The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits
Abstract
1. Introduction
1.1. Use of Small Molecules
1.2. RNA Interference as a Gene Regulating Expression Approach
1.3. Use of Organic Extracts and Natural Supplements as an Alternative Holistic Strategy
1.4. Probiotics and/or Prebiotics
1.5. Other Approaches
2. Results
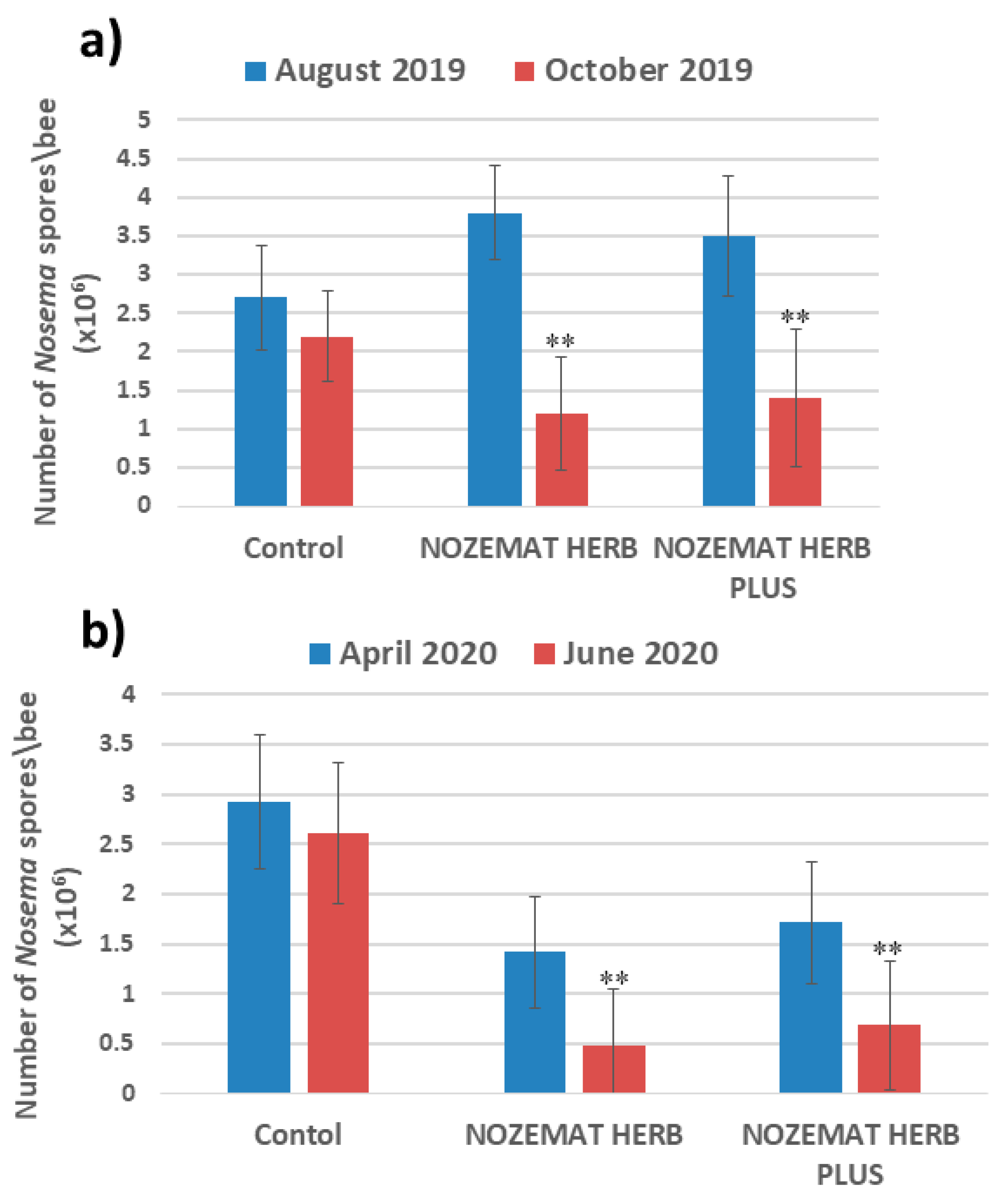
2.1. N. ceranae Infection and Spore Counts
2.2. Effect of Herbal Supplements on Honey Bee Strength and Production Traits
2.2.1. Strength of the Honey Bee Colonies (Estimated Based on Mass)
2.2.2. Sealed Worker Brood Area within the Hives
2.2.3. Amount of Capped Honey in the Beehives
2.2.4. Stored Pollen Area within the Hives
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Experimental Design
4.3. Microscopic Detection of N. ceranae
4.4. DNA Extraction, PCR Amplification and Sequencing
4.5. Evaluation of Honey Bee Strength, Sealed Worker Brood and Food Supplies
- Strength of the bee colony based on its mass (kg)—the mass is calculated based on the number of frames occupied by bees, considering that one frame in a Langstroth–Rut hive contains approximately 200 g of bees [115].
- Sealed worker brood area—a measuring frame with the size of the squares 5 × 5 cm was used. In 1 cm2 there were 4 worker cells in the bee comb. The area of 25 cm2 corresponded to 100 worker cells [115].
- Amount of honey in the beehives—a measuring frame with 5 × 5 cm squares was used to measure the capped honey in the bee combs. Eight squares of the measuring frame corresponded to 0.350 kg honey [115].
- Stored pollen area in the beehives—the amount of the collected pollen was evaluated through direct surface measurements of the comb (cm2) [115].
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paudel, Y.P.; Mackereth, R.; Hanley, R.; Qin, W. Honey Bees (Apis Mellifera L.) and Pollination Issues: Current Status, Impacts and Potential Drivers of Decline. J. Agric. Sci. 2015, 7, 93. [Google Scholar] [CrossRef]
- Sidhu, C.S.; Joshi, N.K. Establishing Wildflower Pollinator Habitats in Agricultural Farmland to Provide Multiple Ecosystem Services. Front. Plant Sci. 2016, 7, 363. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Fournier, V.; Giovenazzo, P. The impact of lowbush blueberry (Vaccinium angustifolium Ait.) and cranberry (Vaccinium macrocarpon Ait.) pollination on honey bee (Apis mellifera L.) colony health status. PLoS ONE 2020, 15, e0227970. [Google Scholar]
- Moritz, R.F.A.; Erler, S. Lost Colonies Found in a Data Mine: Global Honey Trade but Not Pests or Pesticides as a Major Cause of Regional Honeybee Colony Declines. Agric. Ecosyst. Environ. 2016, 216, 44–50. [Google Scholar] [CrossRef]
- Lozier, J.D.; Zayed, A. Bee Conservation in the Age of Genomics. Conserv. Genet. 2017, 18, 713–729. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.A.; Msweli, S.M.; Johnson, S.D. Native Honeybees as Flower Visitors and Pollinators in Wild Plant Communities in a Biodiversity Hotspot. Ecosphere 2020, 11, e02957. [Google Scholar] [CrossRef]
- Aslan, C.E.; Liang, C.T.; Galindo, B.; Kimberly, H.; Topete, W. The Role of Honey Bees as Pollinators in Natural Areas. Nat. Areas J. 2016, 36, 478–488. [Google Scholar] [CrossRef]
- Yohannes, G. Review on Medicinal Value of Aloe Vera in Veterinary Practice. Biomed. J. Sci. Tech. Res. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease Associations between Honeybees and Bumblebees as a Threat to Wild Pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Seitz, N.; Traynor, K.S.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Ellis, J.D. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apic. Res. 2015, 54, 292–304. [Google Scholar] [CrossRef]
- Chauzat, M.P.; Jacques, A.; Laurent, M.; Bougeard, S.; Hendrikx, P.; Ribière-Chabert, M.; EPILOBEE Consortium. Risk indicators affecting honey bee colony survival in Europe: One year of surveillance. Apidologie 2016, 47, 348–378. [Google Scholar] [CrossRef]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. N. ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than N. ceranae apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Fries, I. N. ceranae in European Honey Bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Meana, A.; Bartolomé, C.; Botías, C.; Martín-Hernández, R. N. ceranae (Microsporidia), a Controversial 21st Century Honey Bee Pathogen. Environ. Microbiol. Rep. 2013, 5, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Emsen, B.; Guzman-Novoa, E.; Hamiduzzaman, M.M.; Eccles, L.; Lacey, B.; Ruiz-Pérez, R.A.; Nasr, M. Higher Prevalence and Levels of N. ceranae than N. apis Infections in Canadian Honey Bee Colonies. Parasitol. Res. 2016, 115, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Chemurot, M.; De Smet, L.; Brunain, M.; De Rycke, R.; de Graaf, D.C. N. neumanni n. sp. (Microsporidia, Nosematidae), a New Microsporidian Parasite of Honeybees, Apis mellifera in Uganda. Eur. J. Protistol. 2017, 61, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; Botías, C.; García-Palencia, P.; Meana, A. Regurgitated pellets of Merops apiaster as fomites of infective Nosema ceranae (Microsporidia) spores. Environ. Microbiol. 2008, 10, 1374–1379. [Google Scholar] [CrossRef]
- Plischuk, S.; Martín-Hernández, R.; Prieto, L.; Lucía, M.; Botías, C.; Meana, A.; Abrahamovich, A.H.; Lange, C.; Higes, M. South American native bumblebees (Hymenoptera: Apidae) infected by Nosema ceranae (Microsporidia), an emerging pathogen of honeybees (Apis mellifera). Environ. Microbiol. Rep. 2009, 1, 131–135. [Google Scholar] [CrossRef]
- Plischuk, S.; Lange, C.E. Bombus brasiliensis Lepeletier (Hymenoptera, Apidae) infected with Nosema ceranae (Microsporidia). Rev. Bras. Entomol. 2016, 60, 347–351. [Google Scholar] [CrossRef]
- Porrini, M.P.; Porrini, L.P.; Garrido, P.M.; Silva Neto, K.M.; Porrini, D.P.; Muller, F.; Nuñez, L.A.; Alvarez, L.; Iriarte, P.F.; Eguaras, M.J. Nosema ceranae in South American native stingless bees and social wasp. Microb. Ecol. 2017, 74, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Ravoet, J.; De Smet, L.; Meeus, I.; Smagghe, G.; Wenseleers, T.; de Graaf, D.C. Widespread occurrence of honey bee pathogens in solitary bees. J. Invertebr. Pathol. 2014, 122, 55–58. [Google Scholar] [CrossRef]
- Cilia, G.; Cardaio, I.; dos Santos, P.E.J.; Ellis, J.D.; Nanetti, A. The first detection of Nosema ceranae (Microsporidia) in the small hive beetle, Aethina tumida Murray (Coleoptera: Nitidulidae). Apidologie 2018, 49, 619–624. [Google Scholar] [CrossRef]
- Papini, R.; Mancianti, F.; Canovai, R.; Cosci, F.; Rocchigiani, G.; Benelli, G.; Canale, A. Prevalence of the microsporidian Nosema ceranae in honeybee (Apis mellifera) apiaries in Central Italy. Saudi J. Biol. Sci. 2017, 24, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Sagona, S.; Giusti, M.; Dos Santos, P.E.J.; Nanetti, A.; Felicioli, A. Nosema ceranae infection in honeybee samples from Tuscanian Archipelago (Central Italy) investigated by two qPCR methods. Saudi J. Biol. Sci. 2019, 26, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Gajger, I.T.; Vugrek, O.; Grilec, D.; Petrinec, Z. Prevalence and distribution of Nosema ceranae in Croatian honeybee colonies. Vet. Med. 2010, 55, 457–462. [Google Scholar] [CrossRef]
- Blažytė-Čereškienė, L.; Skrodenytė-Arbačiauskienė, V.; Radžiutė, S.; Nedveckytė, I.; Būda, V. Honey bee infection caused by Nosema spp. in Lithuania. J. Apic. Sci. 2016, 60, 77–88. [Google Scholar] [CrossRef]
- Pacini, A.; Mira, A.; Molineri, A.; Giacobino, A.; Cagnolo, N.B.; Aignasse, A.; Zago, L.; Izaguirre, M.; Merke, J.; Orellano, E.; et al. Distribution and prevalence of Nosema apis and N. ceranae in temperate and subtropical eco-regions of Argentina. J. Invertebr. Pathol. 2016, 141, 34–37. [Google Scholar] [CrossRef]
- Sinpoo, C.; Paxton, R.J.; Disayathanoowat, T.; Krongdang, S.; Chantawannakul, P. Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response. J. Insect Physiol. 2018, 105, 1–8. [Google Scholar] [CrossRef]
- Paris, L.; El Alaoui, H.; Delbac, F.; Diogon, M. Effects of the Gut Parasite N. ceranae ceranae on Honey Bee Physiology and Behavior. Curr. Opin. Insect Sci. 2018, 26, 149–154. [Google Scholar] [CrossRef]
- Arismendi, N.; Caro, S.; Castro, M.P.; Vargas, M.; Riveros, G.; Venegas, T. Impact of Mixed Infections of Gut Parasites Lotmaria passim and N. ceranae ceranae on the Lifespan and Immune-related Biomarkers in Apis mellifera. Insects 2020, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.N.; Jack, C.J.; Bustamante, T.A.; Schmehl, D.R.; Ellis, J.D. Effects of Supplemental Pollen Feeding on Honey Bee (Hymenoptera: Apidae) Colony Strength and N. ceranae spp. Infection. J. Econ. Entomol. 2019, 112, 60–66. [Google Scholar] [CrossRef]
- Farrar, C.L. N. ceranae Losses in Package Bees as Related to Queen Supersedure and Honey Yields. J. Econ. Entomol. 1947, 40, 333–338. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. N. ceranae spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013, 44, 25. [Google Scholar] [CrossRef]
- Mendoza, Y.; Diaz-Cetti, S.; Ramallo, G.; Santos, E.; Porrini, M.; Invernizzi, C. N. ceranae Winter Control: Study of the Effectiveness of Different Fumagillin Treatments and Consequences on the Strength of Honey Bee (Hymenoptera: Apidae) Colonies. J. Econ. Entomol. 2017, 110, 1–5. [Google Scholar] [PubMed]
- Burnham, A.J. Scientific Advances in Controlling N. ceranae (Microsporidia) Infections in Honey Bees (Apis Mellifera). Front. Vet. Sci. 2019, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An Overview of Recent Scientific Advances and Their Significance for Apiculture. J. Agric. Food Chem. 2014, 62, 2728–2737. [Google Scholar] [CrossRef]
- Huang, W.-F.; Solter, L.F.; Yau, P.M.; Imai, B.S. N. ceranae Escapes Fumagillin Control in Honey Bees. PLoS Pathog 2013, 9, e1003185. [Google Scholar] [CrossRef] [PubMed]
- Van den Heever, J.P.; Thompson, T.S.; Otto, S.J.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. The effect of dicyclohexylamine and fumagillin on Nosema ceranae-infected honey bee (Apis mellifera) mortality in cage trial assays. Apidologie 2016, 47, 663–670. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Trytek, M.; Borsuk, G.; Buczek, K.; Rybicka-Jasińska, K.; Gryko, D. Porphyrins Inactivate N. ceranae spp. Microsporidia. Sci. Rep. 2018, 8, 5523. [Google Scholar] [CrossRef] [PubMed]
- Nanetti, A.; Rodriguez-García, C.; Meana, A.; Martín-Hernández, R.; Higes, M. Effect of Oxalic Acid on N. ceranae Infection. Res. Vet. Sci. 2015, 102, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Lodesani, M.; Maistrello, L. Effect of thymol and resveratrol administered with candy or syrup on the development of N. ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 2010, 41, 141–150. [Google Scholar] [CrossRef]
- Jiang, Z.; Cui, W.; Prasad, P.; Touve, M.A.; Gianneschi, N.C.; Mager, J.; Thayumanavan, S. Bait-and-Switch Supramolecular Strategy To Generate Noncationic RNA–Polymer Complexes for RNA Delivery. Biomacromolecules 2019, 20, 435–442. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Davis, M.E. Clinical Experiences with Systemically Administered SiRNA-Based Therapeutics in Cancer. Nat. Rev. Drug Discov. 2015, 14, 843–856. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Robinson, G.E. The Power and Promise of Applying Genomics to Honey Bee Health. Curr. Opin. Insect. Sci. 2015, 10, 124–132. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Virus and DsRNA-Triggered Transcriptional Responses Reveal Key Components of Honey Bee Antiviral Defense. Sci. Rep. 2017, 7, 6448. [Google Scholar] [CrossRef]
- Evans, J.D.; Cook, S.C. Genetics and Physiology of Varroa Mites. Curr. Opin. Insect Sci. 2018, 26, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Evans, J.D.; Li, W.; Branchiccela, B.; Li, J.H.; Heerman, M.C.; Banmeke, O.; Zhao, Y.; Hamilton, M.; Higes, M.; et al. Nosemosis control in European honey bees, Apis mellifera, by silencing the gene encoding N. ceranae polar tube protein 3. J. Exp. Biol. 2018, 221, jeb184606. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, D.; Gwak, W.; Woo, S. Increased Survival of the Honey Bee Apis mellifera Infected with the Microsporidian N. ceranae by Effective Gene Silencing. Arch. Insect Biochem. Physiol. 2020, 105, e21734. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Evans, J.D.; Huang, Q.; Rodríguez-García, C.; Liu, J.; Hamilton, M.; Grozinger, C.M.; Webster, T.C.; Su, S.; Chen, Y.P. Silencing the Honey Bee (Apis mellifera) Naked Cuticle Gene (nkd) Improves Host Immune Function and Reduces N. ceranae Infections. Appl. Environ. Microbiol. 2016, 82, 6779–6787. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Pan, L.; Chen, Z.; Du, H.; Luo, B.; Luo, J.; Pan, G. The Roles of Microsporidia Spore Wall Proteins in the Spore Wall Formation and Polar Tube Anchorage to Spore Wall during Development and Infection Processes. Exp. Parasitol. 2018, 187, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mamta, B.; Rajam, M.V. RNAi Technology: A New Platform for Crop Pest Control. Physiol. Mol. Biol. Plants 2017, 23, 487–501. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/Double-Stranded RNA Nanoparticle-Mediated RNA Interference to Silence Chitin Synthase Genes through Larval Feeding in the African Malaria Mosquito (Anopheles Gambiae): Nanoparticle-Mediated RNAi in Mosquito Larvae. Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian Activity of Sulphated Polysaccharides from Algae and Their Potential to Control Honeybee Nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, S.; Xu, Y.; Gong, H.; Wu, Y.; Chen, Y.; Hu, F.; Zheng, H. Protective potential of Chinese herbal extracts against microsporidian N. ceranae, an emergent pathogen of western honey bees, Apis mellifera L. J. Asia Pac. Entomol. 2019, in press. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising Antimicrobial Activity against the Honey Bee Parasite N. ceranae by Methanolic Extracts from Chilean Native Plants and Propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C. How do bees prevent hive infections? The antimicrobial properties of propolis. Biology 2010, 1, 481–493. [Google Scholar]
- Suwannapong, G.; Maksong, S.; Phainchajoen, M.; Benbow, M.E.; Mayack, C. Survival and Health Improvement of N. ceranae Infected Apis florea (Hymenoptera: Apidae) Bees after Treatment with Propolis Extract. J. Asia Pac. Entomol. 2018, 21, 437–444. [Google Scholar] [CrossRef]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis Consumption Reduces N. ceranae Infection of European Honey Bees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef]
- Gherman, B.I.; Denner, A.; Bobiş, O.; Dezmirean, D.S.; Mărghitaş, L.A.; Schlüns, H.; Moritz, R.F.A.; Erler, S. Pathogen-Associated Self-Medication Behavior in the Honeybee Apis mellifera. Behav. Ecol. Sociobiol. 2014, 68, 1777–1784. [Google Scholar] [CrossRef]
- LoCascio, G.M.; Aguirre, L.; Irwin, R.E.; Adler, L.S. Pollen from Multiple Sunflower Cultivars and Species Reduces a Common Bumblebee Gut Pathogen. R. Soc. Open Sci. 2019, 6, 190279. [Google Scholar] [CrossRef]
- Charistos, L.; Parashos, N.; Hatjina, F. Long Term Effects of a Food Supplement HiveAliveTM on Honey Bee Colony Strength and N. ceranae Spore Counts. J. Apic. Res. 2015, 54, 420–426. [Google Scholar] [CrossRef]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of Api-Bioxal® and ApiHerb® Treatments against N. ceranae Infection in Apis mellifera Investigated by Two QPCR Methods. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary Amino Acid and Vitamin Complex Protects Honey Bee from Immunosuppression Caused by N. ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef]
- Van den Heever, J.P.; Thompson, T.S.; Otto, S.J.G.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Evaluation of Fumagilin-B® and other potential alternative chemotherapies against N. ceranae -infected honeybees (Apis mellifera) in cage trial assays. Apidologie 2016, 47, 617–630. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.; Rivera, R.; Carroll, M.; Chambers, M.; Hidalgo, G.; de Jong, E.W. Honey Bee Colonies Provided with Natural Forage Have Lower Pathogen Loads and Higher Overwinter Survival than Those Fed Protein Supplements. Apidologie 2016, 47, 186–196. [Google Scholar] [CrossRef]
- Alberoni, D.; Baffoni, L.; Gaggìa, F.; Ryan, P.M.; Murphy, K.; Ross, P.R.; Stanton, C.; Di Gioia, D. Impact of Beneficial Bacteria Supplementation on the Gut Microbiota, Colony Development and Productivity of Apis mellifera L. Benef. Microbes 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Li, J.H.; Evans, J.D.; Li, W.F.; Zhao, Y.Z.; DeGrandi-Hoffman, G.; Huang, S.K.; Li, Z.G.; Hamilton, M.; Chen, Y.P. New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to N. ceranae infection. PLoS ONE 2017, 12, e0187505. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggìa, F.; Alberoni, D.; Cabbri, R.; Nanetti, A.; Biavati, B.; Di Gioia, D. Effect of Dietary Supplementation of Bifidobacterium and Lactobacillus Strains in Apis mellifera L. against N. ceranae. Benef. Microbes 2016, 7, 45–51. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Snyder, L.; Meador, C.A.D.; Naldo, R.; Mott, B.; Anderson, K.E. Parasaccharibacter apium, gen. nov., sp. nov., Improves Honey Bee (Hymenoptera: Apidae) Resistance to N. ceranae. J. Econ. Entomol. 2016, 109, 537–543. [Google Scholar] [CrossRef]
- El Khoury, S.; Rousseau, A.; Lecoeur, A.; Cheaib, B.; Bouslama, S.; Mercier, P.L.; Demey, V.; Castex, M.; Giovenazzo, P.; Derome, N. Deleterious interaction between honeybees (Apis mellifera) and its microsporidian intracellular parasite N. ceranae was mitigated by administrating either endogenous or allochthonous gut microbiota strains. Front. Ecol. Evol. 2018, 6, 58. [Google Scholar] [CrossRef]
- Arredondo, D.; Castelli, L.; Porrini, M.P.; Garrido, P.M.; Eguaras, M.J.; Zunino, P.; Antúnez, K. Lactobacillus kunkeei Strains Decreased the Infection by Honey Bee Pathogens Paenibacillus larvae and N. ceranae. Benef. Microbes 2018, 9, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak-Baryczko, M.; Szymaś, B. Improvement of the composition of pollen substitute for honey bee (Apis mellifera L.), through implementation of probiotic preparations. J. Apic. Sci. 2006, 50, 15–23. [Google Scholar]
- Schmidt, K.; Engel, P. Probiotic Treatment with a Gut Symbiont Leads to Parasite Susceptibility in Honey Bees. Trends Parasitol. 2016, 32, 914–916. [Google Scholar] [CrossRef]
- Rubanov, A.; Russell, K.A.; Rothman, J.A.; Nieh, J.C.; McFrederick, Q.S. Intensity of N. ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Fernandez-Botran, R. β-Glucan and Parasites. Helminthologia 2018, 55, 177–184. [Google Scholar] [CrossRef]
- Saltykova, E.S.; Gaifullina, L.R.; Kaskinova, M.D.; Gataullin, A.R.; Matniyazov, R.T.; Poskryakov, A.V.; Nikolenko, A.G. Effect of Chitosan on Development of N. apis Microsporidia in Honey Bees. Microbiology 2018, 87, 738–743. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Ribaric, J.; Matak, M.; Svecnjak, L.; Kozaric, Z.; Nejedli, S.; Smodis Skerl, I. Zeolite Clinoptilolite as a Dietary Supplement and Remedy for Honeybee (Apis mellifera L.) Colonies. Vet. Med. 2017, 60, 696–705. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Meana, A.N. ceranae in Europe: An Emergent Type C Nosemosis. Apidologie 2010, 41, 375–392. [Google Scholar] [CrossRef]
- Fries, I.; Feng, F.; da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. N. ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Medici, S.K.; Sarlo, E.G.; Porrini, M.P.; Braunstein, M.; Eguaras, M.J. Genetic Variation and Widespread Dispersal of N. ceranae in Apis mellifera Apiaries from Argentina. Parasitol. Res. 2012, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Invernizzi, C.; Mendoza, Y.; Zunino, P. Honeybee colony losses in Uruguay during 2013–2014. Apidologie 2017, 48, 364–370. [Google Scholar] [CrossRef]
- Goblirsch, M. N. ceranae Disease of the Honey Bee (Apis mellifera). Apidologie 2018, 49, 131–150. [Google Scholar] [CrossRef]
- Cristina, R.T.; Kovačević, Z.; Cincović, M.; Dumitrescu, E.; Muselin, F.; Imre, K.; Militaru, D.; Mederle, N.; Radulov, I.; Hădărugă, N.; et al. Composition and Efficacy of a Natural Phytotherapeutic Blend against Nosemosis in Honey Bees. Sustainability 2020, 12, 5868. [Google Scholar] [CrossRef]
- Raymann, K.; Shaffer, Z.; Moran, N.A. Antibiotic Exposure Perturbs the Gut Microbiota and Elevates Mortality in Honeybees. PLoS Biol. 2017, 15, e2001861. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Załuski, D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. roots: A new hope against honeybee death caused by nosemosis. Molecules 2020, 25, 4452. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Łoś, A.; Grzybek, M.; Ścibior, R.; Strachecka, A. Piperine as a new natural supplement with beneficial effects on the life-span and defence system of honeybees. J. Agric. Sci. 2019, 157, 140–149. [Google Scholar] [CrossRef]
- Strachecka, A.J.; Olszewski, K.; Paleolog, J. Curcumin stimulates biochemical mechanisms of Apis mellifera resistance and extends the apian life-span. J. Apic. Sci. 2015, 59, 129–141. [Google Scholar] [CrossRef]
- Tasleem, F.; Azhar, I.; Ali, S.N.; Perveen, S.; Mahmood, Z.A. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac. J. Trop. Med. 2014, 7, S461–S468. [Google Scholar] [CrossRef]
- Umar, S.; Sarwar, A.H.M.G.; Umar, K.; Ahmad, N.; Sajad, M.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Piperine ameliorates oxidative stress, inflammation and histological outcome in collagen induced arthritis. Cell. Immunol. 2013, 284, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kurze, C.; Dosselli, R.; Grassl, J.; Le Conte, Y.; Kryger, P.; Baer, B.; Moritz, R.F.A. Differential proteomics reveals novel insights into N. ceranae–honey bee interactions. Insect Biochem. Mol. Biol. 2016, 79, 42–49. [Google Scholar] [CrossRef]
- Cilia, G.; Cabbri, R.; Maiorana, G.; Cardaio, I.; Dall’Olio, R.; Nanetti, A. A novel TaqMan® assay for Nosema ceranae quantification in honey bee, based on the protein coding gene Hsp70. Eur. J. Protistol. 2018, 63, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.S.; Moran, N.A.; Evans, J.D. Early Gut Colonizers Shape Parasite Susceptibility and Microbiota Composition in Honey Bee Workers. Proc. Natl. Acad. Sci. USA 2016, 113, 9345–9350. [Google Scholar] [CrossRef]
- Daisley, B.A.; Chmiel, J.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Missing Microbes in Bees: How Systematic Depletion of Key Symbionts Erodes Immunity. Trends Microbiol. 2020, 28, 1010–1021. [Google Scholar] [CrossRef]
- Paris, L.; Peghaire, E.; Moné, A.; Diogon, M.; Debroas, D.; Delbac, F.; El Alaoui, H. Honeybee Gut Microbiota Dysbiosis in Pesticide/Parasite Co-Exposures Is Mainly Induced by N. ceranae. J. Invertebr. Pathol. 2020, 172, 107348. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antúnez, K. Impact of Nutritional Stress on Honeybee Gut Microbiota, Immunity, and N. ceranae Infection. Microb. Ecol. 2020, 80, 908–919. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal Activity of the Essential Oil Obtained from Cryptocarya alba against Infection in Honey Bees by N. ceranae ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, J.H.; Jo, M.; Rangachari, B.; Park, J.K. Anti-Nosemosis Activity of Aster scaber and Artemisia dubia Aqueous Extracts. J. Apic. Sci. 2018, 62, 27–38. [Google Scholar] [CrossRef]
- Balamurugan, R.; Park, J.K.; Lee, J.K. Anti-Nosemosis Activity of Phenolic Compounds Derived from Artemisia dubia and Aster scaber. J. Apic. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Farjan, M.; Łopieńska-Biernat, E.; Lipiński, Z.; Dmitryjuk, M.; Żółtowska, K. Supplementing with vitamin C the diet of honeybees (Apis mellifera carnica) parasitized with Varroa destructor: Effects on antioxidative status. Parasitology 2014, 141, 770–776. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Lakić, N.; Radović, D.; Ristanić, M.; Tarić, E.; Stevanović, J. Efficacy of Plant-Derived Formulation “Argus Ras” in Varroa destructor Control. Acta Vet. 2017, 67, 191–200. [Google Scholar] [CrossRef]
- Aurori, A.C.; Bobiş, O.; Dezmirean, D.S.; Mărghitaş, L.A.; Erler, S. Bay Laurel (Laurus nobilis) as Potential Antiviral Treatment in Naturally BQCV Infected Honeybees. Virus Res. 2016, 222, 29–33. [Google Scholar] [CrossRef]
- Fernández, N.J.; Damiani, N.; Podaza, E.A.; Martucci, J.F.; Fasce, D.; Quiroz, F.; Meretta, P.E.; Quintana, S.; Eguaras, M.J.; Gende, L.B. Laurus nobilis L. Extracts against Paenibacillus larvae: Antimicrobial Activity, Antioxidant Capacity, Hygienic Behavior and Colony Strength. Saudi J. Biol. Sci. 2019, 26, 906–912. [Google Scholar] [CrossRef]
- Ferreira, T.P.; Oliveira, E.E.; Tschoeke, P.H.; Pinheiro, R.G.; Maia, A.M.S.; Aguiar, R.W.S. Potential Use of Negramina (Siparuna guianensis Aubl.) Essential Oil to Control Wax Moths and Its Selectivity in Relation to Honey Bees. Ind. Crops Prod. 2017, 109, 151–157. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Simeunovic, P.; Lakic, N.; Radovic, I.; Sokovic, M.; Griensven, L.J.V. The Effect of Agaricus brasiliensis Extract Supplementation on Honey Bee Colonies. An. Acad. Bras. Ciênc. 2018, 90, 219–229. [Google Scholar] [CrossRef]
- Lamontagne-Drolet, M.; Samson-Robert, O.; Giovenazzo, P.; Fournier, V. The Impacts of Two Protein Supplements on Commercial Honey Bee (Apis mellifera L.) Colonies. J. Apic. Res. 2019, 58, 800–813. [Google Scholar] [CrossRef]
- Jehlík, T.; Kodrík, D.; Krištůfek, V.; Koubová, J.; Sábová, M.; Danihlík, J.; Tomčala, A.; Frydrychová, R.C. Effects of Chlorella sp. on Biological Characteristics of the Honey Bee Apis mellifera. Apidologie 2019, 50, 564–577. [Google Scholar] [CrossRef]
- Higginson, A.D.; Gilbert, F.S.; Reader, T.; Barnard, C.J. Reduction of visitation rates by honeybees (Apis mellifera) to individual inflorescences of lavender (Lavandula stoechas) upon removal of coloured accessory bracts (Hymenoptera: Apidae). Entomol. Gen. 2007, 29, 165–178. [Google Scholar] [CrossRef]
- Bekret, A.; Çankaya, S.; Silici, S. The Effects of Mixture of Plant Extracts and Oils are added to Syrup on Honey Bee Colony Development and Honey Yield. TURJAF 2015, 3, 365–370. [Google Scholar]
- Utuk, A.E.; Piskin, F.C.; Girisgin, A.O.; Selcuk, O.; Aydin, L. Microscopic and molecular detection of N. ceranae spp. in honeybees of Turkey. Apidologie 2016, 47, 267–271. [Google Scholar] [CrossRef][Green Version]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Bailon, E.G.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed]
- Crozier, R.H.; Crozier, Y.C. The mitochondrial genome of the honeybee Apis mellifera: Complete sequence and genome organization. Genetics 1993, 133, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Delaplane, K.S.; Van Der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumkova, R.; Balkanska, R.; Hristov, P. The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits. Pathogens 2021, 10, 234. https://doi.org/10.3390/pathogens10020234
Shumkova R, Balkanska R, Hristov P. The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits. Pathogens. 2021; 10(2):234. https://doi.org/10.3390/pathogens10020234
Chicago/Turabian StyleShumkova, Rositsa, Ralitsa Balkanska, and Peter Hristov. 2021. "The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits" Pathogens 10, no. 2: 234. https://doi.org/10.3390/pathogens10020234
APA StyleShumkova, R., Balkanska, R., & Hristov, P. (2021). The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits. Pathogens, 10(2), 234. https://doi.org/10.3390/pathogens10020234







