The Dynamics of Hepatic Fibrosis Related to Schistosomiasis and Its Risk Factors in a Cohort of China
Abstract
:1. Introduction
2. Results
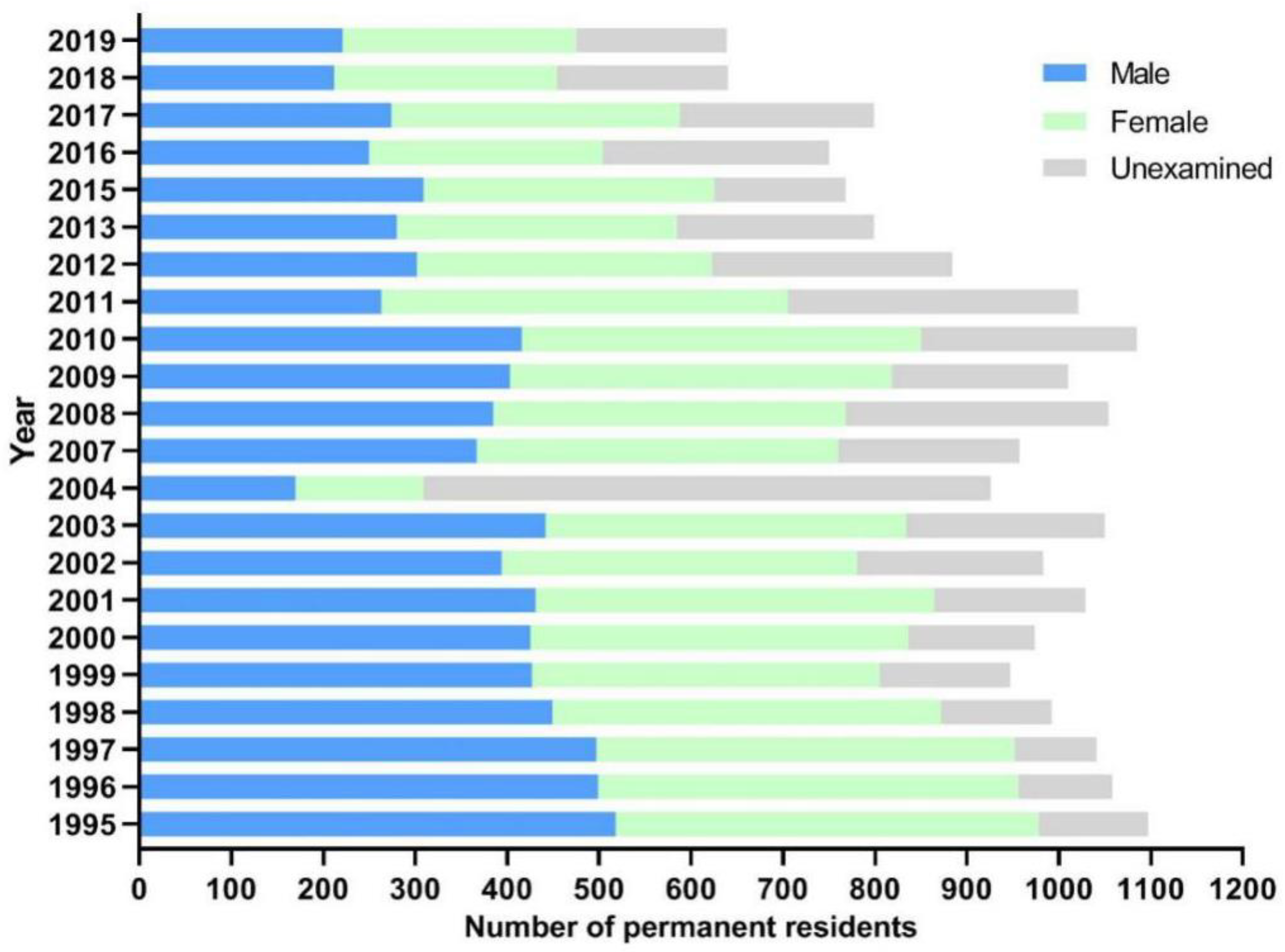
2.1. General Information of Participants
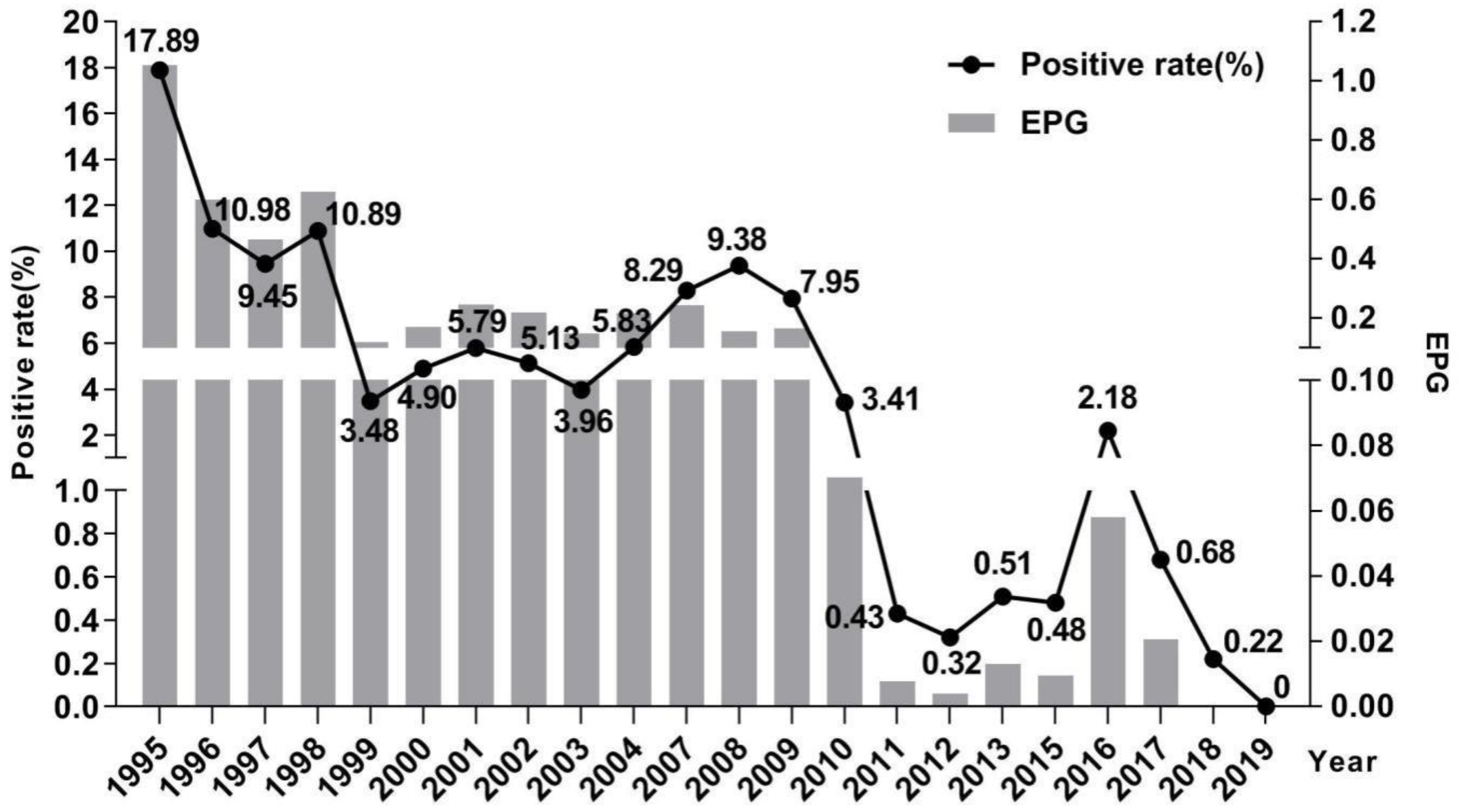
2.2. Changes of Schistosomiasis Prevalence in Residents
2.3. Changes of Hepatic Fibrosis Related to Schistosomiasis in Residents
2.4. Factors Influencing the Evolution of Hepatic Fibrosis
3. Discussion
4. Materials and Methods
4.1. Study Area and Cohort
4.2. Parasitological Survey
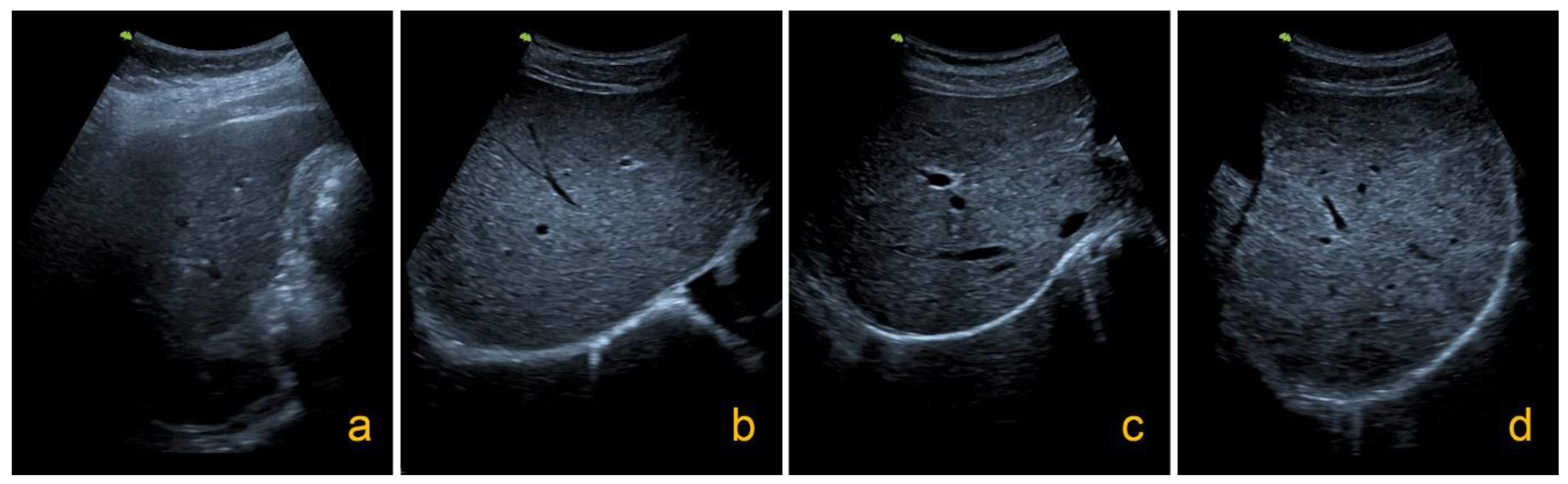
4.3. Ultrasound Examination
4.4. Questionnaire Survey
4.5. Quality Control
4.6. Data Management and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Schistosomiasis. Updated 17 April 2019. Available online: https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis (accessed on 10 September 2019).
- WHO. A Roadmap for Implementation: Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases; WHO/HTM/NTD/2012.1; World Health Organization: Geneva, Switzerland, 2012; Available online: https://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf (accessed on 10 September 2019).
- Zhou, X.N.; Li, S.Z.; Hong, Q.B.; Yang, K.; Lv, S.; Xu, J. Remain true to our original aspiration for farewell to the God of Plague, compose the new chapter for the national schistosomiasis control programme scientifically-Commemoration of 60th anniversary of publishing Chairman Mao Zedong’s two poems “Farewell to the God of Plague”. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2018, 30, 1–4. (In Chinese) [Google Scholar]
- Xu, J.; Lü, S.; Cao, C.L.; Li, S.Z.; Zhou, X.N. Progress and challenges of schistosomiasis elimination in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2018, 30, 605–609. (In Chinese) [Google Scholar]
- Zhang, L.J.; Xu, Z.M.; Dang, H.; Li, Y.L.; Lü, S.; Xu, J.; Li, S.Z.; Zhou, X.N. Endemic status of schistosomiasis in People’s Republic of China in 2019. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2020, 32, 551–558. (In Chinese) [Google Scholar] [PubMed]
- Arnaud, V.; Li, J.; Wang, Y.Y.; Fu, X.; Shi, M.Z.; Luo, X.S.; Hou, X.Y.; Dessein, H.; Zhou, J.; Yu, X.L.; et al. Regulatory Role of interleukin-10 and interferon-γ in severe hepatic central and peripheral fibrosis in uumans infected with Schistosoma japonicum. J. Infect. Dis. 2008, 198, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Yin, A.H.; Hua, H.Y.; Shun, G.X.; Xu, M.Q.; Zhou, W.E.; Zhu, W.C.; Feng, J.Y.; You, L.; Liang, Y.S. Survey on newly-developed advanced schistosomiasis patients in schistosomiasis transmission-interrupted areas. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2013, 25, 477–480+484. (In Chinese) [Google Scholar]
- Silva, P.C.; Leal, T.V.; Domingues, A.L. Treatment and education reduce the severity of schistosomiasis periportal fibrosis. Rev. Soc. Bras. Med. Trop. 2013, 46, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Booth, M.; Vennervald, B.J.; Kabatereine, N.B.; Kazibwe, F.; Ouma, J.H.; Kariuki, C.H.; Muchiri, E.; Kadzo, H.; Ireri, E.; Kimani, G.; et al. Hepatosplenic morbidity in two neighbouring communities in Uganda with high levels of Schistosoma mansoni infection but very different durations of residence. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 125–136. [Google Scholar] [CrossRef]
- Abdel, H.A.; Talaat, M. Histological assessment of tissue repair after treatment of human schistosomiasis. Acta Trop. 2000, 77, 91–96. [Google Scholar] [CrossRef]
- Wiest, P.M.; Wu, G.L.; Zhang, S.J.; McGarvey, S.T.; Tan, E.; Yuan, J.H.; Peters, P.; Olveda, R.M.; Olds, G.R. Schistosomiasis japonica on Jishan Island, Jiangxi Province, People’s Republic of China: Persistence of hepatic fibrosis after reduction of the prevalence of infection with age. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 290–294. [Google Scholar] [CrossRef]
- Qiu, D.C.; Zhang, G.S.; Wen, S.; Zhang, Y.; Wu, Z.S.; Cui, L.N.; Shan, J.Z.; Chai, X.P.; Li, M.S. Observation on changes of liver fibrosis due to schistotoma japonicum after treatment with praziquantel. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2000, 12, 138–140. (In Chinese) [Google Scholar]
- Cai, W.M. Understanding of hepatic fibrosis and acvanced schistosomiasis in the past and future. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2008, 20, 235–240. (In Chinese) [Google Scholar]
- Liu, Y.M.; Lin, D.D.; Hu, F.; Tao, B.; Jiang, Q.L.; Wang, J.M.; Li, J.Y. Utrasonographical characters of liver and spleen of residents due to schistosoma japonicum infection and their changes in Poyang Lake region, Jiaangxi Province. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2010, 22, 31–34. (In Chinese) [Google Scholar]
- Department of Health Disease Control of Health Ministry of the People’s Republic of China. Schistosomiasis Control Manual, 3rd ed.; Shanghai Science and Technology Press: Shanghai, China, 2000; pp. 114–163. (In Chinese)
- Hu, F.; Gao, Z.L.; Yuan, M.; Li, Z.J.; Li, Y.F.; Liu, Y.M.; Li, J.Y.; Xie, S.Y.; Wen, Y.S.; Lin, D.D. Changing trends of schistosome infection and live fibrosis among residents in the Poyang Lake region. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2021, 39, 629–637. (In Chinese) [Google Scholar]
- Xu, J.; Steinman, P.; Maybe, D.; Zhou, X.N.; Lv, S.; Li, S.Z.; Peeling, R. Chapter One—Evolution of the National Schistosomiasis Control Programmes in The People’s Republic of China. Adv. Parasitol. 2016, 92, 1–38. [Google Scholar] [PubMed]
- Xu, J.; Xu, J.F.; Li, S.Z.; Zhang, L.J.; Wang, Q.; Zhu, G.G.; Zhou, X.N. Integrated control programmes for schistosomiasis and other helminth infections in P.R. China. Acta Trop. 2015, 141(Pt. B), 332–341. [Google Scholar] [CrossRef]
- Lin, D.D.; Liu, J.X.; Liu, Y.M.; Hu, F.; Zhang, Y.Y.; Xu, J.M.; Li, J.Y.; Ji, M.J.; Bergquist, R.; Wu, G.L.; et al. Routine Kato–Katz technique underestimates the prevalence of Schistosoma japonicum: A case study in an endemic area of the People’s Republic of China. Parasitol Int. 2008, 57, 281–286. [Google Scholar] [CrossRef]
- Xin, X.; Zhu, H.P.; Yu, C.H.; Xu, X.J.; Li, R.D.; Qiu, J. A Bayesian approach to estimate the prevalence of Schistosomiasis japonica infection in the Hubei Province Lake regions, China. Int. J. Environ. Res. Public Health 2013, 10, 2799–2812. [Google Scholar]
- Zhu, H.Q.; Xu, J.; Zhu, R.; Cao, C.L.; Bao, Z.P.; Yu, Q.; Zhang, L.J.; Xu, X.L.; Feng, Z.; Guo, J.G. Comparison of the miracidium hatching test and modified Kato-Katz method for detecting Schistosoma japonicum in low prevalence areas of China. Southeast Asian J. Trop. Med. Public Health 2014, 45, 20–25. [Google Scholar]
- Wilson, M.S.; Mentink-Kane, M.M.; Pesce, J.T.; Thompson, R.T.; Thompson, R.; Wynn, T.A. Immunopathology of schistosomiasis. Immunol. Cell Biol. 2007, 85, 148–154. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, X.; Chen, X.J.; Zhu, J.F.; Xu, Z.P.; Wang, X.F.; Liu, F.; Hu, W.; Zhou, L.; Su, C. Heat Shock Protein 60 in Eggs Specifically Induces Tregs and Reduces Liver Immunopathology in Mice with Schistosomiasis Japonica. PLoS ONE 2015, 10, e0139133. [Google Scholar]
- Duan, Y.N.; Pan, J.; Chen, J.L.; Zhu, D.D.; Wang, J.X.; Sun, X.L.; Chen, L.T.; Wu, L.T. Soluble Egg Antigens of Schistosoma japonicum Induce Senescence of Activated Hepatic Stellate Cells by Activation of the FoxO3a/SKP2/P27 Pathway. PLoS Negl. Trop. Dis. 2016, 10, e0005268. [Google Scholar] [CrossRef]
- Song, L.G.; Zhang, B.B.; Liu, J.H.; Wang, M.; Ma, X.H.; Wang, L.F.; Wu, X.Y.; Wu, Z.D.; Wang, T.P. Reversal of liver fibrosis after splenectomy in a patient with advanced schistosomiasis japonica: A case report with 4-year follow-up. PLoS Negl. Trop. Dis. 2019, 13, e0007174. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Liu, X.; Dong, H.F.; Xia, D.; Wang, L.X.; Chen, Y.; Xiong, Y. Sorafenib and praziquantel synergistically attenuate Schistosoma japonicum-induced liver fibrosis in mice. Parasitol. Res. 2018, 117, 2831–2839. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission, the People’s Republic of China. Guideline for Providing Medical Assistance to Advanced Schistosomiasis Patients. 2005. Available online: http://www.nhc.gov.cn/wjw/zcjd/201304/9f4a98c0137b42b9951a685eef87458e.shtml (accessed on 15 September 2019).
- Lei, Z.L.; Zheng, H.; Zhang, L.J.; Zhu, R.; Xu, Z.M.; Xu, J.; Fu, Q.; Wang, Q.; Li, S.Z.; Zhou, X.N. Endemic status of schistosomiasis in People’s Republic of China in 2013. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2014, 26, 591–597. (In Chinese) [Google Scholar] [PubMed]
- Lei, Z.L.; Zhang, L.J.; Xu, Z.M.; Dang, H.; Xu, J.; Lv, S.; Cao, C.L.; Li, S.Z.; Zhou, X.N. Endemic status of schistosomiasis in People’s Republic of China in 2014. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2015, 27, 563–569. (In Chinese) [Google Scholar] [PubMed]
- Zhang, L.J.; Xu, Z.M.; Qian, Y.J.; Dang, H.; Lü, S.; Xu, J.; Li, S.Z.; Zhou, X.N. Endemic status of schistosomiasis in People’s Republic of China in 2015. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2016, 28, 611–617. (In Chinese) [Google Scholar]
- Zhang, L.J.; Xu, Z.M.; Qian, Y.J.; Dang, H.; Lü, S.; Xu, J.; Li, S.Z.; Zhou, X.N. Endemic status of schistosomiasis in People’s Republic of China in 2016. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2017, 29, 669–677. (In Chinese) [Google Scholar]
- Hua, H.Y.; Yin, A.H.; Xu, M.H.; Zhou, Z.Y.; You, L.; Guo, H.X. Advanced schisto somiasis reappeared after curing seemingly being cured for over 20 years and without known history of reexposure to Schistosoma japonicum. Parasitol. Res. 2015, 114, 3535–3538. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Q.; Zhao, A.; Zhu, J.; Zhang, G.G. Survey on know ledge and attitude of schistosomiasis control among villages in susceptible zones in Pongyang Lake area. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2011, 23, 93–94+106. (In Chinese) [Google Scholar]
- Hu, G.H.; Hu, J.; Song, K.Y.; Lin, D.D.; Zhang, J.; Cao, C.L.; Xu, J.; Li, D.; Jiang, W.S. The role of health education and health promotion in the control of schistosomiasis: Experiences from a 12-year intervention study in the Poyang Lake area. Acta Trop. 2005, 96, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Rahoud, S.A.; Mergani, A.; Khamis, A.H.; Saeed, O.K.; Mohamed-Ali, Q.; Dessein, A.J.; Nasr Eldin, M.A.; Elwali, N. Factors controlling the effect of praziquantel on liver fibrosis in Schistosoma mansoni-infected patients. FEMS Immunol. Med. Microbiol. 2010, 58, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, M.M.K.; Mahmoud, S.S.; El-Sayed, S.H.; Rizk, E.M.A.; Raafat, A.; Negm, M.S.I. Impact of treatment with a Protein Tyrosine Kinase Inhibitor (Genistein) on acute and chronic experimental Schistosoma mansoni infection. Exp. Parasitol. 2018, 185, 115–123. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Diagnostic Criteria for Schistosomiasis; WS 261-2006S; People’s Medical Publishing House: Beijing, China, 2006.
- Niamey Working Group. Ultrasound in Schistosomiasis: A Practical Guide to the Standardized Use of Ultrasonography for the Assessment of Schistosomiasis-Related Morbidity; TDR/STR/SCH/00.1; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Agbota, G.; Polman, K.; Wieringa, F.T.; Campos-Ponce, M.; Accrombessi, M.; Yovo, E.; Roucher, C.; Ezinmègnon, S.; Marcos, J.Y.; Vachot, L.; et al. Maternal malaria but not schistosomiasis is associated with a higher risk of febrile infection in infant during the first 3 months of life: A mother-child cohort in Benin. PLoS ONE 2019, 14, e0222864. [Google Scholar] [CrossRef] [PubMed]
| 1995 | 2019 | ||||
|---|---|---|---|---|---|
| Grade 0 | Grade I | Grade II | Grade III | Subtotal | |
| Grade 0 | 92 | 26 | 3 | 1 | 122 |
| Grade I | 53 | 38 | 30 | 10 | 131 |
| Grade II | 1 | 7 | 7 | 16 | 31 |
| Grade III | 0 | 1 | 3 | 4 | 8 |
| Total | 146 | 72 | 43 | 31 | 292 |
| Factors | Investigated Number | Recovery (n = 65) | Deterioration (n = 86) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | χ2 | OR | 95% CI | p Value | No. | χ2 | OR | 95% CI | p Value | |||
| Gender | Male | 146 | 27 | 2.395 | 0.645 | 0.369–1.127 | 0.122 | 59 | 16.878 | 2.989 | 1.754–5.092 | 0.000 |
| Female | 146 | 38 | 27 | |||||||||
| Age group | <38 | 149 | 30 | 0.795 | 1.285 | 0.740–2.234 | 0.373 | 31 | 10.948 | 2.379 | 1.415–4.000 | 0.001 |
| ≥38 | 143 | 35 | 55 | |||||||||
| Occupation | Fishermen | 66 | 8 | 5.066 | 0.409 | 0.184–0.908 | 0.024 | 29 | 8.614 | 2.324 | 1.312–4.115 | 0.003 |
| Non-fishermen | 226 | 57 | 57 | |||||||||
| Education | Illiterate | 125 | 34 | 7.689 | 0.104 | 35 | 2.899 | 0.575 | ||||
| Primary school | 106 | 25 | 36 | |||||||||
| Junior high school | 46 | 4 | 10 | |||||||||
| Senior high school | 15 | 2 | 5 | |||||||||
| Egg positive | Yes | 139 | 35 | 0.07 | 0.928 | 0.534–1.613 | 0.791 | 37 | 4.295 | 1.707 | 1.027–2.837 | 0.038 |
| No | 153 | 30 | 49 | |||||||||
| Water contact | Frequently | 137 | 30 | 0.02 | 0.961 | 0.553–1.671 | 0.889 | 40 | 0.008 | 0.977 | 0.590–1.618 | 0.928 |
| Infrequently/No | 155 | 35 | 46 | |||||||||
| No. treatment | <8 | 145 | 39 | 3.578 | 0.584 | 0.333–1.023 | 0.059 | 31 | 9.034 | 2.198 | 1.309–3.694 | 0.003 |
| ≥8 | 147 | 26 | 55 | |||||||||
| Active medication | Yes | 29 | 4 | 1.334 | 0.530 | 0.177–1.582 | 0.248 | 9 | 0.039 | 1.087 | 0.474–2.494 | 0.844 |
| No | 263 | 61 | 77 | |||||||||
| Anti-fibrosis treatment | Yes | 150 | 41 | 4.587 | 1.849 | 1.049–3.261 | 0.032 | 34 | 6.835 | 0.507 | 0.304–0.847 | 0.009 |
| No | 142 | 24 | 52 | |||||||||
| Factors | Recovery | Deterioration | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Gender | 0.964 | 0.471–1.974 | 0.921 | 2.735 | 1.523–4.910 | 0.001 |
| Age group | 2.986 | 1.668–5.347 | 0.000 | |||
| Profession | 0.480 | 0.205–1.122 | 0.090 | 2.416 | 1.229–4.749 | 0.010 |
| Education | 0.322 | |||||
| Egg positive | 1.411 | 0.762–2.611 | 0.273 | |||
| No. treatment | 0.568 | 0.304–1.060 | 0.076 | 1.651 | 0.895–3.045 | 0.108 |
| Anti-fibrosis treatment | 2.277 | 1.249–4.151 | 0.007 | 0.302 | 0.165–0.553 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, F.; Xie, S.-Y.; Yuan, M.; Li, Y.-F.; Li, Z.-J.; Gao, Z.-L.; Lan, W.-M.; Liu, Y.-M.; Xu, J.; Lin, D.-D. The Dynamics of Hepatic Fibrosis Related to Schistosomiasis and Its Risk Factors in a Cohort of China. Pathogens 2021, 10, 1532. https://doi.org/10.3390/pathogens10121532
Hu F, Xie S-Y, Yuan M, Li Y-F, Li Z-J, Gao Z-L, Lan W-M, Liu Y-M, Xu J, Lin D-D. The Dynamics of Hepatic Fibrosis Related to Schistosomiasis and Its Risk Factors in a Cohort of China. Pathogens. 2021; 10(12):1532. https://doi.org/10.3390/pathogens10121532
Chicago/Turabian StyleHu, Fei, Shu-Ying Xie, Min Yuan, Yi-Feng Li, Zhao-Jun Li, Zhu-Lu Gao, Wei-Ming Lan, Yue-Ming Liu, Jing Xu, and Dan-Dan Lin. 2021. "The Dynamics of Hepatic Fibrosis Related to Schistosomiasis and Its Risk Factors in a Cohort of China" Pathogens 10, no. 12: 1532. https://doi.org/10.3390/pathogens10121532
APA StyleHu, F., Xie, S.-Y., Yuan, M., Li, Y.-F., Li, Z.-J., Gao, Z.-L., Lan, W.-M., Liu, Y.-M., Xu, J., & Lin, D.-D. (2021). The Dynamics of Hepatic Fibrosis Related to Schistosomiasis and Its Risk Factors in a Cohort of China. Pathogens, 10(12), 1532. https://doi.org/10.3390/pathogens10121532






