First Case of Autochthonous Equine Theileriosis in Austria
Abstract
:1. Introduction
2. Case Description and Further Examinations
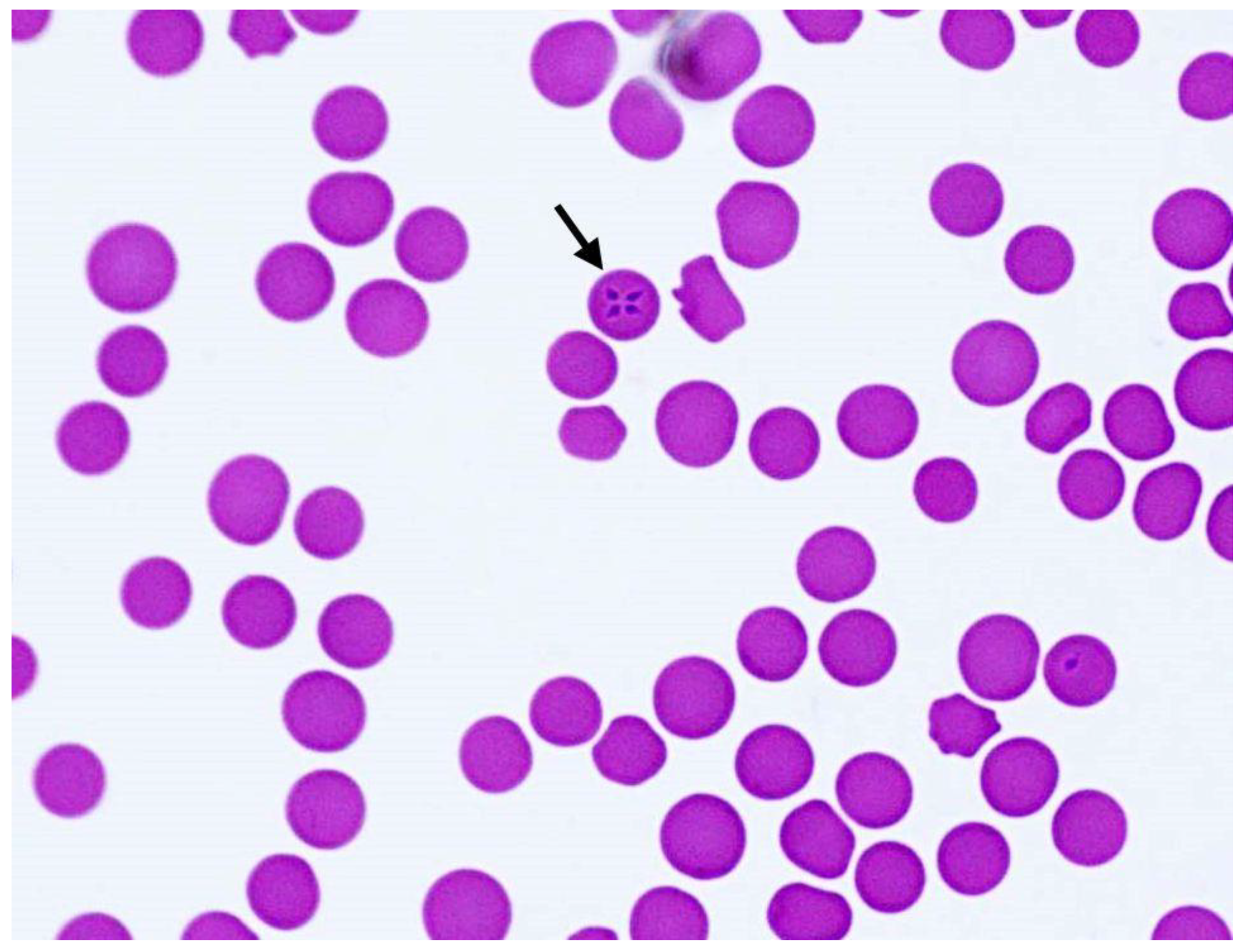
2.1. Clinical Presentation
2.2. Tick Analyses
3. Discussion
4. Materials and Methods
4.1. Vector Search and Examination
4.2. Molecular Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Host (Source) | Country (GenBank® ID) |
|---|---|
| horse (blood) | China (e.g., MT093500), Kazakhstan (e.g., MN857679), Russia (MG551915), Saudi Arabia (KJ801928), South Korea (HM229407), Spain (DQ287951), Portugal (e.g., MT767169), France (MF510476), Ukraine (KP868757) |
| dog (blood) | Saudi Arabia (LC431546) |
| Dermacentor nuttalli (tick) | Mongolia (JQ657703) |
References
- Tirosh-Levy, S.; Gottlieb, Y.; Fry, L.M.; Knowles, D.P.; Steinman, A. Twenty years of equine piroplasmosis research: Global distribution; molecular diagnosis; and phylogeny. Pathogens 2020, 9, 926. [Google Scholar] [CrossRef]
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H.; Schein, E. Redescription of Babesia equi Laveran; 1901 as Theileria equi. Parasitol. Res. 1998, 84, 467–475. [Google Scholar] [CrossRef]
- Wise, L.N.; Pelzel-McCluskey, A.M.; Mealey, R.H.; Knowles, D.P. Equine piroplasmosis. Vet. Clin. N. Am. Equine Pract. 2014, 30, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Kappmeyer, L.S.; Knowles, D.P. Persistently infected horses are reservoirs for intrastadial tick–borne transmission of the apicomplexan parasite Babesia equi. Infect. Immun. 2008, 76, 3525–3529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueti, M.W.; Mealey, R.H.; Kappmeyer, L.S.; White, S.N.; Kumpula–McWhirter, N.; Pelzel, A.M.; Grause, J.F.; Bunn, T.O.; Schwartz, A.; Traub-Dargatz, J.L.; et al. Re-Emergence of the apicomplexan Theileria equi in the United States: Elimination of persistent infection and transmission risk. PLoS ONE 2012, 7, e44713. [Google Scholar] [CrossRef] [Green Version]
- WAHID (World Animal Health Information Database). 2020. Available online: https://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home (accessed on 18 December 2020).
- Onyiche, T.E.; Taioe, M.O.; Molefe, N.I.; Biu, A.A.; Luka, J.; Omeh, I.J.; Yokoyama, N.; Thekisoe, O. Equine piroplasmosis: An insight into global exposure of equids from 1990 to 2019 by systematic review and metaanalysis. Parasitology 2020, 147, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, M.T.E.P.; Lewis, B.D.; Penzhorn, B.L. Molecular evidence for transplacental transmission of Theileria equi from carrier mares to their apparently healthy foals. Vet. Parasitol. 2007, 148, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duscher, G.G.; Feiler, A.; Leschnik, M.; Joachim, A. Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from Eastern Austria and the influence of acaricides/repellents on these parameters. Parasit. Vectors 2013, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Takeet, M.; Adeleye, A.; Adebayo, O.; Akande, F. Haematology and serum biochemical alteration in stress induced equine theileriosis. A case report. Sci. World J. 2010, 4, 19–21. [Google Scholar] [CrossRef]
- Rothschild, C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013, 33, 497–508. [Google Scholar] [CrossRef]
- Zobba, R.; Ardu, M.; Niccolini, S.; Chessa, B.; Manna, L.; Cocco, R.; Pinna Parpaglia, M.L. Clinical and laboratory findings in equine piroplasmosis. J. Equine Vet. Sci. 2008, 28, 301–308. [Google Scholar] [CrossRef]
- Onyiche, T.E.; Suganuma, K.; Igarashi, I.; Yokoyama, N.; Xuan, X.; Thekisoe, O. A review on equine piroplasmosis: Epidemiology; vector ecology; risk factors; host immunity; diagnosis and control. Int. J. Environ. Res. Public Health 2019, 16, 1736. [Google Scholar] [CrossRef] [Green Version]
- Alhassan, A.; Govind, Y.; Tam, N.T.; Thekisoe, O.M.; Yokoyama, N.; Inoue, N.; Igarashi, I. Comparative evaluation of the sensitivity of LAMP; PCR and in vitro culture methods for the diagnosis of equine piroplasmosis. Parasitol. Res. 2007, 100, 1165–1168. [Google Scholar] [CrossRef]
- Ueti, M.W.; Palmer, G.H.; Kappmeyer, L.S.; Scoles, G.A.; Knowles, D.P. Expression of equi merozoite antigen 2 during development of Babesia equi in the midgut and salivary gland of the vector tick Boophilus microplus. J. Clin. Microbiol. 2003, 41, 5803–5809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhoora, R.; Franssen, L.; Closthuizen, M.C.; Guthrie, A.J.; Zweygarth, E.; Penzhorn, B.L.; Jongenjahn, F.; Collins, N.E. Sequence heterogeneity in the 18S rRNA gene within Theileria equi and Babesia caballi from horses in South Africa. Vet. Parasitol. 2009, 159, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, D.P., Jr.; Perryman, L.E.; Kappmeyer, L.S.; Hennager, S.G. Detection of equine antibody to Babesia equi merozoite proteins by a monoclonal antibody–based competitive inhibition enzyme–linked immunosorbent assay. J. Clin. Microbiol. 1991, 29, 2056–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikadai, H.; Osorio, C.R.; Xuan, X.; Igarashi, I.; Kanemaru, T.; Nagasawa, H.; Fujisaki, K.; Suzuki, N.; Mikami, T. Detection of Babesia caballi infection by enzyme-linked immunosorbent assay using recombinant 48–kDa merozoite rhoptry protein. Int. J. Parasitol. 2000, 30, 633–635. [Google Scholar] [CrossRef]
- Bruning, A.; Phipps, P.; Posnett, E.; Canning, E.U. Monoclonal antibodies against Babesia caballi and Babesia equi and their application in serodiagnosis. Vet. Parasitol. 1997, 68, 11–26. [Google Scholar] [CrossRef]
- Diana, A.; Guglielmini, C.; Candini, D.; Pietra, M.; Cipone, M. Cardiac arrhythmias associated with piroplasmosis in the horse: A case report. Vet. J. 2007, 174, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Dvir, E.; Lobetti, R.G.; Jacobson, L.S.; Pearson, J.; Becker, P.J. Electrocardiographic changes and cardiac pathology in canine babesiosis. J. Vet. Cardiol. 2004, 6, 15–23. [Google Scholar] [CrossRef]
- Ahmandpour, S.; Esmaeilneja, B.; Dalir-Naghadeh, B.; Asri-Reyaei, S. Alterations of cardiac and renal biomarkers in horses naturally infected with Theileria equi. Comp. Immunol. Microbiol. Infect. Dis. 2020, 71, 101502. [Google Scholar] [CrossRef] [PubMed]
- Sant, C.; d’Abadie, R.; Pargass, I.; Basu, A.K.; Asgarali, Z.; Charles, R.A.; Georges, K.C. Prospective study investigating transplacental transmission of equine piroplasmosis in thoroughbred foals in Trinidad. Vet. Parasitol. 2016, 226, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.D.; Penzhorn, B.L.; Volkmann, D.H. Could treatment of pregnant mares prevent abortions due to equine piroplasmosis? J. S. Afr. Vet. Assoc. 1999, 70, 90–91. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, I.B.; Câmara, A.C.L.; Bittencourt, M.V.; Marçola, T.G.; Paludo, G.R.; Soto–Blanco, B. Detection of Theileria equi in spleen and blood of asymptomatic piroplasm carrier horses. Acta Parasitol. 2013, 5, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Montes Cortés, M.G.; Fernández–García, J.L.; Habela Martínez–Estéllez, M.Á. A multinested PCR for detection of the equine piroplasmids Babesia caballi and Theileria equi. Ticks Tick Borne Dis. 2019, 10, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Tirosh-Levy, S.; Steinman, A.; Levy, H.; Katz, Y.; Shtilman, M.; Gottlieb, Y. Parasite load and genotype are associated with clinical outcome of piroplasm–infected equines in Israel. Parasit. Vectors 2020, 13, 267. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timoney, P.J. Chapter 69—Infectious diseases and the international movement of horses. In Equine Infectious Diseases; Sellon, D.C., Long, M., Saunders, W.B., Eds.; Elsevier: Philadelphia, PA, USA, 2007; pp. 549–556. ISBN 9781416024064. [Google Scholar]
- Hornok, S.; Edelhofer, R.; Földvári, G.; Joachim, A.; Farkas, R. Serological evidence for Babesia canis infection of horses and an endemic focus of B. caballi in Hungary. Acta Vet. Hung. 2007, 55, 491–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, R.; Tánczos, B.; Gyurkovszky, M.; Földvári, G.; Solymosi, N.; Edelhofer, R.; Hornok, S. Serological and molecular detection of Theileria equi infection in horses in Hungary. Vet. Parasitol. 2013, 192, 143–148. [Google Scholar] [CrossRef]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors 2016, 9, 314. [Google Scholar] [CrossRef] [Green Version]
- Drehmann, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany—evidence of a continuing spread of Dermacentor reticulatus. Front. Vet. Sci. 2020, 7, 66. [Google Scholar] [CrossRef]
- Nosek, J. The ecology and public health importance of Dermacentor marginatus and D. reticulatus ticks in central Europe. Folia Parasitol. 1972, 19, 93–102. [Google Scholar]
- Bullová, E.; Lukán, M.; Stanko, M.; Petko, B. Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet. Parasitol. 2009, 165, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G.; Kiewra, D. New locations of Dermacentor reticulatus ticks in Western Poland: The first evidence of the merge in D. reticulatus occurrence areas? Wiad. Parazytol. 2010, 56, 333–336. [Google Scholar]
- Butler, C.M.; van Oldruitenborgh–Oosterbaan, M.M.; Stou, T.A.E.; van der Kolk, J.H.; van den Wollenberg, L.; Nielen, M.; Jongejan, F.; Werners, A.H.; Houwers, D.J. Prevalence of the causative agents of equine piroplasmosis in the South West of The Netherlands and the identification of two autochthonous clinical Theileria equi infections. Vet. J. 2012, 193, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jongejan, F.; Ringenier, M.; Putting, M.; Berger, L.; Burgers, S.; Kortekaas, R.; Lenssen, J.; van Roessel, M.; Wijnveld, M.; Madder, M. Novel foci of Dermacentor reticulatus ticks infected with Babesia canis and Babesia caballi in the Netherlands and in Belgium. Parasit. Vectors 2015, 8, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetterarchiv Güssing—Meteoblue. Available online: https://www.meteoblue.com/de/ (accessed on 18 December 2020).
- Hodžić, A.; Fuehrer, H.P.; Duscher, G.G. First Molecular evidence of zoonotic bacteria in ticks in Bosnia and Herzegovina. Transbound. Emerg. Dis. 2017, 64, 1313–1316. [Google Scholar] [CrossRef]
- Zintl, A.; Finnerty, E.J.; Murphy, T.M.; de Waal, T.; Gray, J.S. Babesias of red deer (Cervus elaphus) in Ireland. Vet. Res. 2011, 42, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cézanne, R.; Mrowietz, N.; Eigner, B.; Duscher, G.G.; Glawischnig, W.; Fuehrer, H.P. Molecular analysis of Anaplasma phagocytophilum and Babesia divergens in red deer (Cervus elaphus) in Western Austria. Mol. Cell. Probes 2017, 31, 55–58. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Admission (+0d) | +2d | +8d | +17d * | +89d ** | Reference Interval |
|---|---|---|---|---|---|---|
| Erythrocytes [×106 cells/µL] | 3.03 ↓ | 3.89 ↓ | 3.27 ↓ | 5.34 ↓ | 5.5 ↓ | 6.50–11.0 |
| Hemoglobin [g/dL] | 5.0 ↓ | 6.4 ↓ | 5.5 ↓ | 9.4 ↓ | 9.9 ↓ | 10.0–18.0 |
| Hematocrit [%] | 13↓ | 18 ↓ | 16 ↓ | 27 ↓ | 30 ↓ | 32–55 |
| Mean corpuscular volume [fL] | 41.4 | 46.5 | 48.3 | 51.1 | 54.0 | 37.0–55.0 |
| Mean corpuscular hemoglobin [pg/cell] | 16.5 | 16.5 | 16.8 | 17.6 | 18.0 | 13.0–19.0 |
| Mean corpuscular hemoglobin concentration [g/dL] | 40.4 ↑ | 35.4 | 34.8 | 34.4 | 33.3 | 31.0–37.0 |
| Thrombocytes [103 cells/µL] | 53 ↓ | 206 | 90–300 | |||
| Mean platelet volume [fL] | 13.1 ↑ | 5.6–10.4 | ||||
| Leukocytes [cells/µL] | 11,500 ↑ | 9000 | 8270 | 8500 | 12,870 ↑ | 5000–10,000 |
| Neutrophilic granulocytes [cells/µL] | 8730 ↑ | 5285 | 5602 | 10,000 ↑ | 2500–6900 | |
| Monocytes [cell/µL] | 690 ↑ | 675 ↑ | 356 | 187 | 309 | 200–600 |
| Total protein [g/dL] | 7.78 ↑ | 5.50–7.70 | ||||
| Albumin [g/dL] | 2.33 ↓ | 2.40–4.50 | ||||
| Serum amyloid A [mg/L] | >5241 ↑ | >5241 ↑ $ | 478 ↑ | 2.5 | 9 | <10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dirks, E.; de Heus, P.; Joachim, A.; Cavalleri, J.-M.V.; Schwendenwein, I.; Melchert, M.; Fuehrer, H.-P. First Case of Autochthonous Equine Theileriosis in Austria. Pathogens 2021, 10, 298. https://doi.org/10.3390/pathogens10030298
Dirks E, de Heus P, Joachim A, Cavalleri J-MV, Schwendenwein I, Melchert M, Fuehrer H-P. First Case of Autochthonous Equine Theileriosis in Austria. Pathogens. 2021; 10(3):298. https://doi.org/10.3390/pathogens10030298
Chicago/Turabian StyleDirks, Esther, Phebe de Heus, Anja Joachim, Jessika-M. V. Cavalleri, Ilse Schwendenwein, Maria Melchert, and Hans-Peter Fuehrer. 2021. "First Case of Autochthonous Equine Theileriosis in Austria" Pathogens 10, no. 3: 298. https://doi.org/10.3390/pathogens10030298








