Survival of Enterohemorrhagic Escherichia coli O104:H4 Strain C227/11Φcu in Agricultural Soils Depends on rpoS and Environmental Factors
Abstract
:1. Introduction
2. Results
2.1. Construction of Deletion Mutants
2.2. Transcription of Cloned fliC or rpoS Genes on Complementation Plasmids
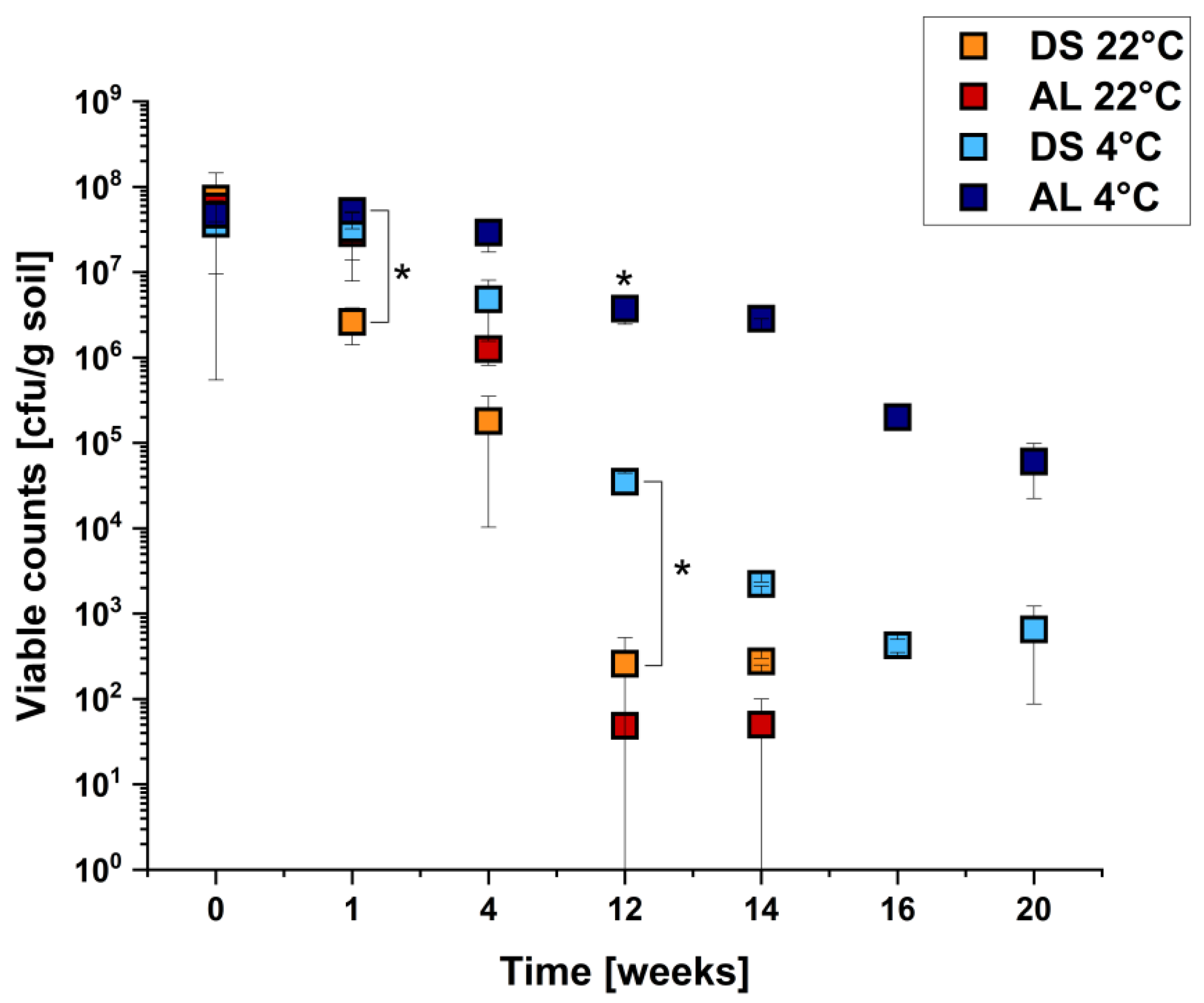
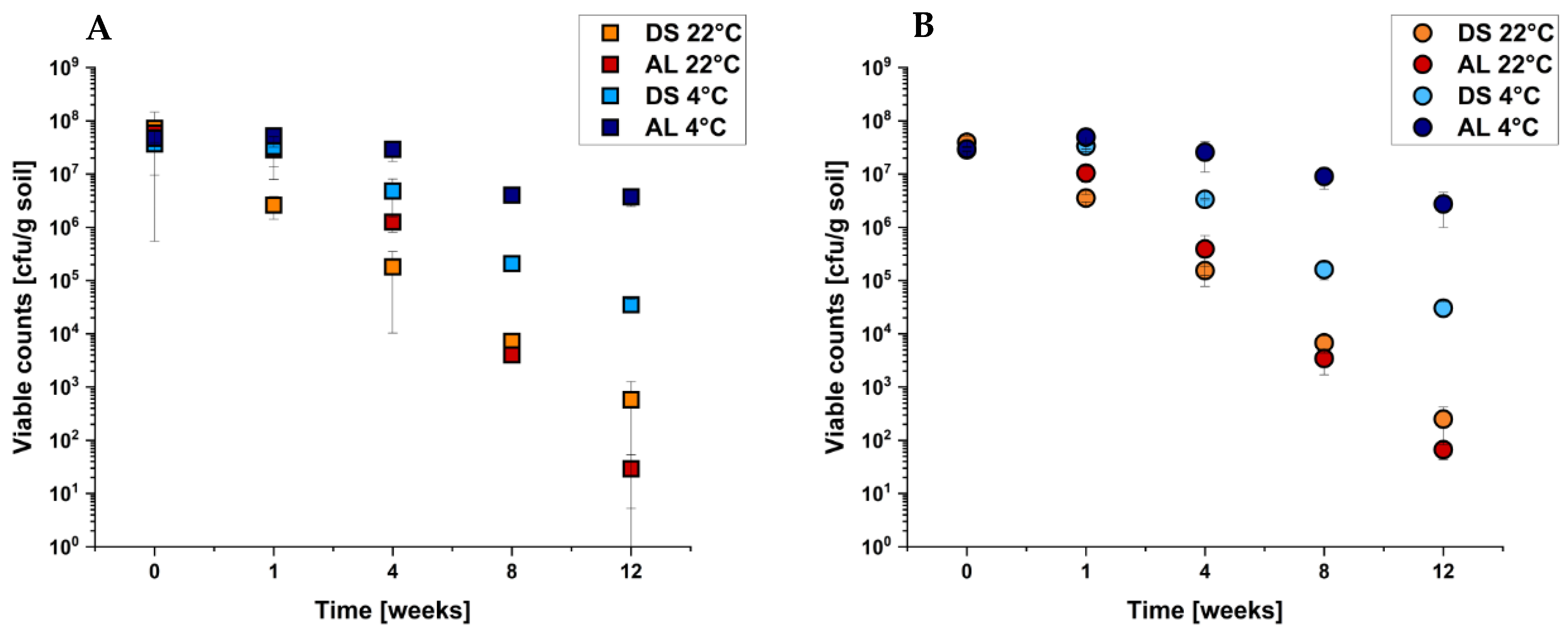
2.3. Survival of E. coli O104:H4 Strain C227/11Φcu in Different Soil Types under Different Temperatures
2.4. Addition of Cattle Manure to Soil Microenvironments Improves Survival of E. coli O104:H4 Strain C227/11Φcu
2.5. Influence of RpoS and FliC on the Survival of E. coli O104:H4 Strain C227/11Φcu in Soil Samples
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Plasmids
4.2. Preparation of Electrocompetent Bacterial Cells and Electroporation
4.3. Construction of Gene Deletion Mutant
4.4. Plasmid Construction
4.5. Complementation of Deletion Mutants
4.6. Analysis of Gene Transcription
4.7. Soil Microenvironment Inoculation Experiments
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaper, J.B. Enterohemorrhagic Escherichia coli. Curr. Opin. Microbiol. 1998, 1, 103–108. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Mermin, J.H.; Mshar, P.A.; Hadler, J.L.; Voetsch, A.; Wojtkunski, C.; Swartz, M.; Mshar, R.; Lambert-Fair, M.-A.; Farrar, J.A.; et al. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Intern. Med. 1999, 159, 1758. [Google Scholar] [CrossRef]
- Currie, A.; MacDonald, J.; Ellis, A.; Siushansian, J.; Chui, L.; Charlebois, M.; Peermohamed, M.; Everett, D.; Fehr, M.; Ng, L.K. Outbreak of Escherichia coli O157:H7 infections associated with consumption of beef donair. J. Food Prot. 2007. [Google Scholar] [CrossRef]
- Grant, J.; Wendelboe, A.M.; Wendel, A.; Jepson, B.; Torres, P.; Smelser, C.; Rolfs, R.T. Spinach-associated Escherichia coli O157:H7 outbreak, Utah and New Mexico, 2006. Emerg. Infect. Dis. 2008, 14, 1633–1636. [Google Scholar] [CrossRef]
- King, L.A.; Mailles, A.; Mariani-Kurkdjian, P.; Vernozy-Rozand, C.; Montet, M.P.; Grimont, F.; Pihier, N.; Devalk, H.; Perret, F.; Bingen, E.; et al. Community-wide outbreak of Escherichia coli O157:H7 associated with consumption of frozen beef burgers. Epidemiol. Infect. 2009, 137, 889–896. [Google Scholar] [CrossRef]
- Greig, J.D.; Ravel, A. Analysis of foodborne outbreak data reported internationally for source attribution. Int. J. Food Microbiol. 2009, 130, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Marder, E.P.; Garman, K.N.; Ingram, L.A.; Dunn, J.R. Multistate outbreak of Escherichia coli O157:H7 associated with bagged salad. Foodborne Pathog. Dis. 2014, 11, 593–595. [Google Scholar] [CrossRef]
- Robert Koch Institute. Final Presentation and Evaluation of Epidemiological Findings in the EHEC O104:H4 Outbreak; Robert Koch Institute: Berlin, Germany, 2011. [Google Scholar]
- King, L.A.; Nogareda, F.; Weill, F.-X.; Mariani-Kurkdjian, P.; Loukiadis, E.; Gault, G.; Jourdan-DaSilva, N.; Bingen, E.; Mace, M.; Thevenot, D.; et al. Outbreak of Shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin. Infect. Dis. 2012, 54, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Robert Koch-Institut. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2011. Krankenh.-Hyg. Infekt. 2012, 34, 174–175. [Google Scholar] [CrossRef]
- Holden, N.; Pritchard, L.; Toth, I. Colonization outwith the colon: Plants as an alternative environmental reservoir for human pathogenic enterobacteria. FEMS Microbiol. Rev. 2009, 33, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Crozier, L.; Marshall, J.; Merget, B.; Holmes, A.; Holden, N.J. Differences in internalization and growth of Escherichia coli O157:H7 within the apoplast of edible plants, spinach and lettuce, compared with the model species Nicotiana benthamiana. Microb. Biotechnol. 2017, 10, 555–569. [Google Scholar] [CrossRef] [Green Version]
- Chitarra, W.; Decastelli, L.; Garibaldi, A.; Gullino, M.L. Potential uptake of Escherichia coli O157:H7 and Listeria monocytogenes from growth substrate into leaves of salad plants and basil grown in soil irrigated with contaminated water. Int. J. Food Microbiol. 2014, 189, 139–145. [Google Scholar] [CrossRef]
- Wright, K.M.; Holden, N.J. Quantification and colonisation dynamics of Escherichia coli O157:H7 inoculation of microgreens species and plant growth substrates. Int. J. Food Microbiol. 2018, 273, 1–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation; Food and Agriculture Organization. Microbiological Hazards in Fresh Leafy Vegetables and Herbs; Microbiological Riskassessment Series; Meeting Report. 2008. Available online: https://www.fao.org/3/i0452e/i0452e.pdf (accessed on 4 November 2021).
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Renter, D.G.; Sargeant, J.M.; Oberst, R.D.; Samadpour, M. Diversity, frequency, and persistence of Escherichia coli O157 strains from range cattle environments. Appl. Environ. Microbiol. 2003, 69, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Fegan, N.; Vanderlinde, P.; Higgs, G.; Desmarchelier, P. The prevalence and concentration of Escherichia coli O157 in faeces of cattle from different production systems at slaughter. J. Appl. Microbiol. 2004, 97, 362–370. [Google Scholar] [CrossRef]
- McCabe, E.; Burgess, C.M.; Lawal, D.; Whyte, P.; Duffy, G. An investigation of shedding and super-shedding of Shiga toxigenic Escherichia coli O157 and E. coli O26 in cattle presented for slaughter in the Republic of Ireland. Zoonoses Public Health 2019, 66, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, J.V.; Karns, J.S. Persistence of Escherichia coli O157:H7 in soil and on plant roots. Environ. Microbiol. 2002, 4, 89–96. [Google Scholar] [CrossRef]
- Ongeng, D.; Geeraerd, A.H.; Springael, D.; Ryckeboer, J.; Muyanja, C.; Mauriello, G. Fate of Escherichia coli O157:H7 and Salmonella enterica in the manure-amended soil-plant ecosystem of fresh vegetable crops: A review. Crit. Rev. Microbiol. 2015, 41, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C.; Webb, C.C.; Diaz-Perez, J.C.; Phatak, S.C.; Silvoy, J.J.; Davey, L.; Payton, A.S.; Liao, J.; Ma, L.; Doyle, M.P. Surface and internalized Escherichia coli O157:H7 on field-grown spinach and lettuce treated with spray-contaminated irrigation water. J. Food Prot. 2010, 73, 1023–1029. [Google Scholar] [CrossRef]
- Islam, M.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Persistence of enterohemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. J. Food Prot. 2004, 67, 1365–1370. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, H.; Wu, L.; Lou, J.; Wu, J.; Brookes, P.C.; Xu, J. Survival of Escherichia coli O157:H7 in soils from Jiangsu Province, China. PLoS ONE 2013, 8, e81178. [Google Scholar] [CrossRef] [PubMed]
- Franz, E.; van Hoek, A.H.A.M.; Bouw, E.; Aarts, H.J.M. Variability of Escherichia coli O157 strain survival in manure-amended soil in relation to strain origin, virulence profile, and carbon nutrition profile. Appl. Environ. Microbiol. 2011, 77, 8088–8096. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Morgan, J.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Persistence of Salmonella enterica serovar Typhimurium on lettuce and parsley and in soils on which they were grown in fields treated with contaminated manure composts or irrigation water. Foodborne Pathog. Dis. 2004, 1, 27–35. [Google Scholar] [CrossRef]
- Locatelli, A.; Spor, A.; Jolivet, C.; Piveteau, P.; Hartmann, A. Biotic and abiotic soil properties influence survival of Listeria monocytogenes in soil. PLoS ONE 2013, 8, e75969. [Google Scholar] [CrossRef] [Green Version]
- NandaKafle, G.; Christie, A.A.; Vilain, S.; Brözel, V.S. Growth and extended survival of Escherichia coli O157:H7 in soil organic matter. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, E.B.; Yaron, S.; Matthews, K.R. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 2002, 68, 397–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Bauchan, G.; Nichols-Russell, L.; Luo, Y.; Wang, Q.; Nou, X. Proliferation of Escherichia coli O157:H7 in soil-substitute and hydroponic microgreen production systems. J. Food Prot. 2015, 78, 1785–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, M.C.; Webb, C.C.; Davey, L.E.; Payton, A.S.; Flitcroft, I.D.; Doyle, M.P. Biotic and abiotic variables affecting internalization and fate of Escherichia coli O157:H7 isolates in leafy green roots. J. Food Prot. 2014, 77, 872–879. [Google Scholar] [CrossRef]
- Zangari, T.; Melton-Celsa, A.R.; Panda, A.; Boisen, N.; Smith, M.A.; Tatarov, I.; De Tolla, L.J.; Nataro, J.P.; O’Brien, A.D. Virulence of the Shiga toxin type 2-expressing Escherichia coli O104:H4 German outbreak isolate in two animal models. Infect. Immun. 2013, 81, 1562–1574. [Google Scholar] [CrossRef] [Green Version]
- Rühlmann, J.; Ruppel, S. Effects of organic amendments on soil carbon content and microbial biomass—Results of the long-term box plot experiment in Grossbeeren. Arch. Agron. Soil Sci. 2005, 51, 163–170. [Google Scholar] [CrossRef]
- Schreiter, S.; Ding, G.-C.; Heuer, H.; Neumann, G.; Sandmann, M.; Grosch, R.; Kropf, S.; Smalla, K. Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce. Front. Microbiol. 2014, 5, 144. [Google Scholar] [CrossRef]
- Eißenberger, K.; Drissner, D.; Walsh, F.; Weiss, A.; Schmidt, H. Plant variety and soil type influence Escherichia coli O104:H4 strain C227/11ϕcu adherence to and internalization into the roots of lettuce plants. Food Microbiol. 2020, 86, 103316. [Google Scholar] [CrossRef]
- Eißenberger, K.; Moench, D.; Drissner, D.; Weiss, A.; Schmidt, H. Adherence factors of enterohemorrhagic Escherichia coli O157:H7 strain Sakai influence its uptake into the roots of Valerianella locusta grown in soil. Food Microbiol. 2018, 76, 245–256. [Google Scholar] [CrossRef]
- Jechalke, S.; Schierstaedt, J.; Becker, M.; Flemer, B.; Grosch, R.; Smalla, K.; Schikora, A. Salmonella establishment in agricultural soil and colonization of crop plants depend on soil type and plant species. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schierstaedt, J.; Jechalke, S.; Nesme, J.; Neuhaus, K.; Sørensen, S.J.; Grosch, R.; Smalla, K.; Schikora, A. Salmonella persistence in soil depends on reciprocal interactions with indigenous microorganisms. Environ. Microbiol. 2020, 22, 2639–2652. [Google Scholar] [CrossRef] [Green Version]
- Battesti, A.; Majdalani, N.; Gottesman, S. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 2011, 65, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, H.; Polen, T.; Heuveling, J.; Wendisch, V.; Hengge, R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 2005, 187, 1591–1603. [Google Scholar] [CrossRef] [Green Version]
- Hengge, R. Proteolysis of σS (RpoS) and the general stress response in Escherichia coli. Res. Microbiol. 2009, 160, 667–676. [Google Scholar] [CrossRef]
- Van Hoek, A.H.A.M.; Aarts, H.J.M.; Bouw, E.; van Overbeek, W.M.; Franz, E. The role of rpoS in Escherichia coli O157 manure-amended soil survival and distribution of allelic variations among bovine, food and clinical isolates. FEMS Microbiol. Lett. 2013, 338, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Sauer, K.; Camper, A.K. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 2001, 183, 6579–6589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pion, M.; Bshary, R.; Bindschedler, S.; Filippidou, S.; Wick, L.Y.; Job, D.; Junier, P. Gains of bacterial flagellar motility in a fungal world. Appl. Environ. Microbiol. 2013, 79, 6862–6867. [Google Scholar] [CrossRef] [Green Version]
- Haiko, J.; Westerlund-Wikström, B. The role of the bacterial flagellum in adhesion and virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef] [Green Version]
- Duan, Q.; Zhou, M.; Zhu, L.; Zhu, G. Flagella and bacterial pathogenicity. J. Basic Microbiol. 2013, 53, 1–8. [Google Scholar] [CrossRef]
- Saile, N.; Schwarz, L.; Eißenberger, K.; Klumpp, J.; Fricke, F.W.; Schmidt, H. Growth advantage of Escherichia coli O104:H4 strains on 5-N-acetyl-9-O-acetyl neuraminic acid as a carbon source is dependent on heterogeneous phage-borne nanS-p esterases. Int. J. Med. Microbiol. 2018, 308, 459–468. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [Green Version]
- Bolivar, F.; Rodriguez, R.L.; Greene, P.J.; Betlach, M.C.; Heyneker, H.L.; Boyer, H.W.; Crosa, J.H.; Falkow, S. Construction and characterization of new cloning vehicle. II. A multipurpose cloning system. Gene 1977, 2, 95–113. [Google Scholar] [CrossRef]
- Reddy, K.R.; Khaleel, R.; Overcash, M.R. Behavior and transport of microbial pathogens and indicator organisms in soils treated with organic wastes. J. Environ. Qual. 1981, 10, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Beuchat, L.R. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2002, 4, 413–423. [Google Scholar] [CrossRef]
- Persad, A.K.; LeJeune, J.T. Animal Reservoirs of Shiga Toxin-Producing Escherichia coli. Microbiol. Spectr. 2014, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Fegan, N.; Gobius, K.S.; Dykes, G.A. Pathogenic Escherichia coli. Encycl. Meat Sci. 2014, 2, 357–361. [Google Scholar] [CrossRef]
- Ogden, I.D.; Hepburn, N.F.; MacRae, M.; Strachan, N.J.C.; Fenlon, D.R.; Rusbridge, S.M.; Pennington, T.H. Long-term survival of Escherichia coli O157 on pasture following an outbreak associated with sheep at a scout camp. Lett. Appl. Microbiol. 2002, 34, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Cheasty, T.; Stuart, J.; Crampin, M.; Willshaw, G.; Djuretic, T.; Hancock, R.; Elstob, C.; Rouse, A. Outbreak of Escherichia coli O157 infection associated with a music festival. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Morgan, J.; Doyle, M.P. Fate of Escherichia coli O157:H7 in manure-amended soil. Appl. Environ. Microbiol. 2002, 68, 2605–2609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, H.P.; Casey, P.G.; Cotter, J.; Gahan, C.G.M.; Hill, C. Factors affecting survival of Listeria monocytogenes and Listeria innocua in soil samples. Arch. Microbiol. 2011, 193, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Underthun, K.; De, J.; Gutierrez, A.; Silverberg, R.; Schneider, K.R. Survival of Salmonella and Escherichia coli in two different soil types at various moisture levels and temperatures. J. Food Prot. 2018, 81, 150–157. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Garbeva, P.; Salles, J. Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 2002, 13, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, E.L.; Richards, K.G.; Ritz, K.; Tyrrel, S.F.; Brennan, F.P. Impact of soil type, biology and temperature on the survival of non-toxigenic Escherichia coli O157. Biol. Environ. Proc. R. Irish Acad. 2013, 113, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Semenov, A.V.; Van Bruggen, A.H.C.; Van Overbeek, L.; Termorshuizen, A.J.; Semenov, A.M. Influence of temperature fluctuations on Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in cow manure. FEMS Microbiol. Ecol. 2007, 60, 419–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and their contaminants in soils, surface and groundwater. In Encyclopedia of the Anthropocene; Elsevier: Oxford, UK, 2018; Volume 5, pp. 225–240. [Google Scholar] [CrossRef]
- Besser, T.E.; Hancock, D.D.; Pritchett, L.C.; McRae, E.M.; Rice, D.H.; Tarr, P.I. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 1997, 175, 726–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shere, J.A.; Bartlett, K.J.; Kaspar, C.W. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl. Environ. Microbiol. 1998, 64, 1390–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Doyle, M.P.; Shere, J.; Garber, L. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 1995, 61, 1290–1293. [Google Scholar] [CrossRef] [Green Version]
- Chase-Topping, M.E.; McKendrick, I.J.; Pearce, M.C.; MacDonald, P.; Matthews, L.; Halliday, J.; Allison, L.; Fenlon, D.; Low, J.C.; Gunn, G.; et al. Risk factors for the presence of high-level shedders of Escherichia coli O157 on Scottish farms. J. Clin. Microbiol. 2007, 45, 1594–1603. [Google Scholar] [CrossRef] [Green Version]
- LeJeune, J.T.; Besser, T.E.; Rice, D.H.; Berg, J.L.; Stilborn, R.P.; Hancock, D.D. Longitudinal study of fecal shedding of Escherichia coli O157:H7 in feedlot cattle: Predominance and persistence of specific clonal types despite massive cattle population turnover. Appl. Environ. Microbiol. 2004, 70, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Naylor, S.W.; Low, J.C.; Besser, T.E.; Mahajan, A.; Gunn, G.J.; Pearce, M.C.; McKendrick, I.J.; Smith, D.G.E.; Gally, D.L. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 2003, 71, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Franz, E.; Semenov, A.V.; Termorshuizen, A.J.; de Vos, O.J.; Bokhorst, J.G.; van Bruggen, A.H.C. Manure-amended soil characteristics affecting the survival of E. coli O157:H7 in 36 Dutch soils. Environ. Microbiol. 2008, 10, 313–327. [Google Scholar] [CrossRef]
- Brennan, F.P.; Moynihan, E.; Griffiths, B.S.; Hillier, S.; Owen, J.; Pendlowski, H.; Avery, L.M. Clay mineral type effect on bacterial enteropathogen survival in soil. Sci. Total Environ. 2014, 468–469, 302–305. [Google Scholar] [CrossRef]
- Dowe, M.J.; Jackson, E.D.; Mori, J.G.; Bell, C.R. Listeria monocytogenes survival in soil and incidence in agricultural soils. J. Food Prot. 1997, 60, 1201–1207. [Google Scholar] [CrossRef]
- Cai, P.; Huang, Q.; Walker, S.L. Deposition and survival of Escherichia coli O157:H7 on clay minerals in a parallel plate flow system. Environ. Sci. Technol. 2013, 47, 1896–1903. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, H.; Cai, P.; Fein, J.B.; Chen, W. Atomic force microscopy measurements of bacterial adhesion and biofilm formation onto clay-sized particles. Sci. Rep. 2015, 5, 16857. [Google Scholar] [CrossRef]
- Stotzky, G. Influence of soil mineral colloids on metabolic processes, growth, adhesion, and ecology of microbes and viruses. In Interactions of Soil Minerals with Natural Organics and Microbes; Huang, P., Schnitzer, M., Eds.; Soil Science Society of America: Madison, WI, USA, 1986; Volume 17, pp. 305–428. [Google Scholar] [CrossRef] [Green Version]
- Courvoisier, E.; Dukan, S. Improvement of Escherichia coli growth by kaolinite. Appl. Clay Sci. 2009, 44, 67–70. [Google Scholar] [CrossRef]
- McCaulou, D.R.; Bales, R.C.; Arnold, R.G. Effect of temperature-controlled motility on transport of bacteria and microspheres through saturated sediment. Water Resour. Res. 1995, 31, 271–280. [Google Scholar] [CrossRef]
- Lunsdorf; Erb; Abraham; Timmis ‘Clay hutches’: A novel interaction between bacteria and clay minerals. Environ. Microbiol. 2000, 2, 161–168. [CrossRef]
- Coldewey, S.M.; Hartmann, M.; Schmidt, D.S.; Engelking, U.; Ukena, S.N.; Gunzer, F. Impact of the rpoS genotype for acid resistance patterns of pathogenic and probiotic Escherichia coli. BMC Microbiol. 2007, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Stasic, A.J.; Wong, A.C.L.; Kaspar, C.W. Osmotic and desiccation tolerance in Escherichia coli O157:H7 requires rpoS (σ38). Curr. Microbiol. 2012, 65, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Hryckowian, A.J.; Welch, R.A. RpoS contributes to phagocyte oxidase-mediated stress resistance during urinary tract infection by Escherichia coli CFT073. mBio 2013, 4, e00023-13. [Google Scholar] [CrossRef] [Green Version]
- Somorin, Y.; Abram, F.; Brennan, F.; O’Byrne, C. The general stress response is conserved in long-term soil-persistent strains of Escherichia coli. Appl. Environ. Microbiol. 2016, 82, 4628–4640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saile, N.; Voigt, A.; Kessler, S.; Stressler, T.; Klumpp, J.; Fischer, L.; Schmidt, H. Escherichia coli O157:H7 strain EDL933 harbors multiple functional prophage-associated genes necessary for the utilization of 5-N-acetyl-9-O-acetyl neuraminic acid as a growth substrate. Appl. Environ. Microbiol. 2016, 82, 5940–5950. [Google Scholar] [CrossRef] [Green Version]
- Nübling, S.; Eisele, T.; Stöber, H.; Funk, J.; Polzin, S.; Fischer, L.; Schmidt, H. Bacteriophage 933W encodes a functional esterase downstream of the Shiga toxin 2a operon. Int. J. Med. Microbiol. 2014, 304, 269–274. [Google Scholar] [CrossRef]
- Bondì, R.; Chiani, P.; Michelacci, V.; Minelli, F.; Caprioli, A.; Morabito, S. The gene tia, harbored by the subtilase-encoding pathogenicity island, is involved in the ability of locus of enterocyte effacement-negative Shiga toxin-producing Escherichia coli strains to invade monolayers of epithelial cells. Infect. Immun. 2017, 85, e00613-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinisch, L.; Zoric, K.; Krause, M.; Schmidt, H. Transcription of the subtilase cytotoxin gene subAB1 in Shiga toxin-producing Escherichia coli is dependent on hfq and hns. Appl. Environ. Microbiol. 2019, 85, 85. [Google Scholar] [CrossRef] [PubMed]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef]

| Strain or Plasmid | * Characteristics | Origin |
|---|---|---|
| Strains | ||
| E. coli DH5α | tonA lacZΔM15 endA1 recA1 thi-1 supE44 phoA gyrA96 hsdR17 Δ(lacZYA-argF)U169 relA1 | Invitrogen |
| E. coli O104:H4 strain C227/11Φcu | Serotype O104:H4, cured from the stx2a-phage | [33] |
| E. coli O104:H4 strain C227/11Φcu/ΔrpoS | Deletion of rpoS | this study |
| E. coli O104:H4 strain C227/11Φcu/ΔrpoS/pFJ02 | Deletion of rpoS, complemented, camR | this study |
| E. coli O104:H4 strain C227/11Φcu/ΔfliC | Deletion of fliC | this study |
| E. coli O104:H4 strain C227/11Φcu/ΔfliC/pFJ03 | Deletion of fliC, complemented, camR | this study |
| Plasmids | ||
| pKEC1.5 | Derivative of plasmid pKD46, ampR replaced by camR | [48] |
| pKD4 | Carries kanR flanked by FRT sites | [49] |
| pCP20 | Encoding for FLP recombinase, temperature-sensitive, camR/ampR | [49] |
| pBR322 | Cloning vector, pMB1 origin of replication, ampR, tet R | [50] |
| pFJ01 | pBR322 origin of replication, camR instead of ampR | this study |
| pFJ02 | pBR322 origin of replication, camR instead of ampR, rpoS gene from E. coli O104:H4 C227/11Φcu | this study |
| pFJ03 | pBR322 origin of replication, camR instead of ampR, fliC gene from E. coli O104:H4 C227/11Φcu | this study |
| Name | * Sequence (5′-3′) | Function | Reference |
|---|---|---|---|
| P-cat PvuI for | ATACGATCGAGCGCTGATGTCCGGC | Exchange of resistance | [37] |
| cat PvuI rev | ATACGATCGTTACGCCCCGCCCTGCCA | Exchange of resistance | [37] |
| fliCdel-O104-for | AGCCCAATACTTAAACCGTAGACTTGAAAACAGGAAAATGgcgattgtgtaggctggagc | Mutagenesis | This study |
| fliCdel-O104-rev | GCAGAAAAAACCCCGCCGGTAGCGGGGTCAGGCAGGTTAAcatggtccatatgaatatcctcc | Mutagenesis | This study |
| rpoSdel-O104-for | TTGAATGTTCCGTCAAGGGATCACGGGTAGGAGCCACCTTgcgattgtgtaggctggagc | Mutagenesis | This study |
| rpoSdel-O104-rev | CCAGCCTCGCTTGAGACTGGCCTTTCTGACAGATGCTTACcatggtccatatgaatatcctcc | Mutagenesis | This study |
| fliC-O104-for | CCCAAGCGTTGAAATACTAGCCA | Confirmation of mutagenesis | This study |
| fliC-O104-rev | CTTCAGCGGTATAGAGTGAATTCA | Confirmation of mutagenesis | This study |
| rpoS-O104-for | CTGCGTTATTTGCCGCAGCG | Confirmation of mutagenesis | This study |
| rpoS-O104-rev | GTGCGCAAGATGATGAACGCAT | Confirmation of mutagenesis | This study |
| fliC-HindIII-O104-for | CGCAAGCTTATGGCACAAGTCATTAATA | Complementation | This study |
| fliC-BamHI-O104-rev | TATGGATCCTTAGCCTTGTAACAGAGA | Complementation | This study |
| rpoS-HindIII-O104-for | CCCAAGCTTATGAGTCAGAATACGCTGAAA | Complementation | This study |
| rpoS-BamHI-O104-rev | AATGGATCCTTACTCGCGGAACAGCG | Complementation | This study |
| C227/11Φcu in | δ | p | R2 | Time for 4 Log Reduction (Weeks) | Time to Reach Detection Limit of 102 cfu/g Soil (Weeks) |
|---|---|---|---|---|---|
| DS 22 °C | 1.73 | 0.83 | 0.9642 | ±9.2 weeks | ±13.3 |
| AL 22 °C | 1.15 | 0.74 | 0.9678 | ±7.6 weeks | ±12.3 |
| DS 4 °C | 2.89 | 0.88 | 0.9639 | ±14 weeks | - |
| AL 4 °C | 11.11 | 1.87 | 0.9657 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detert, K.; Schmidt, H. Survival of Enterohemorrhagic Escherichia coli O104:H4 Strain C227/11Φcu in Agricultural Soils Depends on rpoS and Environmental Factors. Pathogens 2021, 10, 1443. https://doi.org/10.3390/pathogens10111443
Detert K, Schmidt H. Survival of Enterohemorrhagic Escherichia coli O104:H4 Strain C227/11Φcu in Agricultural Soils Depends on rpoS and Environmental Factors. Pathogens. 2021; 10(11):1443. https://doi.org/10.3390/pathogens10111443
Chicago/Turabian StyleDetert, Katharina, and Herbert Schmidt. 2021. "Survival of Enterohemorrhagic Escherichia coli O104:H4 Strain C227/11Φcu in Agricultural Soils Depends on rpoS and Environmental Factors" Pathogens 10, no. 11: 1443. https://doi.org/10.3390/pathogens10111443






