Immunization against a Conserved Surface Polysaccharide Stimulates Bovine Antibodies with Opsonic Killing Activity but Does Not Protect against Babesia bovis Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunofluorescence Assays
2.2. Pathogen and Animals
2.3. Immunization
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Opsonophagocytic Killing Assay. In Vitro Killing of Staphylococcus aureus
2.6. Challenge with B. bovis Texas T2Bo Stabilate
2.7. PCR to Detect B. bovis Post-Challenge
2.8. Statistical Analysis
2.9. Ethics Statement
3. Results
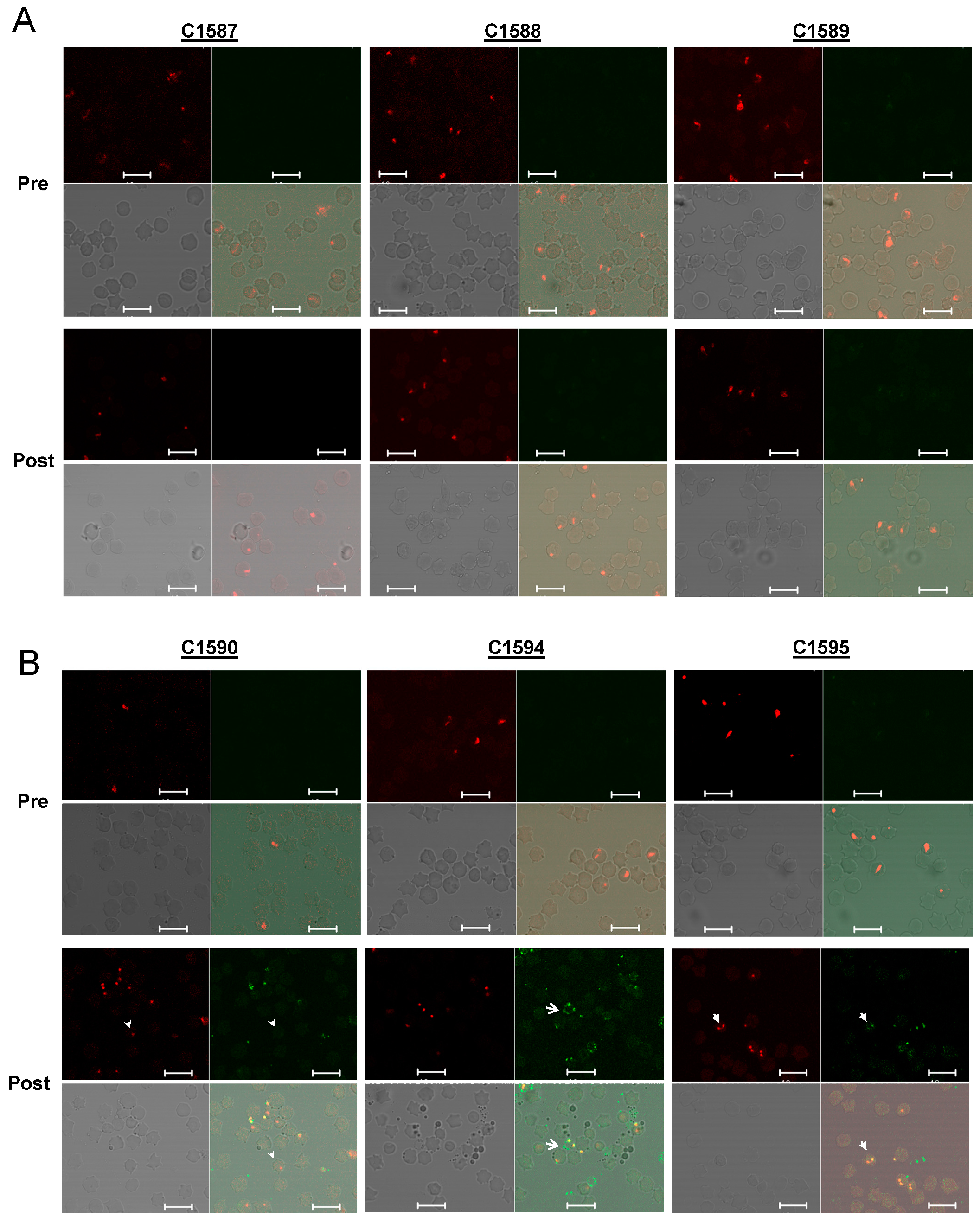
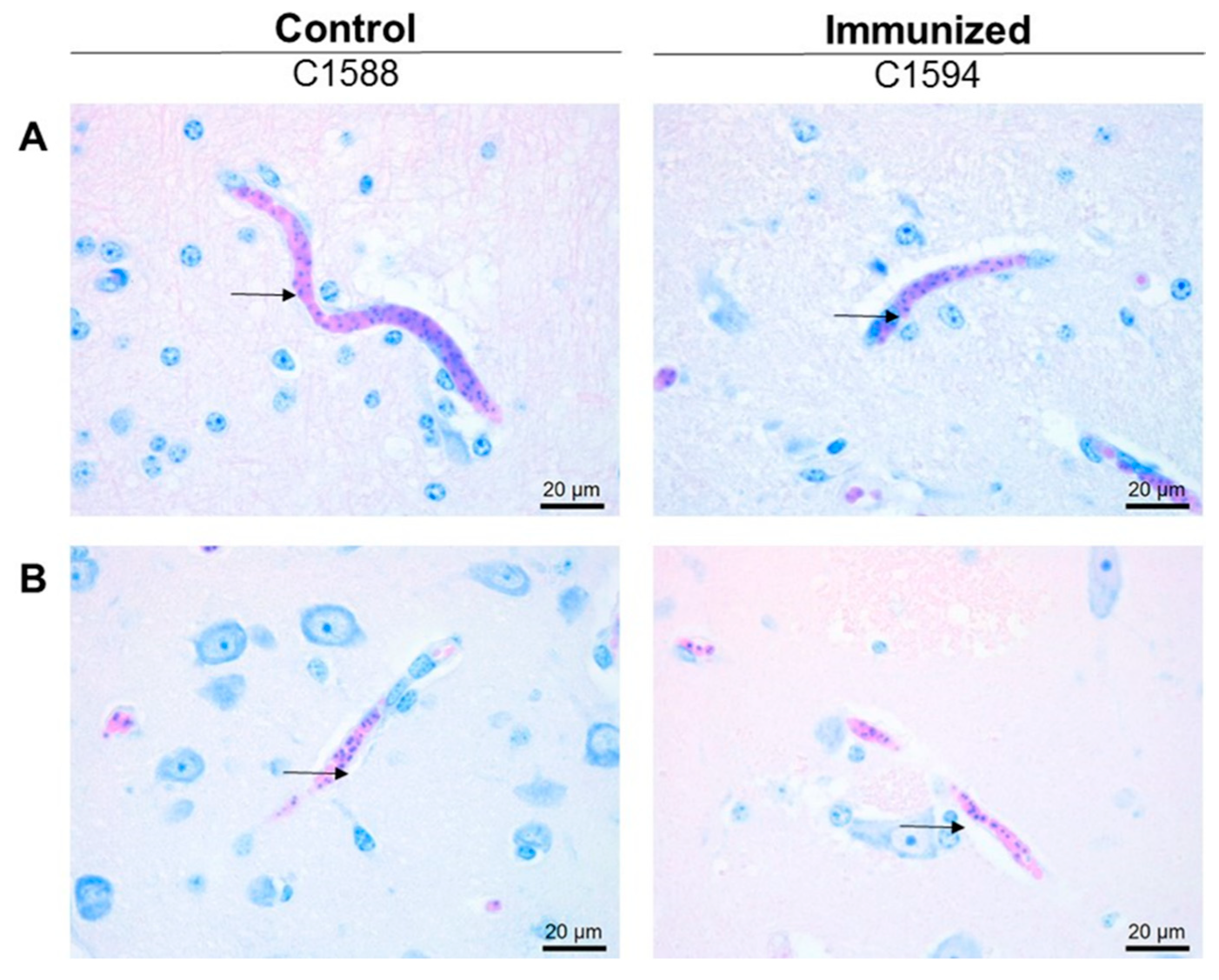
3.1. Expression of PNAG on Babesia parasites
3.2. Characterization of PNAG-Specific Antibodies in Immunized Calves
3.3. Development of Babesiosis in Immunized Calves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PNAG | poly-N-acetylglucosamine |
| dPNAG | deactylated poly-N-acetylglucosamine |
| TT | tetanus toxoid |
| BSA | bovine serum albumin |
| PCV | packed cell volume |
References
- Suarez, C.E.; Noh, S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011, 180, 109–125. [Google Scholar] [CrossRef]
- Suarez, C.E.; Alzan, H.F.; Silva, M.G.; Rathinasamy, V.; Poole, W.A.; Cooke, B.M. Unravelling the cellular and molecular pathogenesis of bovine babesiosis: Is the sky the limit? Int. J. Parasitol. 2019, 49, 183–197. [Google Scholar] [CrossRef]
- Brown, W.C.; Palmer, G.H. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol. Today 1999, 15, 275–281. [Google Scholar] [CrossRef]
- Bock, R.; Jackson, L.; de Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vivas, R.I.; Jonsson, N.N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2018, 117, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Florin-Christensen, M.; Suarez, C.E.; Rodriguez, A.E.; Flores, D.A.; Schnittger, L. Vaccines against bovine babesiosis: Where we are now and possible roads ahead. Parasitology 2014, 1–30. [Google Scholar] [CrossRef]
- Shkap, V.; Leibovitz, B.; Krigel, Y.; Hammerschlag, J.; Marcovics, A.; Fish, L.; Molad, T.; Savitsky, I.; Mazuz, M. Vaccination of older Bos taurus bulls against bovine babesiosis. Vet. Parasitol. 2005, 129, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Trueman, K.F.; Blight, G.W. The effect of age on resistance of cattle to Babesia bovis. Aust. Vet. J. 1978, 54, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Cywes-Bentley, C.; Skurnik, D.; Zaidi, T.; Roux, D.; Deoliveira, R.B.; Garrett, W.S.; Lu, X.; O’Malley, J.; Kinzel, K.; Zaidi, T.; et al. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, E2209–E2218. [Google Scholar] [CrossRef] [Green Version]
- Skurnik, D.; Cywes-Bentley, C.; Pier, G.B. The exceptionally broad-based potential of active and passive vaccination targeting the conserved microbial surface polysaccharide PNAG. Expert Rev. Vaccines 2016, 15, 1041–1053. [Google Scholar] [CrossRef] [Green Version]
- Kelly-Quintos, C.; Cavacini, L.A.; Posner, M.R.; Goldmann, D.; Pier, G.B. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect. Immun. 2006, 74, 2742–2750. [Google Scholar] [CrossRef] [Green Version]
- Skurnik, D.; Kropec, A.; Roux, D.; Theilacker, C.; Huebner, J.; Pier, G.B. Natural antibodies in normal human serum inhibit Staphylococcus aureus capsular polysaccharide vaccine efficacy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55, 1188–1197. [Google Scholar] [CrossRef] [Green Version]
- Skurnik, D.; Merighi, M.; Grout, M.; Gadjeva, M.; Maira-Litran, T.; Ericsson, M.; Goldmann, D.A.; Huang, S.S.; Datta, R.; Lee, J.C.; et al. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J. Clin. Investig. 2010, 120, 3220–3233. [Google Scholar] [CrossRef]
- Cywes-Bentley, C.; Rocha, J.N.; Bordin, A.I.; Vinacur, M.; Rehman, S.; Zaidi, T.S.; Meyer, M.; Anthony, S.; Lambert, M.; Vlock, D.R.; et al. Antibody to Poly-N-acetyl glucosamine provides protection against intracellular pathogens: Mechanism of action and validation in horse foals challenged with Rhodococcus equi. PLoS. Pathog. 2018, 14, e1007160. [Google Scholar] [CrossRef] [Green Version]
- Goff, W.L.; Wagner, G.G.; Craig, T.M.; Long, R.F. The bovine immune response to tick-derived Babesia bovis infection: Serological studies of isolated immunoglobulins. Vet. Parasitol. 1982, 11, 109–120. [Google Scholar] [CrossRef]
- Goff, W.L.; Johnson, W.C.; Cluff, C.W. Babesia bovis immunity. In vitro and in vivo evidence for IL-10 regulation of IFN-gamma and iNOS. Ann. N. Y. Acad. Sci. 1998, 849, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.E.; Palmer, G.H.; Jasmer, D.P.; Hines, S.A.; Perryman, L.E.; McElwain, T.F. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol. Biochem. Parasitol. 1991, 46, 45–52. [Google Scholar] [CrossRef]
- Bohaliga, G.A.R.; Johnson, W.C.; Taus, N.S.; Hussein, H.E.; Bastos, R.G.; Suarez, C.E.; O’Connor, R.; Ueti, M.W. Identification of a putative methyltransferase gene of Babesia bigemina as a novel molecular biomarker uniquely expressed in parasite tick stages. Parasites Vectors 2018, 11, 480. [Google Scholar] [CrossRef]
- Goff, W.L.; Jessup, D.A.; Waldrup, K.A.; Thomford, J.W.; Conrad, P.A.; Boyce, W.M.; Gorham, J.R.; Wagner, G.G. The isolation and partial characterization of a Babesia sp. from desert bighorn sheep (Ovis canadensis nelsoni). J. Eukaryot. Microbiol. 1993, 40, 237–243. [Google Scholar] [CrossRef]
- Holman, P.J.; Spencer, A.M.; Telford, S.R., 3rd; Goethert, H.K.; Allen, A.J.; Knowles, D.P.; Goff, W.L. Comparative infectivity of Babesia divergens and a zoonotic Babesia divergens-like parasite in cattle. Am. J. Trop. Med. Hyg. 2005, 73, 865–870. [Google Scholar] [CrossRef]
- Herwaldt, B.; Persing, D.H.; Précigout, E.A.; Goff, W.L.; Mathiesen, D.A.; Taylor, P.W.; Eberhard, M.L.; Gorenflot, A.F. A fatal case of babesiosis in Missouri: Identification of another piroplasm that infects humans. Ann. Intern. Med. 1996, 124, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Pier, G.B.; Boyer, D.; Preston, M.; Coleman, F.T.; Llosa, N.; Mueschenborn-Koglin, S.; Theilacker, C.; Goldenberg, H.; Uchin, J.; Priebe, G.P.; et al. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 2004, 173, 5671–5678. [Google Scholar] [CrossRef] [Green Version]
- Goff, W.L.; Molloy, J.B.; Johnson, W.C.; Suarez, C.E.; Pino, I.; Rhalem, A.; Sahibi, H.; Ceci, L.; Carelli, G.; Adams, D.S.; et al. Validation of a competitive enzyme-linked immunosorbent assay for detection of antibodies against Babesia bovis. Clin. Vaccine Immunol. CVI 2006, 13, 1212–1216. [Google Scholar] [CrossRef] [Green Version]
- Suarez, C.E.; Laughery, J.M.; Schneider, D.A.; Sondgeroth, K.S.; McElwain, T.F. Acute and persistent infection by a transfected Mo7 strain of Babesia bovis. Mol. Biochem. Parasitol. 2012, 185, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; Slamti, L.; Avci, F.Y.; Pier, G.B.; Maira-Litrán, T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 2009, 191, 5953–5963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maira-Litran, T.; Kropec, A.; Goldmann, D.A.; Pier, G.B. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated Staphylococcal Poly-N-acetyl-beta-(1–6)-glucosamine. Infect. Immun. 2005, 73, 6752–6762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merck Veterinary Manual. Available online: https://merckvetmanual.com/special-subjects/reference-guides/normal-rectal-temperature-ranges (accessed on 30 November 2021).
- Bastos, R.G.; Ueti, M.W.; Guerrero, F.D.; Knowles, D.P.; Scoles, G.A. Silencing of a putative immunophilin gene in the cattle tick Rhipicephalus (Boophilus) microplus increases the infection rate of Babesia bovis in larval progeny. Parasites Vectors 2009, 2, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, J.M.; Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Knowles, D.P. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J. Clin. Microbiol. 2007, 45, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.C.; Norimine, J.; Knowles, D.P.; Goff, W.L. Immune control of Babesia bovis infection. Vet. Parasitol. 2006, 138, 75–87. [Google Scholar] [CrossRef]
- Goff, W.L.; Bastos, R.G.; Brown, W.C.; Johnson, W.C.; Schneider, D.A. The bovine spleen: Interactions among splenic cell populations in the innate immunologic control of hemoparasitic infections. Vet. Immunol. Immunopathol. 2010, 138, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goff, W.L.; Johnson, W.C.; Parish, S.M.; Barrington, G.M.; Tuo, W.; Valdez, R.A. The age-related immunity in cattle to Babesia bovis infection involves the rapid induction of interleukin-12, interferon-gamma and inducible nitric oxide synthase mRNA expression in the spleen. Parasite Immunol. 2001, 23, 463–471. [Google Scholar] [CrossRef]
- Jaramillo, O.J.M.; Paoletta, M.S.; Gravisaco, M.J.; López, A.L.S.; Montenegro, V.N.; de la Fournière, S.A.M.; Valenzano, M.N.; Guillemi, E.C.; Valentini, B.; Echaide, I.; et al. Immunisation of cattle against Babesia bovis combining a multi-epitope modified vaccinia Ankara virus and a recombinant protein induce strong Th1 cell responses but fails to trigger neutralising antibodies required for protection. Ticks Tick-Borne Dis. 2019, 10, 101270. [Google Scholar] [CrossRef] [PubMed]


| Adjuvant 1 | PNAG 2 | Test, p Value | |
|---|---|---|---|
| Parameter | |||
| Median days to detect parasitemia | 6 DPI 3 | 7 DPI | Mann–Whitney rank sum test, p = 0.2 |
| Mean peak decrease in PCV 4 | 37.76% | 45.43% | Student’s t-test, p = 0.537 |
| Mean days to develop fever ≥ 39.4 °C | 7.7 | 8.3 | Student’s t-test, p = 0.519 |
| Mean peak parasite copy numbers ± SD | 1.82 × 106 ± 2.26 × 106 | 8.64 × 105 ± 2.26 × 105 | Student’s t-test, p = 0.508 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taus, N.S.; Cywes-Bentley, C.; Johnson, W.C.; Pier, G.B.; Fry, L.M.; Mousel, M.R.; Ueti, M.W. Immunization against a Conserved Surface Polysaccharide Stimulates Bovine Antibodies with Opsonic Killing Activity but Does Not Protect against Babesia bovis Challenge. Pathogens 2021, 10, 1598. https://doi.org/10.3390/pathogens10121598
Taus NS, Cywes-Bentley C, Johnson WC, Pier GB, Fry LM, Mousel MR, Ueti MW. Immunization against a Conserved Surface Polysaccharide Stimulates Bovine Antibodies with Opsonic Killing Activity but Does Not Protect against Babesia bovis Challenge. Pathogens. 2021; 10(12):1598. https://doi.org/10.3390/pathogens10121598
Chicago/Turabian StyleTaus, Naomi S., Colette Cywes-Bentley, Wendell C. Johnson, Gerald B. Pier, Lindsay M. Fry, Michelle R. Mousel, and Massaro W. Ueti. 2021. "Immunization against a Conserved Surface Polysaccharide Stimulates Bovine Antibodies with Opsonic Killing Activity but Does Not Protect against Babesia bovis Challenge" Pathogens 10, no. 12: 1598. https://doi.org/10.3390/pathogens10121598
APA StyleTaus, N. S., Cywes-Bentley, C., Johnson, W. C., Pier, G. B., Fry, L. M., Mousel, M. R., & Ueti, M. W. (2021). Immunization against a Conserved Surface Polysaccharide Stimulates Bovine Antibodies with Opsonic Killing Activity but Does Not Protect against Babesia bovis Challenge. Pathogens, 10(12), 1598. https://doi.org/10.3390/pathogens10121598






