Expression Patterns of miR398, miR167, and miR159 in the Interaction between Bread Wheat (Triticum aestivum L.) and Pathogenic Fusarium culmorum and Beneficial Trichoderma Fungi
Abstract
:1. Introduction
2. Results
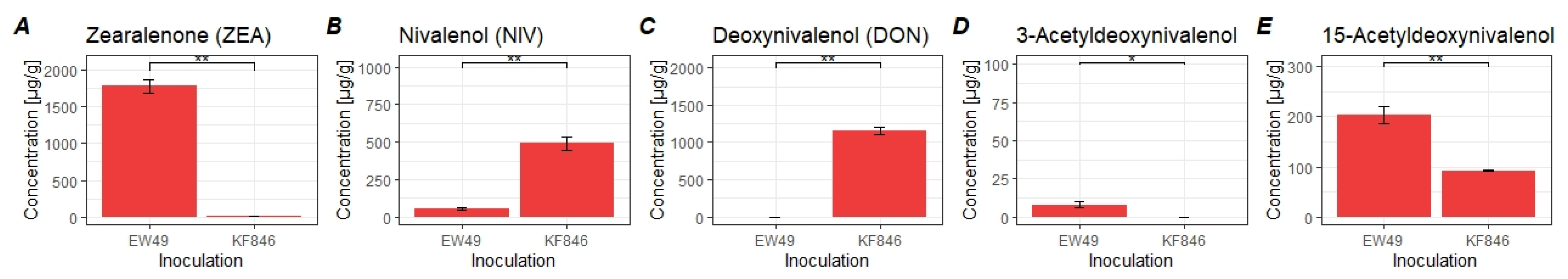
2.1. Interactions between Triticum aestivum L. and Selected Fungi
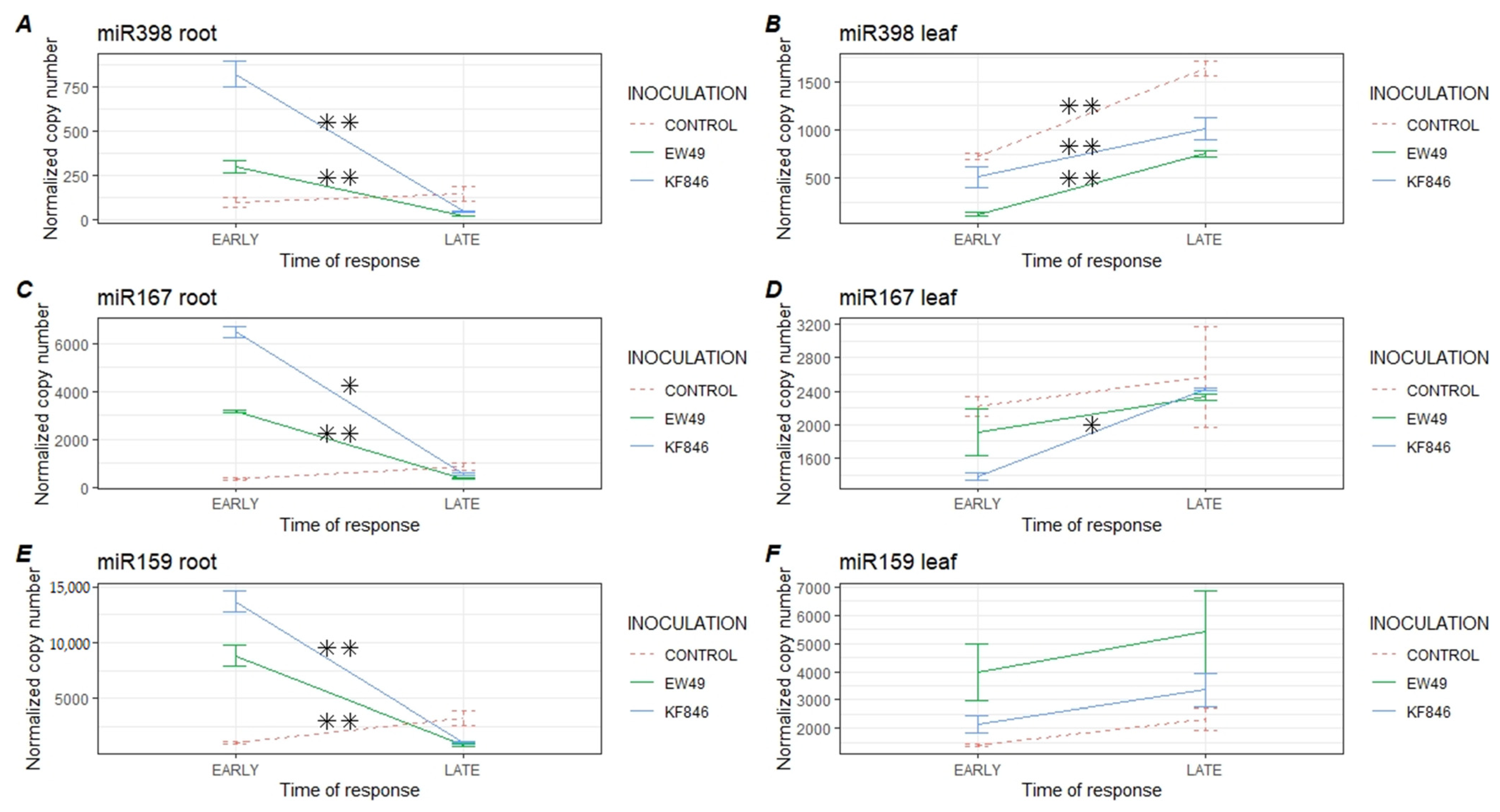
2.2. Early Response of Wheat’s miR398, miR167, and miR159 to Fusarium culmorum Infection
2.3. Late Response of Wheat’s miR398, miR167, and miR159 to Fusarium culmorum Infection
2.4. Comparison of the Temporal Response of Wheat’s miR398, miR167, and miR159 to Fusarium culmorum Infection
2.5. Analysis of the Response of Wheat miR398, miR167 and miR159 to Inoculation of Trichoderma Species and Comparison to the Profiles Formed by Pathogenic Fusarium Strains
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Fungal Collection
4.3. Mycotoxin Analysis
4.4. Inoculum Preparation and Plant Inoculation
4.5. Sampling, Disease Symptoms Assessment
4.6. Total RNA Isolation
4.7. Stem Loop Pulsed RT-PCR
4.8. Reverse Transcription
4.9. Droplet Digital PCR
4.10. Statistical Analysis
| Target Name | Primer Sequence | References |
|---|---|---|
| miR398 | F:GTATACTGTGTTCTCAGGTCG | This study |
| R:GTGCAGGGTCCGAGGT | ||
| miR167 | F:CGCGATGAAGCTGCCAGCAT R:CAGTGCAGGGTCCGAGGT | [61] |
| miR159 | F: CGCGCTTTGGATTGAAGGGA | [61] |
| R:CAGTGCAGGGTCCGAGGT | ||
| ADP-ribosyltransferase | F:GCTCTCCAACAACATTGCCAAC R: GCTTCTGCCTGTCACATACGC | [65] |
4.11. Results Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Katiyar-Agarwal, S.; Jin, H. Role of small RNAs in host-microbe interactions. Annu. Rev. Phytopathol. 2010, 48, 225. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs–big players in plant-microbe interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Lin, L.; Sui, N. Regulation mechanism of microRNA in plant response to abiotic stress and breeding. Mol. Biol. Rep. 2019, 46, 1447–1457. [Google Scholar] [CrossRef]
- Gupta, O.P.; Meena, N.L.; Sharma, I.; Sharma, P. Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat. Mol. Biol. Rep. 2014, 41, 4623–4629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, S.; Zhou, X.; Chellappan, P.; Chen, Z.; Zhou, X.; Jin, H. Bacteria-responsive microRNAs regulate plant innate immunity by modulating plant hormone networks. Plant Mol. Biol. 2011, 75, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaubert-Possamai, S.; Noureddine, Y.; Favery, B. MicroRNAs, new players in the plant–nematode interaction. Front. Plant Sci. 2019, 10, 1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wong, A.Y.; Wang, S.; Jia, Q.; Chuang, W.P.; Bendena, W.G.; Hui, J.H. miRNA-mediated interactions in and between plants and insects. Int. J. Mol. Sci. 2018, 19, 3239. [Google Scholar] [CrossRef] [Green Version]
- Weiberg, A.; Wang, M.; Bellinger, M.; Jin, H. Small RNAs: A new paradigm in plant-microbe interactions. Annu. Rev. Phytopathol. 2014, 52, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, Y.; Song, N.; Zhao, M.; Liu, R.; Feng, H.; Wang, X.; Kang, Z. Puccinia striiformis f. sp. tritici microRNA-like RNA 1 (Pst-milR1), an important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene. New Phytol. 2017, 215, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. World Food and Agriculture-Statistical Yearbook 2020; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal Agent of Foot and Root Rot and Head Blight on Wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef]
- Wiśniewska, H.; Kowalczyk, K. Resistance of cultivars and breeding lines of spring wheat to Fusarium culmorum and powdery mildew. J. Appl. Genet. 2005, 46, 35–40. [Google Scholar]
- Golinski, P.; Waskiewicz, A.; Wisniewska, H.; Kiecana, I.; Mielniczuk, E.; Gromadzka, K.; Rymaniak, E. Reaction of winter wheat (Triticum aestivum L.) cultivars to infection with Fusarium spp.: Mycotoxin contamination in grain and chaff. Food Addit. Contam. 2010, 27, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Góral, T.; Walentyn-Góral, D. Variation for resistance to Fusarium head blight in winter and spring wheat varieties studied in 2009–2016. Biul. Inst. Hod. I Aklim. Roślin 2019, 284, 3–11. [Google Scholar]
- Błaszczyk, L.; Basińska-Barczak, A.; Ćwiek-Kupczyńska, H.; Gromadzka, K.; Popiel, D.; Stępień, Ł. Suppressive Effect of Trichoderma spp. on toxigenic Fusarium species. Pol. J. Microbiol. 2017, 66, 85–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-pentyl-2H-pyran-2-one (6-PAP) and other volatiles by different Trichoderma species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Błaszczyk, L.; Strakowska, J.; Chełkowski, J.; Gąbka-Buszek, A.; Kaczmarek, J. Trichoderma species occurring on wood with decay symptoms in mountain forests in Central Europe: Genetic and enzymatic characterization. J. Appl. Genet. 2016, 57, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Strakowska, J.; Mazzei, P.; Piccolo, A.; Marra, R.; Lombardi, N.; Lorito, M. Cremenolide, a new antifungal, 10-member lactone from Trichoderma cremeum with plant growth promotion activity. Nat. Prod. Res. 2016, 30, 2575–2581. [Google Scholar] [CrossRef] [Green Version]
- Basińska-Barczak, A.; Błaszczyk, L.; Szentner, K. Plant Cell Wall Changes in Common Wheat Roots as a Result of Their Interaction with Beneficial Fungi of Trichoderma. Cells 2020, 9, 2319. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.F.; Song, N.; Wei, J.P.; Wang, X.J.; Feng, H.; Kang, Z.S. MicroRNAs involving in cold, wounding and salt stresses in Triticum aestivum L. Plant Physiol. Biochem. 2014, 80, 90–96. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, R.; Albrecht, U.; Padmanabhan, C.; Wang, A.; Coffey, M.D.; Jin, H. Small RNA profiling reveals phosphorus deficiency as a contributing factor in symptom expression for citrus huanglongbing disease. Mol. Plant 2013, 6, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Xu, K.; Chen, A.; Zhu, Y.; Tang, G.; Xu, G. Expression analysis suggests potential roles of microRNAs for phosphate and arbuscular mycorrhizal signaling in Solanum lycopersicum. Physiol. Plant 2010, 138, 226–237. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, Y.; Yin, M.; Yang, E.; Gong, W.; Liu, C.; Zhang, Y. Identification of novel miRNAs and miRNA expression profiling in wheat hybrid necrosis. PLoS ONE 2015, 10, e0117507. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Shen, C.-H.; Lin, Y.; Chen, P.J.; Xu, X.; Oelmüller, R.; Yeh, K.-W.; Lai, Z. Growth promotion-related miRNAs in Oncidium orchid roots colonized by the endophytic fungus Piriformospora indica. PLoS ONE 2014, 9, e84920. [Google Scholar] [CrossRef]
- Phookaew, P.; Netrphan, S.; Sojikul, P.; Narangajavana, J. Involvement of miR164-and miR167-mediated target gene expressions in responses to water deficit in cassava. Biol. Plant. 2014, 58, 469–478. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, P.; Fang, Y.Y.; Gao, F.; Guo, H.S.; Zhao, J.H. Genome-wide profiling of sRNAs in the Verticillium dahliae-infected Arabidopsis roots. Mycology 2018, 9, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Hai, B.; Guo, J.; Li, Y.; Zhang, L. Characterization of wheat miRNAs and their target genes responsive to cadmium stress. Plant Physiol. Biochem. 2016, 101, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Sun, Y.F.; Song, N.; Wang, X.J.; Feng, H.; Huang, L.L.; Kang, Z.S. Identification of UV-B-induced microRNAs in wheat. Genet. Mol. Res. 2013, 12, 4213–4221. [Google Scholar] [CrossRef]
- Akdogan, G.; Tufekci, E.D.; Uranbey, S.; Unver, T. miRNA-based drought regulation in wheat. Funct. Integr. Genomic 2016, 16, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Wang, Y.; Yao, Y.; Xie, C.; Peng, H.; Ni, Z.; Sun, Q. Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.). BMC Plant Biol. 2010, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Guo, F.; Xu, Q.; Cang, J. LncRNA improves cold resistance of winter wheat by interacting with miR398. Funct. Plant Biol. 2020, 47, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, Y.; Zhu, J.; Liu, S.; Zeng, N.; Zhan, X. miR398 is involved in the relief of phenanthrene-induced oxidative toxicity in wheat roots. Environ. Pollut. 2020, 258, 113701. [Google Scholar] [CrossRef]
- Sunkar, R.; Kapoor, A.; Zhu, J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Ghany, S.E.; Pilon, M. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J. Biol. Chem. 2008, 283, 15932–15945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.J.P.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Sharma, S.; Taneja, M.; Kumar, R.; Sembi, J.K.; Upadhyay, S.K. Superoxide dismutases in bread wheat (Triticum aestivum L.): Comprehensive characterization and expression analysis during development and, biotic and abiotic stresses. Agric. Gene 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, Z.; Bian, L.; Xie, H.; Liang, J. miR398 regulation in rice of the responses to abiotic and biotic stresses depends on CSD1 and CSD2 expression. Funct. Plant Biol. 2011, 38, 44. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Saini, A.; Sunkar, R. Biotic and abiotic stress down-regulate miR398 expression in Arabidopsis. Planta 2009, 229, 1009–1014. [Google Scholar] [CrossRef]
- Luan, Y.; Wang, W.; Liu, P. Identification and functional analysis of novel and conserved microRNAs in tomato. Mol. Biol. Rep. 2014, 41, 5385–5394. [Google Scholar] [CrossRef]
- Naya, L.; Paul, S.; Valdés-López, O.; Mendoza-Soto, A.B.; Nova-Franco, B.; Sosa-Valencia, G.; Hernández, G. Regulation of copper homeostasis and biotic interactions by microRNA 398b in common bean. PLoS ONE 2014, 9, e84416. [Google Scholar]
- Qiu, Z.; He, Y.; Zhang, Y.; Guo, J.; Wang, L. Characterization of miRNAs and their target genes in He-Ne laser pretreated wheat seedlings exposed to drought stress. Ecotoxicol. Environ. Saf. 2018, 164, 611–617. [Google Scholar] [CrossRef]
- Han, Y.; Luan, F.; Zhu, H.; Shao, Y.; Chen, A.; Lu, C.; Zhu, B. Computational identification of microRNAs and their targets in wheat (Triticum aestivum L.). Sci. China Life Sci. 2009, 52, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Tian, Q.; Reed, J.W. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 2006, 133, 4211–4218. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Wang, J.; Wang, W.; Xu, L.A. ARF family identification in Tamarix chinensis reveals the salt responsive expression of TcARF6 targeted by miR167. Plant Biol. 2020, 8, e8829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, N.; Wang, H.; Kasahara, H.; Liu, J.; MacPherson, C.; Machida, Y.; Chua, N.H. IAA-Ala Resistant3, an evolutionarily conserved target of miR167, mediates Arabidopsis root architecture changes during high osmotic stress. Plant Cell 2012, 24, 3590–3602. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Agnew, J.L.; Cohen, J.D.; He, P.; Shan, L.; Sheen, J.; Kunkel, B.N. Pseudomonas syringae type III effector AvrRpt2 alters Arabidopsis thaliana auxin physiology. Proc. Natl. Acad. Sci. USA 2007, 104, 20131–20136. [Google Scholar] [CrossRef] [Green Version]
- Kidd, B.N.; Kadoo, N.Y.; Dombrecht, B.; Tekeoglu, M.; Gardiner, D.M.; Thatcher, L.F.; Aitken, E.A.B.; Schenk, P.M.; Manners, J.M.; Kazan, K. Auxin signaling and transport promote susceptibility to the root-infecting fungal pathogen Fusarium oxysporum in Arabidopsis. Mol. Plant. Microbe Interact. 2011, 24, 733–748. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Satapathy, L.; Basu, S.; Jha, S.K.; Kumar, M.; Mukhopadhyay, K. Characterization of the leaf rust responsive ARF genes in wheat (Triticum aestivum L.). Plant Cell Rep. 2020, 39, 1639–1654. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Chua, N.H. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef]
- Pavitra, K.; Rekha, A.; Ravishankar, K.V. MicroRNA mediated regulation of gene expression in response to soil-borne fungus Fusarium oxysporum f. sp. cubense (Foc1) infection in two contrasting banana genotypes. J. Appl. Hortic. 2017, 19, 191–195. [Google Scholar]
- Inal, B.; Türktaş, M.; Eren, H.; Ilhan, E.; Okay, S.; Atak, M.; Unver, T. Genome-wide fungal stress responsive miRNA expression in wheat. Planta 2014, 240, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.P.; Permar, V.; Koundal, V.; Singh, U.D.; Praveen, S. MicroRNA regulated defense responses in Triticum aestivum L. during Puccinia graminis f. sp. tritici infection. Mol. Biol. Rep. 2012, 39, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jia, L.; Wang, Y.; Li, B.; Sun, D.; Chen, X. Identification of Fusarium graminearum-responsive miRNAs and their targets in wheat by sRNA sequencing and degradome analysis. Funct. Integr. Genom. 2020, 20, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.; Bhuju, S.; Shrestha, J. Effect of Trichoderma viride as biofertilizer on growth and yield of wheat. Malays. J. Sustain. Agric. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Buśko, M.; Chełkowski, J.; Popiel, D.; Perkowski, J. Solid substrate bioassay to evaluate impact of Trichoderma on trichothecene mycotoxin production by Fusarium species. J. Sci. Food Agric. 2008, 88, 536–541. [Google Scholar] [CrossRef]
- Popiel, D.; Kwaśna, H.; Chełkowski, J.; Stępień, S.; Laskowska, M. Impact of selected antagonistic fungi on Fusarium species-toxigenic cereal pathogens. Acta Mycol. 2008, 43, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Beccari, G.; Covarelli, L.; Nicholson, P. Infection process and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant. Pathol. 2011, 60, 671–684. [Google Scholar] [CrossRef]
- Smoczynska, A.; Sega, P.; Stepien, A.; Knop, K.; Jarmołowski, A.; Pacak, A.; Szweykowska-Kulinska, Z. miRNA Detection by Stem-Loop RT-qPCR in Studying microRNA Biogenesis and microRNA Responsiveness to Abiotic Stresses. In Plant MicroRNAs; Humana Press: New York, NY, USA, 2019; pp. 131–150. [Google Scholar]
- Varkonyi-Gasic, E. Stem-loop qRT-PCR for the detection of plant microRNAs. In Plant Epigenetics; Humana Press: Boston, MA, USA, 2017; pp. 163–175. [Google Scholar]
- Feng, H.; Huang, X.; Zhang, Q.; Wei, G.; Wang, X.; Kang, Z. Selection of suitable inner reference genes for relative quantification expression of microRNA in wheat. Plant. Physiol. Biochem. 2012, 51, 116–122. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dinno, A. Dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums, R Package Version 1.3.5. 2017. Available online: https://cran.r-project.org/package=dunn.test (accessed on 8 October 2021).
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis, R Package Version 0.8.32. 2021. Available online: https://github.com/droglenc/FSA (accessed on 8 October 2021).
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]




| miRNA (miRBase Accession No.) | miRNA Sequence | Stem Loop Primer Sequence |
|---|---|---|
| miR398 (MIMAT0018225) | uguguucucaggucgcccccg | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCGGGGG |
| miR167 (MIMAT0005347) | ugaagcugccagcaugaucua | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAGATC |
| miR159 (MIMAT0005343) | uuuggauugaagggagcucug | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAGAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamon, S.; Żok, J.; Gromadzka, K.; Błaszczyk, L. Expression Patterns of miR398, miR167, and miR159 in the Interaction between Bread Wheat (Triticum aestivum L.) and Pathogenic Fusarium culmorum and Beneficial Trichoderma Fungi. Pathogens 2021, 10, 1461. https://doi.org/10.3390/pathogens10111461
Salamon S, Żok J, Gromadzka K, Błaszczyk L. Expression Patterns of miR398, miR167, and miR159 in the Interaction between Bread Wheat (Triticum aestivum L.) and Pathogenic Fusarium culmorum and Beneficial Trichoderma Fungi. Pathogens. 2021; 10(11):1461. https://doi.org/10.3390/pathogens10111461
Chicago/Turabian StyleSalamon, Sylwia, Julia Żok, Karolina Gromadzka, and Lidia Błaszczyk. 2021. "Expression Patterns of miR398, miR167, and miR159 in the Interaction between Bread Wheat (Triticum aestivum L.) and Pathogenic Fusarium culmorum and Beneficial Trichoderma Fungi" Pathogens 10, no. 11: 1461. https://doi.org/10.3390/pathogens10111461






