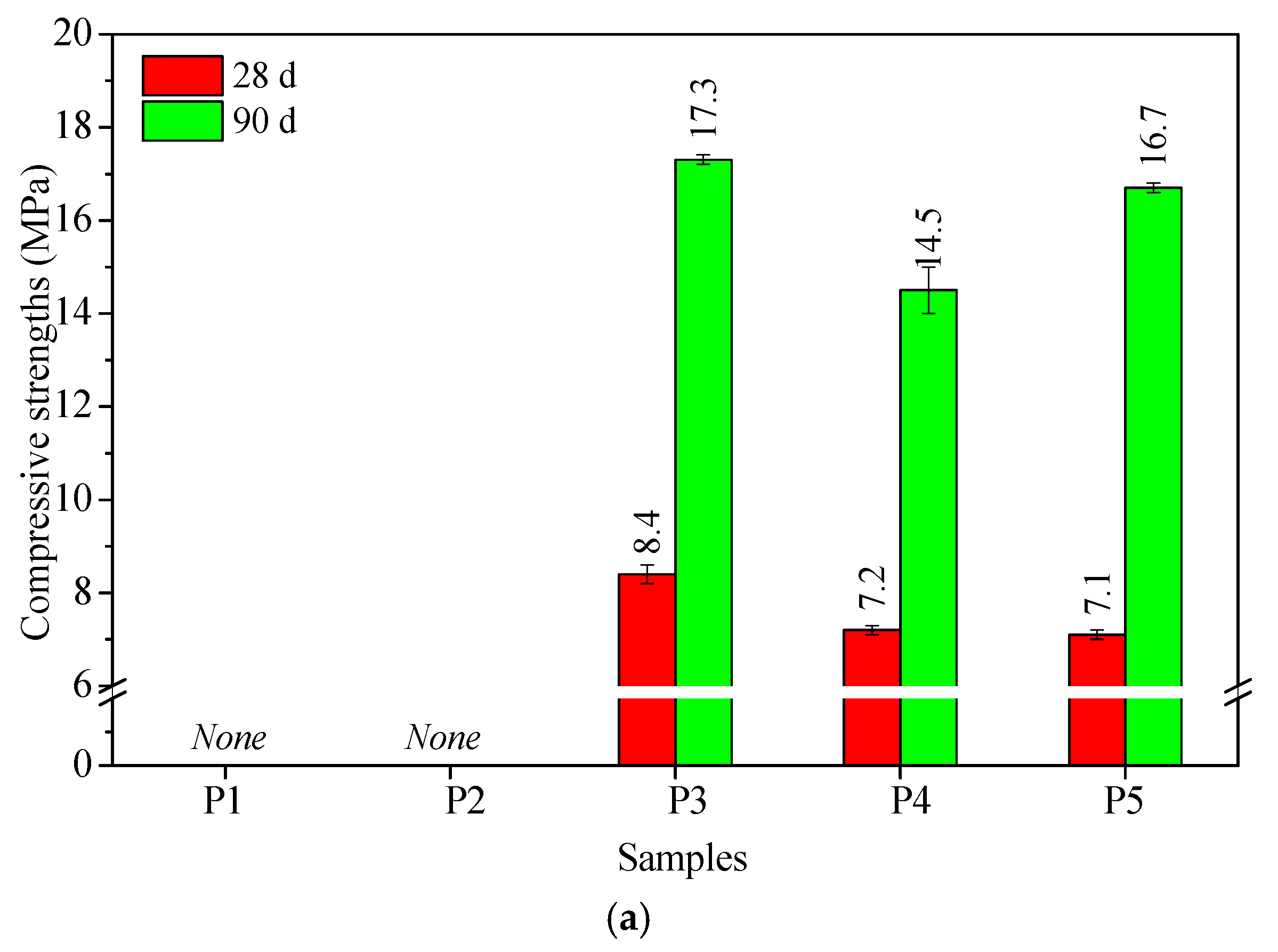
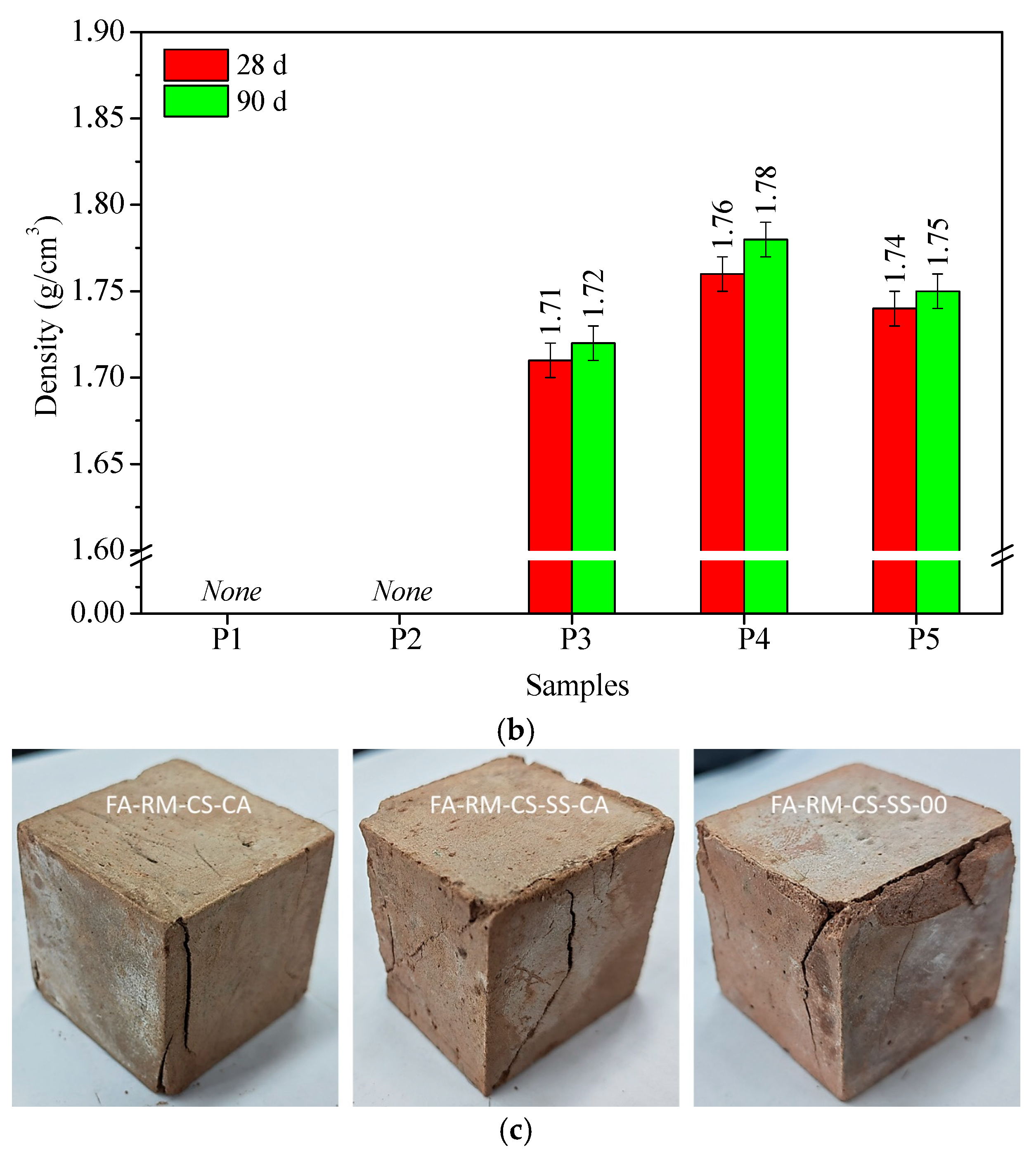
3.2. Hardened Properties of All-Solid-Waste Materials
3.3. Drying Shrinkage Properties of All-Solid-Waste Materials
3.4. Micro-Characterization of All-Solid-Waste Materials
3.4.1. Morphological and Compositional Analysis via SEM-EDS
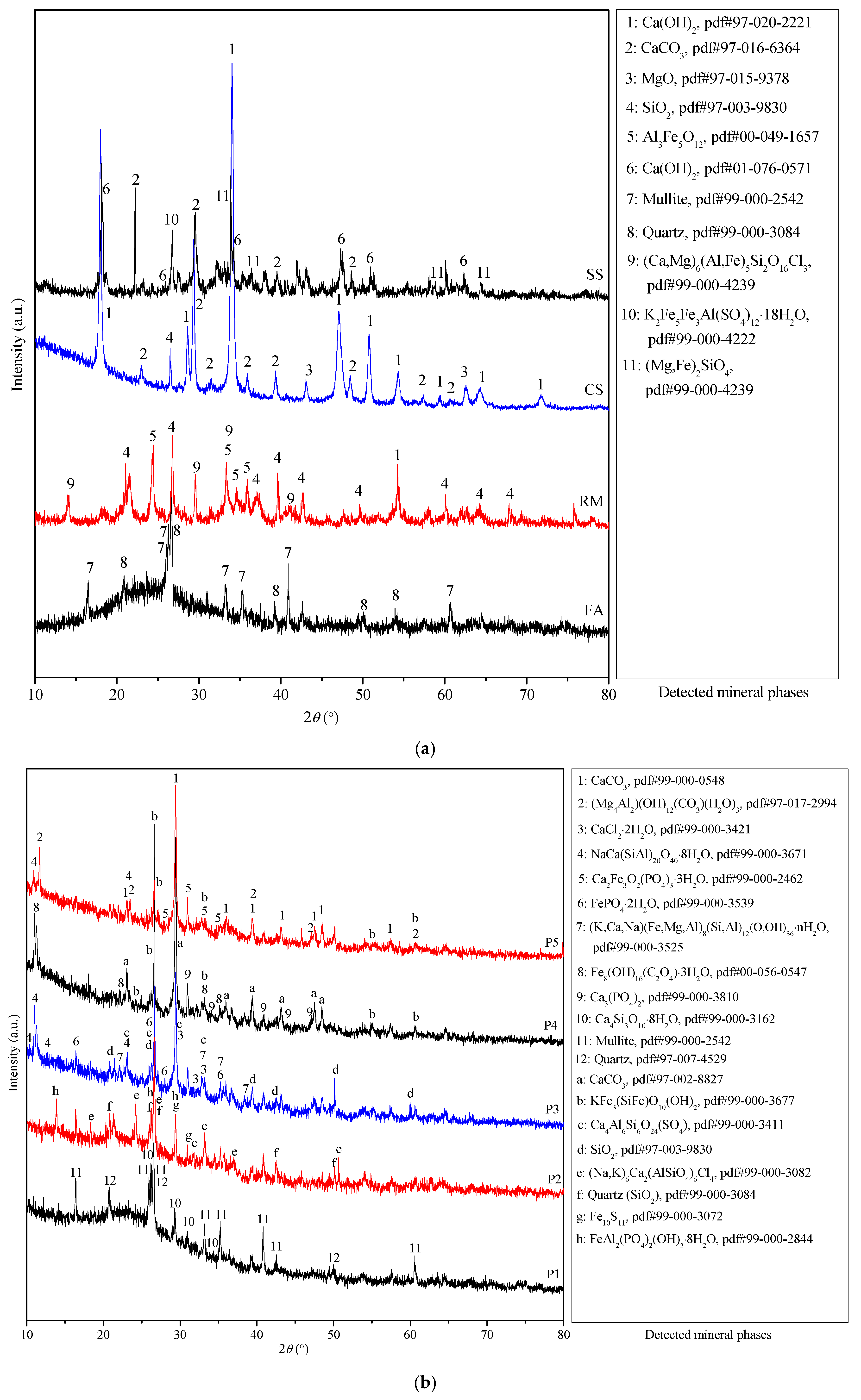
3.4.2. Mineralogical Phase Analysis via XRD
3.4.3. Chemical Bond Analysis via FTIR
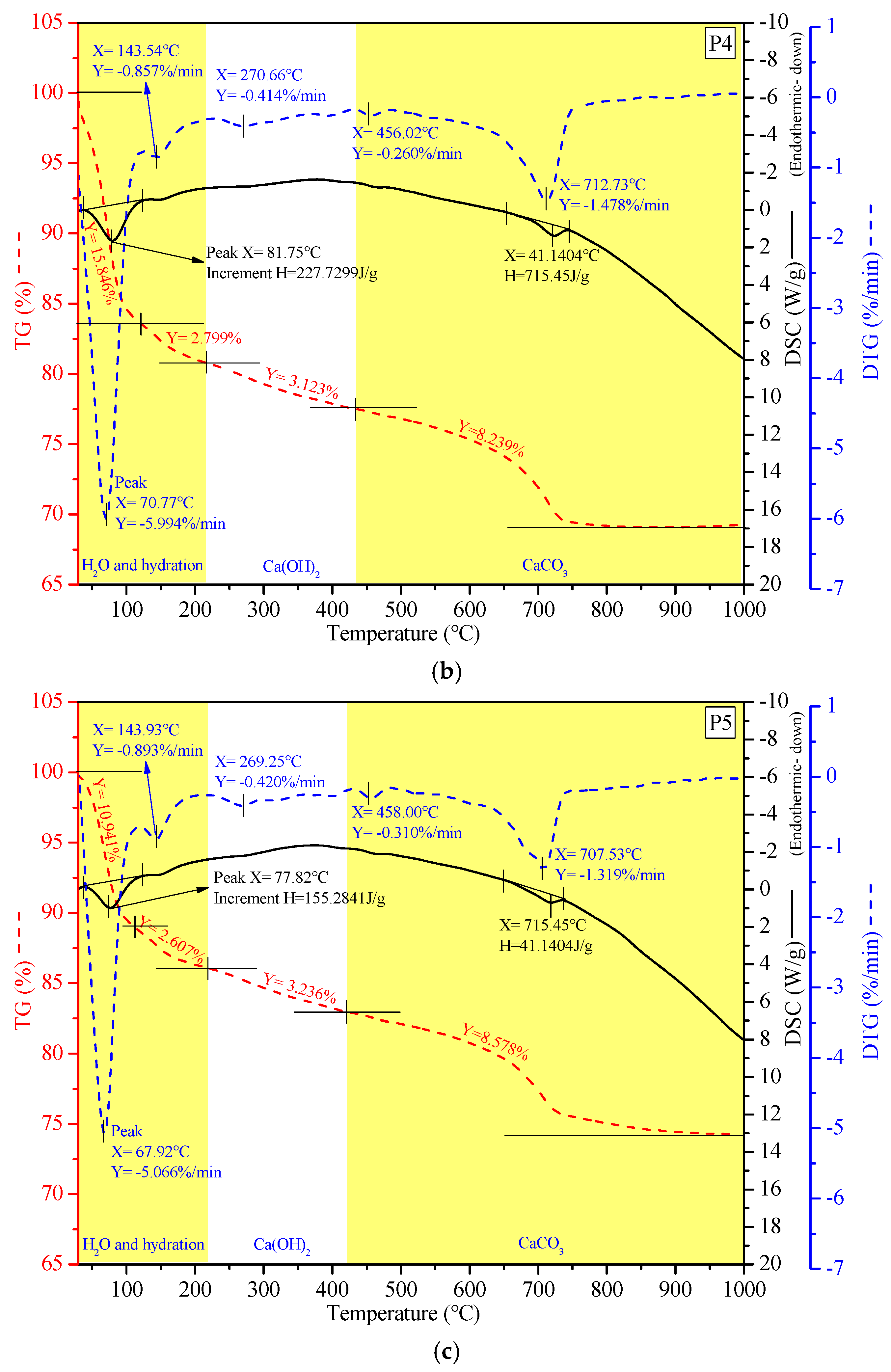
3.4.4. Thermal Behavior Analysis via TG-DSC
4. Discussion
| Cases | Full-Solid-Waste Compositions | 28 d Compressive Strengths (MPa) | References |
|---|---|---|---|
| Binary | FA-SS | 18.8 | [73] |
| Binary | GGBFS-SR | 2.4 | [83] |
| Ternary | GGBS-CS-SR | 43.0 | [81] |
| Ternary | GGBFS-FA-RM | ~9.5 | [84] |
| Ternary | GGBFS-SS–Desulfurized gypsum | — | [85] |
| Ternary | BFS-APG-CS | 51.42 | [25] |
| Ternary | FA-SS–Desulfurization gypsum | 5.0–50.0 | [73] |
| Ternary | GGBFS-SS–β-hemihydrate phosphogypsum | 1.9–31.6 | [86] |
| Ternary | GGBFS-SS-CS | 41.5 | [87] |
| Ternary | GGBFS-CS–Iron tailings | 2.89 | [88] |
| Quaternary | GGBFS-SR-CS-PG | 43.9 | [19] |
| Quaternary | GGBFS-FA-SS-RM | ~13.5 | [45] |
| Quaternary | GGBFS-FA-RM–Eggshell particles | 8.0–12.0 | [84] |
| Quaternary | GGBFS-FA-RM-CS | ~6.5 | [43] |
| Quaternary | BFS-RM-SS–Flue gas desulfurization gypsum | 3.0–18.0 | [23] |
| Quaternary | GGBFS-FA-RM-CS | 6.0 | [82] |
| Quaternary | GGBFS-FA-SF-CS–Gypsum | 15.0–30.0 | [89] |
| Quaternary | GGBFS-MK-CS–Waste mud | >3.0 | [90] |
- I.
- II.
- Ca3(PO4)2 reacted with Ca(OH)2 to produce hydroxyapatite, stabilizing pH at ~12.5 and inhibiting dissolution–reprecipitation shrinkage [93]:
5. Conclusions
- (1)
- A novel cementitious system was developed through the synergistic integration of four low-activity industrial by-products (FA, RM, CS, and SS), achieving 16.7 MPa compressive strength (higher than other samples) with 100% solid-waste utilization (without any additives). This breakthrough demonstrates the feasibility of creating high-performance construction materials without conventional binders, addressing both waste valorization and carbon footprint reduction;
- (2)
- The dual additives Ca(ClO)2 and Ca3(PO4)2 were shown to enable multifunctional enhancement, reducing drying shrinkage while immobilizing Cl− and PO43− pollutants. Ca(ClO)2 accelerated C-S-H gel formation, whereas Ca3(PO4)2 stabilized pH through hydroxyapatite precipitation, collectively mitigating chemical shrinkage mechanisms;
- (3)
- An optimal SS content (≤20%) was identified as critical for balancing micro-aggregate reinforcement and reactivity suppression. While SS addition delayed long-term strength development due to inert C2S components, its role in reducing drying shrinkage and maintaining dimensional stability proved essential for practical applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoro, K.; Daramola, M. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Fankhauser, S.; Smith, S.M.; Allen, M.; Axelsson, K.; Hale, T.; Hepburn, C.; Kendall, J.M.; Khosla, R.; Lezaun, J.; Mitchell-Larson, E.; et al. The meaning of net zero and how to get it right. Nat. Clim. Change 2022, 12, 15–21. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, H.; Miao, E.; Wang, Y.; Zhang, T.; Xiao, Y.; Liu, Z.; Ma, J.; Xiong, Z.; Zhao, Y.; et al. Accelerated CO2 mineralization technology using fly ash as raw material: Recent research advances. Chem. Eng. J. 2024, 488, 150676. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.Ó.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef]
- Chen, M.; Wang, C.; Shi, Q.; Hu, H.; Zhang, Q.; Li, Z. Enhanced simultaneous CO2 mineralization and cadmium immobilization in a wide pH range by using ball-milled serpentine. Chem. Eng. J. 2023, 474, 145558. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, W.; Xu, H.; Liu, Z.; Li, X.; Shi, H.; Yu, Y.; Qu, Z.; Yan, N. CO2 sequestration and CaCO3 recovery with steel slag by a novel two-step leaching and carbonation method. Sci. Total Environ. 2023, 891, 164203. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Zhu, W.; Zeng, G.; Ouyang, Z.; Cheng, M.; Jiang, Z. Carbon dioxide cured building materials as an approach to decarbonizing the calcium carbide related industry. Renew. Sustain. Energy Rev. 2023, 186, 113688. [Google Scholar] [CrossRef]
- Ren, S.; Aldahri, T.; Liu, W.; Liang, B. CO2 mineral sequestration by using blast furnace slag: From batch to continuous experiments. Energy 2021, 214, 118975. [Google Scholar] [CrossRef]
- Renforth, P. The negative emission potential of alkaline materials. Nat. Commun. 2019, 10, 1401. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chen, Y.-H.; Fan, L.-S.; Kim, H.; Gao, X.; Ling, T.-C.; Chiang, P.-C.; Pei, S.-L.; Gu, G. CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. Nat. Sustain. 2020, 3, 399–405. [Google Scholar] [CrossRef]
- Grabias-Blicharz, E.; Franus, W. A critical review on mechanochemical processing of fly ash and fly ash-derived materials. Sci. Total Environ. 2023, 860, 160529. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C.W. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Madej, J.; Franus, W. Synthesis of zeolite-carbon composites using high-carbon fly ash and their adsorption abilities towards petroleum substances. Fuel 2021, 283, 119173. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, X.; Wei, C.; Liu, X.; Zhang, Z. Activation of low-activity calcium silicate in converter steelmaking slag based on synergy of multiple solid wastes in cementitious material. Constr. Build. Mater. 2022, 351, 128925. [Google Scholar] [CrossRef]
- Namarak, C.; Satching, P.; Tangchirapat, W.; Jaturapitakkul, C. Improving the compressive strength of mortar from a binder of fly ash-calcium carbide residue. Constr. Build. Mater. 2017, 147, 713–719. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Guo, W.; Li, J.; Li, X.; Zhao, Q. A novel all-solid-waste binder prepared by salt-alkali synergistic activation system constructed from phosphogypsum, soda residue and calcium carbide slag. Cem. Concr. Compos. 2025, 155, 105841. [Google Scholar] [CrossRef]
- Kumar, A.; Saravanan, T.J.; Bisht, K.; Kabeer, K.I.S.A. A review on the utilization of red mud for the production of geopolymer and alkali activated concrete. Constr. Build. Mater. 2021, 302, 124170. [Google Scholar] [CrossRef]
- Niu, A.; Lin, C. Trends in research on characterization, treatment and valorization of hazardous red mud: A systematic review. J. Environ. Manag. 2024, 351, 119660. [Google Scholar] [CrossRef]
- Qingke, N.; Haiqing, Z.; Haipeng, Y.; Xiangxin, J.; Rihua, Z. Development and field test of Red mud-Fly Ash Geopolymer pile (RFP). Clean. Waste Syst. 2024, 9, 100184. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z. Investigation the synergistic effects in quaternary binder containing red mud, blast furnace slag, steel slag and flue gas desulfurization gypsum based on artificial neural networks. J. Clean. Prod. 2020, 273, 122972. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, X.; Zhou, Q.; Zhu, H.; Cheng, F.; Chen, H. Development of full-solid waste environmentally binder for cemented paste backfill. Constr. Build. Mater. 2024, 443, 137689. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, P.; Shen, X.; Cai, Y.; Zhen, H.; Liu, Z. Comparative analysis of carbonation strengthening mechanisms in full solid waste materials: Steel slag vs. carbide slag. Cem. Concr. Compos. 2025, 157, 105927. [Google Scholar] [CrossRef]
- Li, L.; Xie, J.; Zhang, B.; Feng, Y.; Yang, J. A state-of-the-art review on the setting behaviours of ground granulated blast furnace slag- and metakaolin-based alkali-activated materials. Constr. Build. Mater. 2023, 368, 130389. [Google Scholar] [CrossRef]
- Nasir, M.; Johari, M.A.M.; Maslehuddin, M.; Yusuf, M.O.; Al-Harthi, M.A. Influence of heat curing period and temperature on the strength of silico-manganese fume-blast furnace slag-based alkali-activated mortar. Constr. Build. Mater. 2020, 251, 118961. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, P.; Li, S.; Shen, X.; Fang, J.; Gu, Z.; Wang, Y. Enhancing the performance of NaOH-activated slag using waste green tea extract as a multi-function admixture. Case Stud. Constr. Mater. 2024, 21, e03605. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Jiang, L.; Meng, L.; Zhou, B.; Zhang, J. Long-Term Physical and Mechanical Properties and Microstructures of Fly-Ash-Based Geopolymer Composite Incorporating Carbide Slag. Materials 2021, 14, 6692. [Google Scholar] [CrossRef]
- Bai, B.; Chen, J.; Bai, F.; Nie, Q.; Jia, X. Corrosion effect of acid/alkali on cementitious red mud-fly ash materials containing heavy metal residues. Environ. Technol. Innov. 2024, 33, 103485. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Gao, H.; Yuan, T.; Zhang, X. The effects of salt-loss soda residue and oxalate acid on property and structure of fly ash-based geopolymer. Constr. Build. Mater. 2023, 366, 130214. [Google Scholar] [CrossRef]
- Xiaoshuang, S.; Yanpeng, S.; Jinqian, L.; Yuhao, Z.; Ruihan, H. Preparation and performance optimization of fly ash- slag- red mud based geopolymer mortar: Simplex-centroid experimental design method. Constr. Build. Mater. 2024, 450, 138573. [Google Scholar] [CrossRef]
- Sun, B.; Ye, G.; de Schutter, G. A review: Reaction mechanism and strength of slag and fly ash-based alkali-activated materials. Constr. Build. Mater. 2022, 326, 126843. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Yang, T.; Zhou, S.; Wei, H.; Zhang, Z. Calcium carbide residue as auxiliary activator for one-part sodium carbonate-activated slag cements: Compressive strength, phase assemblage and environmental benefits. Constr. Build. Mater. 2021, 308, 125015. [Google Scholar] [CrossRef]
- Ye, N.; Yang, J.; Liang, S.; Hu, Y.; Hu, J.; Xiao, B.; Huang, Q. Synthesis and strength optimization of one-part geopolymer based on red mud. Constr. Build. Mater. 2016, 111, 317–325. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Gao, H.; Liang, L.; Yang, J. Synthesis, Stability and Microstructure of a One-Step Mixed Geopolymer Backfill Paste Derived from Diverse Waste Slags. Sustainability 2023, 15, 6708. [Google Scholar] [CrossRef]
- Ren, S.; Li, L.; Zhao, X.; Wang, H.; Zhao, R. Unveiling the Potential of Civil Briquette Furnace Slag as a Silico-Aluminon Additive in Alkali-Activated Materials. Materials 2024, 17, 6188. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, S.; Gao, Y.; Liu, C.; Qi, Y. Effect of different gypsums on the workability and mechanical properties of red mud-slag based grouting materials. J. Clean. Prod. 2020, 245, 118759. [Google Scholar] [CrossRef]
- Yao, X.L.; Wang, W.L.; Liu, M.; Yao, Y.G.; Wu, S. Synergistic use of industrial solid waste mixtures to prepare ready-to-use lightweight porous concrete. J. Clean. Prod. 2019, 211, 1034–1043. [Google Scholar] [CrossRef]
- Zhang, M.; He, M.; Zhang, J. Mitigation of efflorescence for multi-componential geopolymer: Influence of steel slag, flue gas desulfurization gypsum and pre-curing periods. J. Clean. Prod. 2023, 403, 136835. [Google Scholar] [CrossRef]
- JC/T 603-2004; Standard Test Method for Drying Shinkage of Mortar. Technical Supervision & Research Center for China Building Materials Industry: Beijing, China, 2004. Available online: https://www.cssn.net.cn/app/home/productDetail/a8105c02d10d9a7a92a9fef608f6879d (accessed on 18 June 2024). (In Chinese)
- Li, L.; Zhao, X.; Wang, H.; Cao, J.; Zhao, X.E. Properties characterization and microstructural analysis of alkali-activated solid waste-based materials with sawdust and wastewater integration. PLoS ONE 2025, 20, e0313413. [Google Scholar] [CrossRef] [PubMed]
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. China Building Materials Federation: Beijing, China, 2005. Available online: https://www.cssn.net.cn/cssn/front/6824280.html (accessed on 18 June 2024). (In Chinese)
- Wang, H.; Zhao, X.; Gao, H.; Yuan, T.; Liu, X.; Zhang, W. Effects of alkali-treated plant wastewater on the properties and microstructures of alkali-activated composites. Ceram. Int. 2023, 49, 8583–8597. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Method of Testing Cements—Determination of Strength (Idt ISO 679: 2021). China Building Materials Federation: Beijing, China, 2021. Available online: https://www.cssn.net.cn/cssn/front/4311243.html (accessed on 18 June 2024). (In Chinese)
- Fu-Quan, C.; Cui-Lan, C.; Ben-Shan, Z. A Novel Method for Calculating Starch Crystallinity. Food Sci. 2011, 32, 68–81. [Google Scholar]
- Zhang, Y.L.; Liu, X.M.; Xu, Y.T.; Tang, B.W.; Wang, Y.G.; Mukiza, E. Synergic effects of electrolytic manganese residue-red mud-carbide slag on the road base strength and durability properties. Constr. Build. Mater. 2019, 220, 364–374. [Google Scholar] [CrossRef]
- Riley, A.L.; Mayes, W.M. Long-term evolution of highly alkaline steel slag drainage waters. Environ. Monit. Assess. 2015, 187, 463. [Google Scholar] [CrossRef]
- Wang, S.; Pan, H.M.; Xiao, C.; Zhao, Q.X.; Wang, J.X. Preparation and mix proportion optimization of red mud-fly ash-based cementitious material synergistic activated by carbide slag and MSWIFA. Constr. Build. Mater. 2024, 415, 135032. [Google Scholar] [CrossRef]
- Thomas, J.J.; Ghazizadeh, S.; Masoero, E. Kinetic mechanisms and activation energies for hydration of standard and highly reactive forms of β-dicalcium silicate (C2S). Cem. Concr. Res. 2017, 100, 322–328. [Google Scholar] [CrossRef]
- Huang, D.W.; Yuan, Q.M.; Chen, P.; Tian, X.; Peng, H. Effect of activator properties on drying shrinkage of alkali-activated fly ash and slag. J. Build. Eng. 2022, 62, 105341. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Cheng, Y.Z.; Huseien, G.F.; Shah, K.W. Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Mo, L.W.; Yang, S.; Huang, B.; Xu, L.L.; Feng, S.F.; Deng, M. Preparation, microstructure and property of carbonated artificial steel slag aggregate used in concrete. Cem. Concr. Comp. 2020, 113, 103715. [Google Scholar] [CrossRef]
- Li, M.H.; Lu, Y.J.; Liu, Y.J.; Chu, J.J.; Zhang, T.S.; Wang, W. Influence of the Steel Slag Particle Size on the Mechanical Properties and Microstructure of Concrete. Sustainability 2024, 16, 2083. [Google Scholar] [CrossRef]
- Lu, J.; Shen, Y.; Wang, Y.; Zhang, H.; Guan, X.; Zhu, J.; Liu, S. In-situ wet carbonation activation of red mud waste for sustainable grout materials. J. Ind. Eng. Chem. 2024, 136, 453–464. [Google Scholar] [CrossRef]
- Liu, S.; Shen, Y.; Wang, Y.; Shen, P.; Xuan, D.; Guan, X.; Shi, C. Upcycling sintering red mud waste for novel superfine composite mineral admixture and CO2 sequestration. Cem. Concr. Compos. 2022, 129, 104497. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Ke, Y.; Liu, D.G.; Tang, C.J. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef]
- Wang, Q.P.; Liao, Z.Y.; Yao, D.X.; Yang, Z.J.; Wu, Y.H.; Tang, C.L. Phosphorus immobilization in water and sediment using iron-based materials: A review. Sci. Total Environ. 2021, 767, 144246. [Google Scholar] [CrossRef]
- Siauciunas, R.; Hilbig, H.; Prichockiene, E.; Smigelskyte, A.; Takulinskas, Z. Accelerated carbonation of C2SH based dense concrete. Ceram. Int. 2020, 46, 29436–29442. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y.X. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Li, W.Z.; Cao, M.L.; Liu, F.Y.; Wang, D.; Chang, J. Pretreatment of alkali activation and carbonation of steel slag for using as binding material. Cem. Concr. Comp. 2024, 149, 105521. [Google Scholar] [CrossRef]
- Duque-Redondo, E.; Yamada, K.; Manzano, H. Effect of Chloride and Sulfate in the Immobilization of Cs-137 in C-S-H Gel. J. Adv. Concr. Technol. 2021, 19, 95–105. [Google Scholar] [CrossRef]
- Yusuf, M.O. Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy. Appl. Sci. 2023, 13, 3353. [Google Scholar] [CrossRef]
- Feng, H.; Wen, J.X.; Shao, Q.; Yang, Y.Y.; Yao, X.P. Carbonation resistance of fly ash/slag based engineering geopolymer composites. Constr. Build. Mater. 2024, 449, 138471. [Google Scholar] [CrossRef]
- Jhang, J.H.; Boscoboinik, J.A.; Altman, E.I. Ambient pressure x-ray photoelectron spectroscopy study of water formation and adsorption under two-dimensional silica and aluminosilicate layers on Pd(111). J. Chem. Phys. 2020, 152, 084705. [Google Scholar] [CrossRef] [PubMed]
- Efimov, A.M.; Pogareva, V.G.; Shashkin, A.V. Water-related bands in the IR absorption spectra of silicate glasses. J. Non-Cryst. Solids 2003, 332, 93–114. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Co-existence of aluminosilicate and calcium silicate gel characterized through selective dissolution and FTIR spectral subtraction. Cem. Concr. Res. 2015, 70, 39–49. [Google Scholar] [CrossRef]
- Bossard, C.; Granel, H.; Jallot, É.; Montouillout, V.; Fayon, F.; Soulié, J.; Drouet, C.; Wittrant, Y.; Lao, J. Mechanism of Calcium Incorporation Inside Sol-Gel Silicate Bioactive Glass and the Advantage of Using Ca(OH)2 over Other Calcium Sources. ACS Biomater. Sci. Eng. 2019, 5, 5906–5915. [Google Scholar] [CrossRef]
- Wang, H.; Gillott, J.E. Mechanism of Alkali-Silica Reaction and the Significance of Calcium Hydroxide. Cem. Concr. Res. 1991, 21, 647–654. [Google Scholar] [CrossRef]
- Glasser, L.S.D.; Kataoka, N. On the Role of Calcium in the Alkali-Aggregate Reaction. Cem. Concr. Res. 1982, 12, 321–331. [Google Scholar] [CrossRef]
- Amirhossein, M.; Wei, J.Q. Characterization of Calcium Silicate Hydrate Gels with Different Calcium to Silica Ratios and Polymer Modifications. Gels 2022, 8, 75. [Google Scholar] [CrossRef]
- Duan, S.Y.; Liao, H.Q.; Cheng, F.Q.; Song, H.P.; Yang, H.Q. Investigation into the synergistic effects in hydrated gelling systems containing fly ash, desulfurization gypsum and steel slag. Constr. Build. Mater. 2018, 187, 1113–1120. [Google Scholar] [CrossRef]
- Monteagudo, S.M.; Moragues, A.; Gávez, J.C.; Casati, M.J.; Reyes, E. The degree of hydration assessment of blended cement pastes by differential thermal and thermogravimetric analysis. Morphological evolution of the solid phases. Thermochim. Acta 2014, 592, 37–51. [Google Scholar] [CrossRef]
- Maitra, S.; Bose, S.; Bandyopadhyay, N.; Roychoudhury, A. Dehydration kinetics of calcium aluminate cement hydrate under non-isothermal conditions. Ceram. Int. 2005, 31, 371–374. [Google Scholar] [CrossRef]
- Vaiciukyniene, D.; Nizeviciene, D.; Kiele, A.; Janavicius, E.; Pupeikis, D. Effect of phosphogypsum on the stability upon firing treatment of alkali-activated slag. Constr. Build. Mater. 2018, 184, 485–491. [Google Scholar] [CrossRef]
- van Riessen, A.; Jamieson, E.; Kealley, C.S.; Hart, R.D.; Williams, R.P. Bayer-geopolymers: An exploration of synergy between the alumina and geopolymer industries. Cem. Concr. Comp. 2013, 41, 29–33. [Google Scholar] [CrossRef]
- Li, M.G.; Tan, H.B.; Zhang, J.J.; Deng, X.F.; Kong, X.H.; Chen, P.; Jian, S.W.; He, X.Y.; Yang, J. Enhancement in compressive strength of carbide slag activated ground granulated blast furnace slag by introducing CaCl2 and NaCl. Constr. Build. Mater. 2023, 385, 131071. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Neupane, K. Fly ash and GGBFS based powder-activated geopolymer binders: A viable sustainable alternative of portland cement in concrete industry. Mech. Mater. 2016, 103, 110–122. [Google Scholar] [CrossRef]
- Guo, W.C.; Zhang, Z.Y.; Bai, Y.Y.; Zhao, G.Q.; Sang, Z.H.; Zhao, Q.X. Development and characterization of a new multi-strength level binder system using soda residue-carbide slag as composite activator. Constr. Build. Mater. 2021, 291, 123367. [Google Scholar] [CrossRef]
- Jiang, H.S.; Ke, G.J.; Li, Z.Y. Synergistic activation mechanism and long-term properties of a solid waste cementitious materials based on red mud and calcium carbide slag. Constr. Build. Mater. 2024, 438, 137313. [Google Scholar] [CrossRef]
- Ren, Q.S.; Qi, W.Y.; Zhao, Q.X.; Jia, Y.L.; Feng, Y.B.; Han, Y.J.; Duan, G.; Pang, H.T. Preparation and Characterization of Low-Carbon Cementitious Materials Based on Soda-Residue-Activated Ground Granulated Blast-Furnace Slag: A Case Study on Cemented Paste Backfills. Metals 2023, 13, 694. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Wang, X.; Zhao, X.-e. Sustainable application of waste eggshell as fillers in alkali-activated solid waste-based materials: Varying treated methods and particle sizes. Constr. Build. Mater. 2024, 425, 136040. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.J.; Zhang, S.Q.; Huang, X.Y.; Wang, X.; Wang, Y.; Ni, W. Research Progress of Low-Carbon Cementitious Materials Based on Synergistic Industrial Wastes. Energies 2023, 16, 2376. [Google Scholar] [CrossRef]
- Chen, P.; Ma, B.; Tan, H.; Wu, L.; Zheng, Z.; He, X.; Li, H.; Jin, Z.; Li, M.; Lv, Z. Improving the mechanical property and water resistance of β-hemihydrate phosphogypsum by incorporating ground blast-furnace slag and steel slag. Constr. Build. Mater. 2022, 344, 128265. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Gu, X.W.; Liu, J.P.; Xu, X.C. Study on the Properties and Hydration Mechanism of Calcium Carbide Residue-Based Low-Carbon Cementitious Materials. Buildings 2024, 14, 1259. [Google Scholar] [CrossRef]
- Feng, Y.B.; Li, F.; Qi, W.Y.; Ren, Q.S.; Qi, W.Y.; Duan, G.; Zheng, K.L.; Han, Y.J.; Pang, H.T. Mechanical Properties and Microstructure of Iron Tailings Cemented Paste Backfills Using Carbide Slag-Activated Ground Granulated Blast-Furnace Slag as Alternative Binder. Minerals 2022, 12, 1549. [Google Scholar] [CrossRef]
- Cong, P.L.; Mei, L.N. Using silica fume for improvement of fly ash/slag based geopolymer activated with calcium carbide residue and gypsum. Constr. Build. Mater. 2021, 275, 122171. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.B.; Guo, J.W.; Cai, G.J.; Li, Y.F. Study on mechanical properties and microstructure of metakaolin-slag-calcium carbide residue synergistic solidifying waste engineering mud. Constr. Build. Mater. 2024, 438, 137135. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Luo, J.Y.; Cao, J.S.; Kang, C.J.; Wang, S.N.; Li, K.Y.; Zhao, J.N.; Aleem, M.; Wang, D.B. Enhanced volatile fatty acids production from waste activated sludge with synchronous phosphorus fixation and pathogens inactivation by calcium hypochlorite stimulation. Sci. Total Environ. 2020, 712, 136500. [Google Scholar] [CrossRef]
- Yin, W.Z.; Yang, B.; Fu, Y.F.; Chu, F.D.; Yao, J.; Cao, S.H.; Zhu, Z.L. Effect of calcium hypochlorite on flotation separation of covellite and pyrite. Powder Technol. 2019, 343, 578–585. [Google Scholar] [CrossRef]
- Kim, W.; Saito, F. Sonochemical synthesis of hydroxyapatite from H3PO4 solution with Ca(OH)2. Ultrason. Sonochem. 2001, 8, 85–88. [Google Scholar] [CrossRef]
- Dou, W.X.; Zhou, Z.; Jiang, L.M.; Jiang, A.J.; Huang, R.W.; Tian, X.C.; Zhang, W.; Chen, D.Q. Sulfate removal from wastewater using ettringite precipitation: Magnesium ion inhibition and process optimization. J. Environ. Manag. 2017, 196, 518–526. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, J.; Cao, J.X.; Li, C.; Shi, D.; Gao, M.R.; Wang, Y.; Zhang, C.S.; Ding, S.M. A new method to overall immobilization of phosphorus in sediments through combined application of capping and oxidizing agents. Sci. Total Environ. 2019, 694, 133770. [Google Scholar] [CrossRef]
- Fang, D.X.; Huang, L.P.; Fang, Z.Y.; Zhang, Q.; Shen, Q.S.; Li, Y.M.; Xu, X.Y.; Ji, F.Y. Evaluation of porous calcium silicate hydrate derived from carbide slag for removing phosphate from wastewater. Chem. Eng. J. 2018, 354, 1–11. [Google Scholar] [CrossRef]
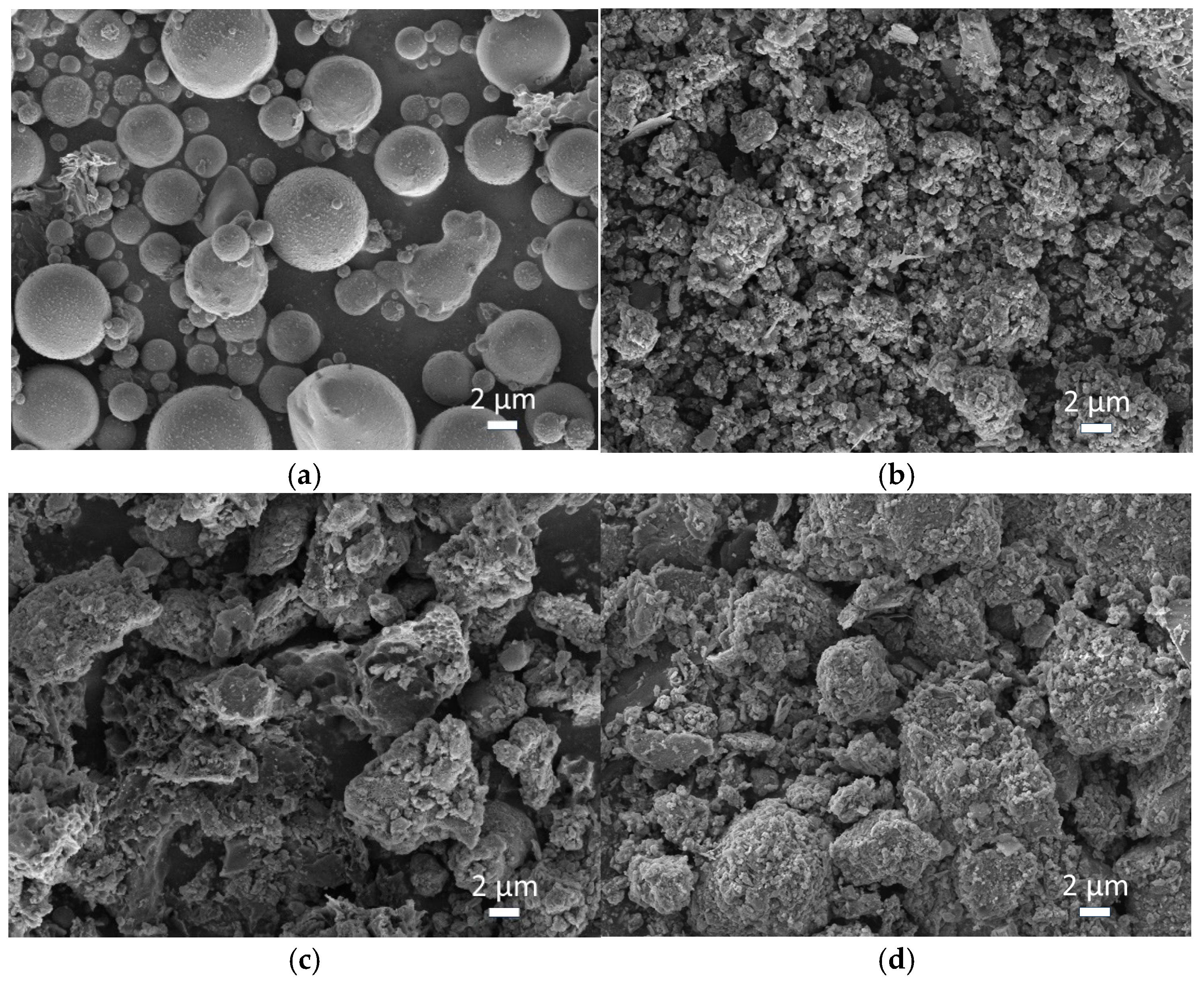



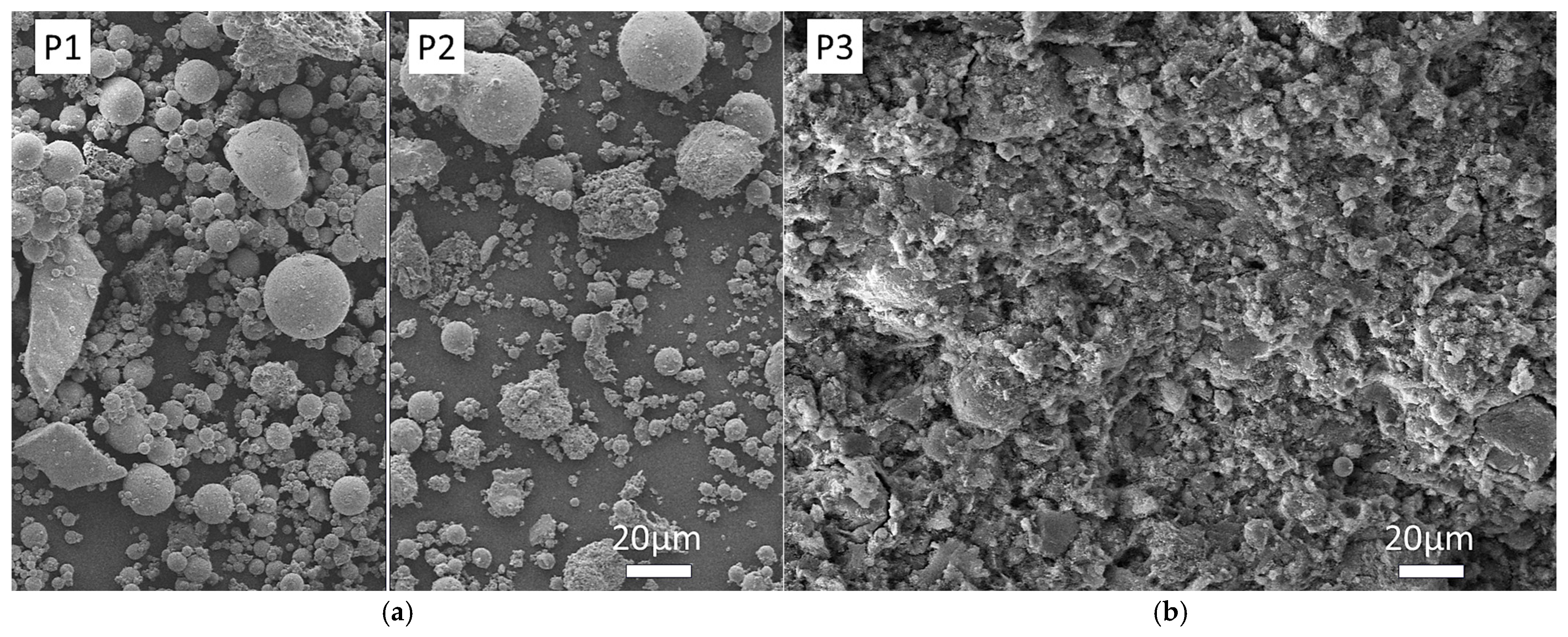

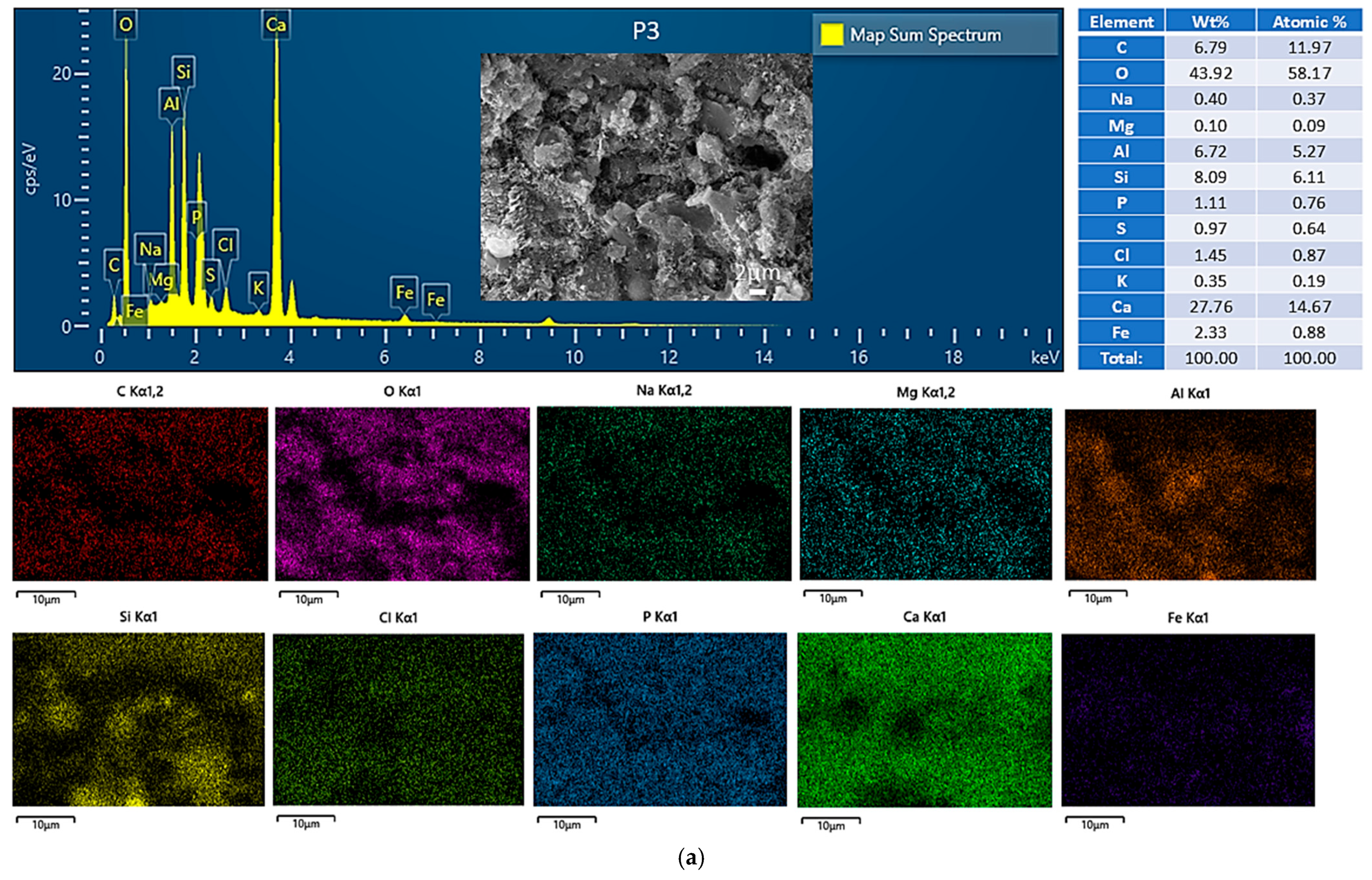
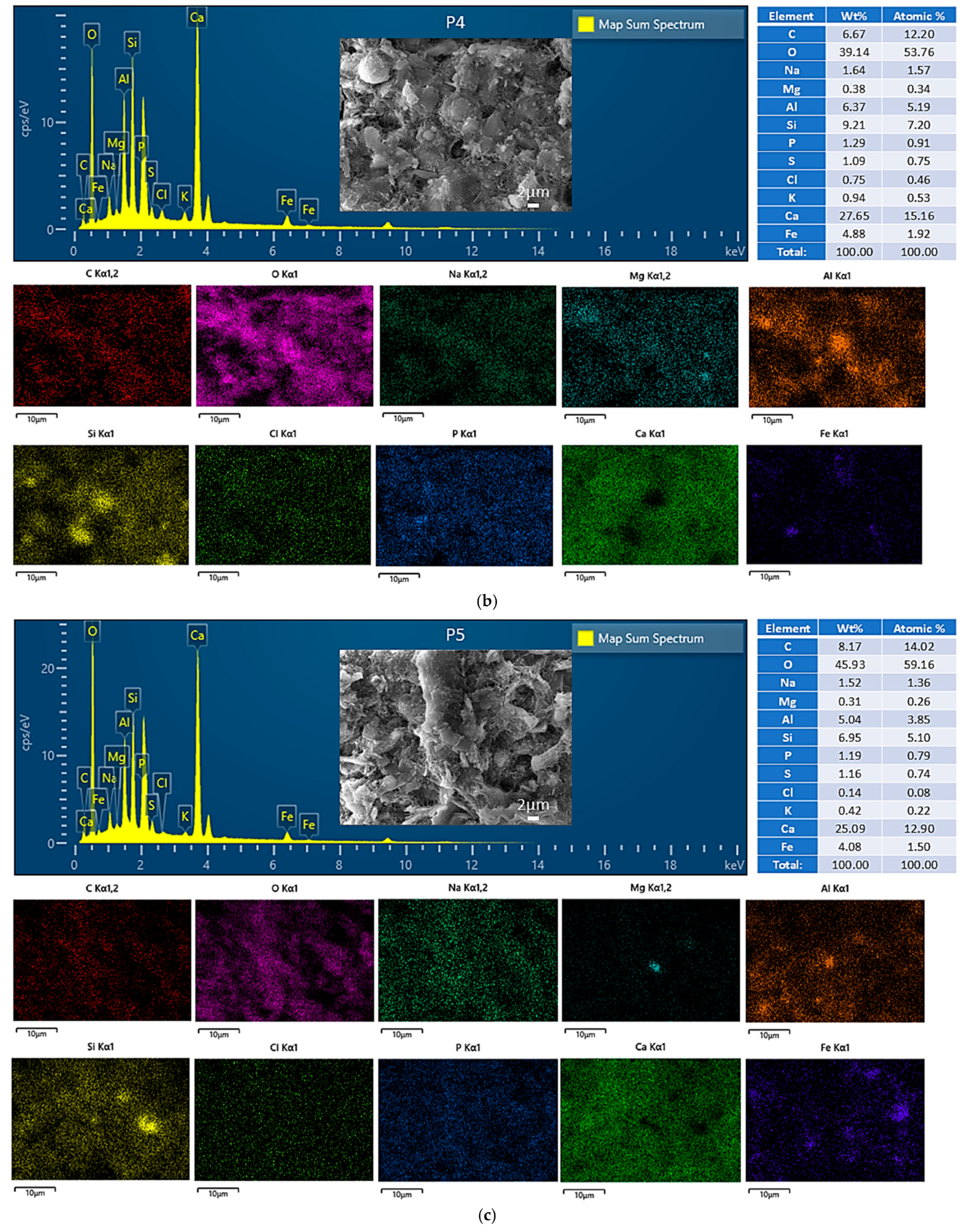


| Index | Parameters | Fly Ash (FA) | Red Mud (RM) | Carbide Slag (CS) | Steel Slag (SS) |
|---|---|---|---|---|---|
| Chemical (mass%) | SiO2 | 45.10 | 27.50 | 2.72 | 16.00 |
| Al2O3 | 25.32 | 28.40 | 2.28 | 5.68 | |
| CaO | 5.32 | 2.50 | 78.86 | 43.60 | |
| MgO | 1.60 | 0.20 | 0.00 | 10.20 | |
| Fe2O3 | 9.50 | 25.80 | 1.02 | 16.50 | |
| K2O | 0.00 | 0.10 | 0.05 | 0.37 | |
| Na2O | 0.00 | 14.70 | 0.00 | 0.24 | |
| Others | 13.16 | 0.80 | 15.07 | 7.41 | |
| Physical | Specific gravity (−) | 2.45 | 2.56 | 1.80 | 2.01 |
| Specific surface area (m2/kg) | 640 | 360 | 420 | 445 |
| Roles | Solid Substances | Solid Mass (g) | Water Mass (g) | Electrical Conductivity (mS/cm) | pH Value (-) |
|---|---|---|---|---|---|
| Water | — | — | 160.0 | 1.29 | 9.03 |
| By-products | Fly ash | 80.0 | 160.0 | 1.68 | 9.89 |
| Red mud | 80.0 | 160.0 | 3.05 | 9.56 | |
| Carbide slag | 80.0 | 160.0 | 3.52 | 12.22 | |
| Steel slag | 80.0 | 160.0 | 2.97 | 12.13 | |
| Additives | Ca3(PO4)2 | 2.0 | 160.0 | 1.28 | 8.71 |
| Ca(ClO)2 | 2.0 | 160.0 | 6.53 | 11.32 |
| Name of Additives | Calcium Hypochlorite | Tricalcium Phosphate |
|---|---|---|
| Chemical formula | Ca(ClO)2 | Ca3(PO4)2 |
| Molecular mass (g/mol) | 142.920 | 310.000 |
| Effective chlorine Cl | ≥35.000% | — |
| Effective content | — | ≥34.000% |
| Hydrochloric acid insoluble | ≤0.050% | ≤0.040% |
| Ammonia precipitate | ≤0.200% | — |
| Nitrate (NO3) | — | ≤0.200% |
| Arsenic (As) | — | ≤0.002% |
| Sulphate (SO4) | ≤0.100% | ≤0.020% |
| Iron (Fe) | ≤0.005% | ≤0.010% |
| Heavy metals (as Pb) | ≤0.002% | ≤0.002% |
| Magnesium and metal salts | ≤0.500% | — |
| Moisture content | — | ≤1.000% |
| No. | Sample | By-Products (g) | Additives (g) with a 1:1 Mass Blend | Tap Water (g) | River Sand (g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fly Ash | Red Mud | Carbide Slag | Steel Slag | Ca3(PO4)2 | Ca(ClO)2 | ||||
| P1 | FA-CA | 400 | — | — | — | 5 | 5 | 182 | — |
| P2 | FA-RM-CA | 200 | 200 | — | — | 5 | 5 | 182 | — |
| P3 | FA-RM-CS-CA | 200 | 100 | 100 | — | 5 | 5 | 182 | — |
| P4 | FA-RM-CS-SS-CA | 100 | 100 | 100 | 100 | 5 | 5 | 182 | — |
| P5 | FA-RM-CS-SS-00 | 100 | 100 | 100 | 100 | 0 | 0 | 182 | — |
| DP1 | FA-CA | 400 | — | — | — | 5 | 5 | 200 | 800 |
| DP2 | FA-RM-CA | 200 | 200 | — | — | 5 | 5 | 200 | 800 |
| DP3 | FA-RM-CS-CA | 200 | 100 | 100 | — | 5 | 5 | 200 | 800 |
| DP4 | FA-RM-CS-SS-CA | 100 | 100 | 100 | 100 | 5 | 5 | 200 | 800 |
| DP5 | FA-RM-CS-SS-00 | 100 | 100 | 100 | 100 | 0 | 0 | 200 | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Zhao, X.; Wang, H. Synthesis and Property Characterization of Low-Activity Waste-Derived Quaternary Cementitious Materials. Buildings 2025, 15, 1426. https://doi.org/10.3390/buildings15091426
Jiang L, Zhao X, Wang H. Synthesis and Property Characterization of Low-Activity Waste-Derived Quaternary Cementitious Materials. Buildings. 2025; 15(9):1426. https://doi.org/10.3390/buildings15091426
Chicago/Turabian StyleJiang, Linlin, Xianhui Zhao, and Haoyu Wang. 2025. "Synthesis and Property Characterization of Low-Activity Waste-Derived Quaternary Cementitious Materials" Buildings 15, no. 9: 1426. https://doi.org/10.3390/buildings15091426
APA StyleJiang, L., Zhao, X., & Wang, H. (2025). Synthesis and Property Characterization of Low-Activity Waste-Derived Quaternary Cementitious Materials. Buildings, 15(9), 1426. https://doi.org/10.3390/buildings15091426







