Fungicidal Activity of a Safe 1,3,4-Oxadiazole Derivative Against Candida albicans
Abstract
:1. Introduction
2. Results
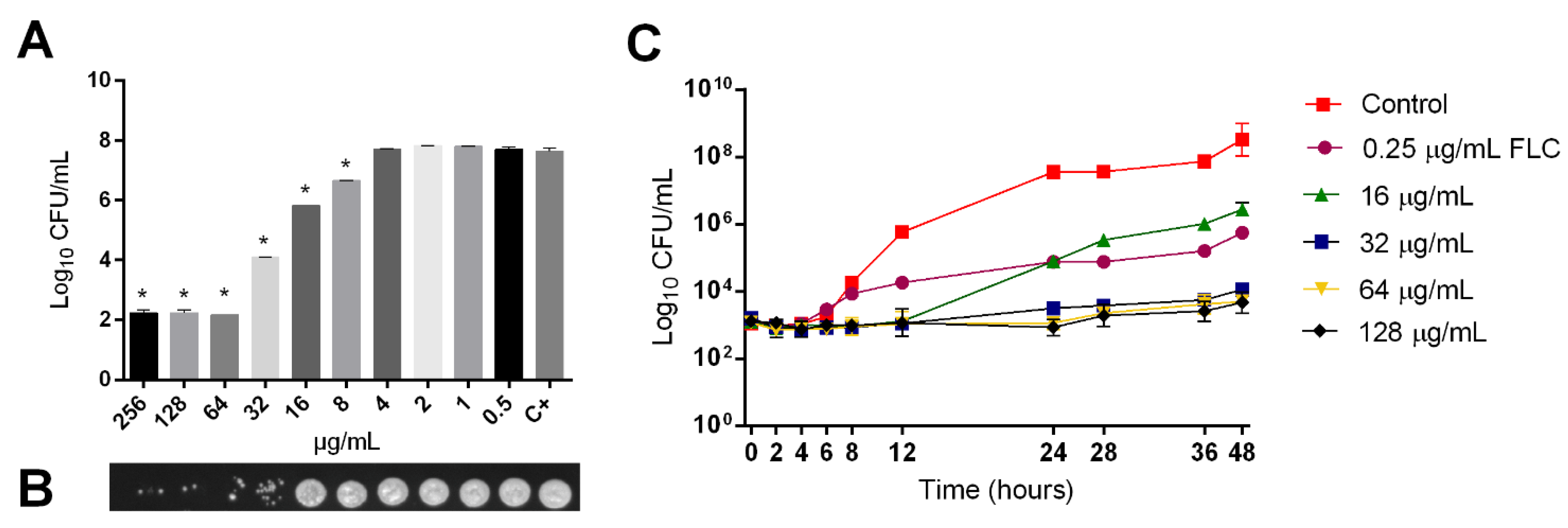
2.1. Fungicidal Activity of LMM6
2.2. Synergistic Effect between LMM6 and Conventional Antifungals
2.3. LMM6 Anti-Biofilm Effect
2.4. LMM6 Low Toxicity in Male Balb/c Mice
2.5. Efficacy of LMM6 on the Treatment of Systemic Candidiasis in a Murine Model
3. Discussion
4. Materials and Methods
4.1. Strains and Growth Conditions
4.2. Chemical Compounds
4.3. Antifungal Susceptibility Testing
4.4. Time-Kill Curve Assay
4.5. Checkerboard Assay and Bliss-Independent Interactions Analysis
4.6. Effects of LMM6 on Biofilm Formation
4.6.1. Determination of Total Biomass by Crystal Violet
4.6.2. Quantification of Viable Biofilm Cells
4.6.3. Effect of LMM6 on Biofilm Structure
4.7. Ethics Statement
4.8. Evaluation of the Acute Toxicity
Biochemical and Hematological Analyzes
4.9. LMM6 Antifungal Activity in a Murine Model of Systemic Candidiasis
4.9.1. Cytokines Detection by Flow Cytometry
4.9.2. Histopathological Analysis
4.10. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camargo, L.F.; Marra, A.R.; Pignatari, A.C.; Sukiennik, T.; Behar, P.P.; Medeiros, E.A.; Ribeiro, J.; Girão, E.; Correa, L.; Guerra, C.; et al. Nosocomial bloodstream infections in a nationwide study: Comparison between solid organ transplant patients and the general population. Transpl. Infect. Dis. 2015, 17, 308–313. [Google Scholar] [CrossRef]
- Braga, I.A.; Campos, P.A.; Gontijo-Filho, P.P.; Ribas, R.M. Multi-hospital point prevalence study of healthcare-associated infections in 28 adult intensive care units in Brazil. J. Hosp. Infect. 2018, 99, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Loubon, C.; Cano-Hernández, B.; Poves-Alvarez, R.; Muñoz-Moreno, M.F.; Román-García, P.; Balbás-Alvarez, S.; de la Varga-Martínez, O.; Gómez-Sánchez, E.; Gómez-Pesquera, E.; Lorenzo-López, M.; et al. The Overlooked Immune State in Candidemia: A Risk Factor for Mortality. J. Clin. Med. 2019, 8, 1512. [Google Scholar] [CrossRef] [Green Version]
- Ghrenassia, E.; Mokart, D.; Mayaux, J.; Demoule, A.; Rezine, I.; Kerhuel, L.; Calvet, L.; De Jong, A.; Azoulay, E.; Darmon, M. Candidemia in critically ill immunocompromised patients: Report of a retrospective multicenter cohort study. Ann. Intensive Care 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, J.A.; Corrales, I.F. Invasive candidiasis: Epidemiology and risk factors. In Fungal Infection; InTechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Tascini, C.; Sozio, E.; Corte, L.; Sbrana, F.; Scarparo, C.; Ripoli, A.; Bertolino, G.; Merelli, M.; Tagliaferri, E.; Corcione, A.; et al. The role of biofilm forming on mortality in patients with candidemia: A study derived from real world data. Infect. Dis. 2018, 50, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Sandai, D.; Tabana, Y.M.; Ouweini, A.E.; Ayodeji, I.O. Resistance of Candida albicans biofilms to drugs and the host immune system. Jundishapur J. Microbiol. 2016, 9, e37385. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Von Lilienfeld-Toal, M.; Wagener, J.; Einsele, H.; Cornely, O.A.; Kurzai, O. Invasive fungal infection. Dtsch. Arztebl. Int. 2019, 116, 271–278. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Brüggemann, R.J.; Alffenaar, J.W.; Blijlevens, N.M.; Billaud, E.M.; Kosterink, J.G.; Verweij, P.E.; Burger, D.M. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin. Infect. Dis. 2009, 48, 1441–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [Green Version]
- Castanheira, M.; Deshpande, L.M.; Davis, A.P.; Rhomberg, P.R.; Pfaller, M.A. Monitoring antifungal resistance in a global collection of invasive yeasts and molds: Application of CLSI epidemiological cutoff values and whole-genome sequencing analysis for detection of azole resistance in Candida albicans. Antimicrob. Agents Chemother. 2017, 61, e00906–e00917. [Google Scholar] [CrossRef] [Green Version]
- Campitelli, M.; Zeineddine, N.; Samaha, G.; Maslak, S. Combination antifungal therapy: A review of current data. J. Clin. Med. Res. 2017, 9, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Scorzoni, L.; de Paula, E.; Silva, A.C.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batool, M.; Ahmad, B.; Choi, S. A structure-based drug discovery paradigm. Int. J. Mol. Sci. 2019, 20, 2783. [Google Scholar] [CrossRef] [Green Version]
- Mariappan, G.; Kumari, A. Virtual screening and its applications in drug discovery process. In Computer Applications in Drug Discovery and Development; IGI Global: Hershey, PA, USA, 2019; pp. 101–126. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Kioshima, E.S.; Svidzinski, T.I.; Bonfim-Mendonça, P.S.; Capoci, I.R.; Faria, D.R.; Sakita, K.M.; Morelli, F.; Rodrigues, F.A.; Felipe, M.S.; Chaucanés, C.P.; et al. Composição Farmacêutica Baseada em Compostos 1,3,4-Oxadiazólicos e Seu Uso na Preparação de Medicamentos para Tratamento de Infecções Sistêmicas. Brazil Patent BR 10 2018 009020 8, 3 May 2018. [Google Scholar]
- Rodrigues-Vendramini, F.A.; Faria, D.R.; Arita, G.S.; Capoci, I.R.; Sakita, K.M.; Caparroz-Assef, S.M.; Becker, T.; Bonfim-Mendonça, P.S.; Felipe, M.S.; Svidzinski, T.I.; et al. Antifungal activity of two oxadiazole compounds for the paracoccidioidomycosis treatment. PLoS Negl. Trop. Dis. 2019, 13, e0007441. [Google Scholar] [CrossRef] [Green Version]
- Capoci, I.R.; Sakita, K.M.; Faria, D.R.; Rodrigues-Vendramini, F.A.; Arita, G.S.; de Oliveira, A.G.; Felipe, M.S.; Maigret, B.; Bonfim-Mendonça, P.S.; Kioshima, E.S.; et al. Two new 1,3,4-oxadiazoles with effective antifungal activity against Candida albicans. Front. Microbiol. 2019, 10, 2130. [Google Scholar] [CrossRef] [PubMed]
- Faria, D.R.; Sakita, K.M.; Capoci, I.R.; Arita, G.S.; Rodrigues-Vendramini, F.A.; de Oliveira Junior, A.G.; Felipe, M.S.; Bonfim-Mendonça, P.S.; Svidzinski, T.I.; Kioshima, E.S. Promising antifungal activity of new oxadiazole against Candida krusei. PLoS ONE 2020, 15, e0227876. [Google Scholar] [CrossRef] [PubMed]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Abadio, A.K.; Kioshima, E.S.; Leroux, V.; Martins, N.F.; Maigret, B.; Felipe, M.S. Identification of new antifungal compounds targeting thioredoxin reductase of Paracoccidioides genus. PLoS ONE 2015, 10, e0142926. [Google Scholar] [CrossRef] [PubMed]
- Dhara, D.; Sunil, D.; Kamath, P.R.; Ananda, K.; Shrilakshmi, S.; Balaji, S. New oxadiazole derivatives: Synthesis and appraisal of their potential as antimicrobial agents. Lett. Drug. Des. Discov. 2018, 15, 21–30. [Google Scholar] [CrossRef]
- Çavuşoğlu, B.K.; Yurttaş, L.; Cantürk, Z. The synthesis, antifungal and apoptotic effects of triazole-oxadiazoles against Candida species. Eur. J. Med. Chem. 2018, 144, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Karaburun, A.Ç.; Çavuşoğlu, B.K.; Çevik, U.A.; Osmaniye, D.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Atlı, Ö.; Koparal, A.S.; Kaplancıklı, Z.A. Synthesis and antifungal potential of some novel benzimidazole-1,3,4-oxadiazole compounds. Molecules 2019, 24, 191. [Google Scholar] [CrossRef] [Green Version]
- Verma, G.; Khan, M.F.; Akhtar, W.; Alam, M.M.; Akhter, M.; Shaquiquzzaman, M. A review exploring therapeutic worth of 1,3,4-oxadiazole tailored compounds. Mini Rev. Med. Chem. 2019, 19, 477–509. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zarychanski, R.; Pisipati, A.; Kumar, A.; Kethireddy, S.; Bow, E.J. Fungicidal versus fungistatic therapy of invasive Candida infection in non-neutropenic adults: A meta-analysis. Mycology 2018, 9, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Calvo, J.M.; Barbero, G.R.; Guerrero-Vásquez, G.; Durán, A.G.; Macías, M.; Rodríguez-Iglesias, M.A.; Molinillo, J.M.; Macías, F.A. Synthesis, antibacterial and antifungal activities of naphthoquinone derivatives: A structure–activity relationship study. Med. Chem. Res. 2016, 25, 1274–1285. [Google Scholar] [CrossRef]
- Li, W.S.; Chen, Y.C.; Kuo, S.F.; Chen, F.J.; Lee, C.H. The impact of biofilm formation on the persistence of candidemia. Front. Microbiol. 2018, 9, 1196. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, D.R.; Feresin, L.P.; Arias, L.S.; Barão, V.A.; Barbosa, D.B.; Delbem, A.C. Effect of tyrosol on adhesion of Candida albicans and Candida glabrata to acrylic surfaces. Med. Mycol. 2015, 53, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Ostrosky-Zeichner, L. Combination antifungal therapy: A critical review of the evidence. Clin. Microbiol. Infect. 2008, 14, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.A.; Reyes, G.H.; Long, L.A.; Mukherjee, P.K.; Ghannoum, M.A. Efficacy of caspofungin combined with amphotericin B against azole-resistant Candida albicans. J. Antimicrob. Chemother. 2003, 51, 1427–1429. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Liao, K.; Hang, C. Caffeic acid phenethyl ester synergistically enhances the antifungal activity of fluconazole against resistant Candida albicans. Phytomedicine 2018, 40, 55–58. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Xu, J.; Wu, J.; Wang, Y.; Qiu, X.; Zhang, Y.; Hou, W.; Yan, L.; An, M.; et al. The synergism of the small molecule ENOblock and fluconazole against fluconazole-resistant Candida albicans. Front. Microbiol. 2019, 10, 2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, H.O.; Majima, H.; Watanabe, A.; Kamei, K. In Vitro Characterization of Twenty-One Antifungal Combinations against Echinocandin-Resistant and -Susceptible Candida glabrata. J. Fungi 2021, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Tonholo, D.R.; Maltarollo, V.G.; Kronenberger, T.; Silva, I.R.; Azevedo, P.O.; Oliveira, R.B.; Souza, L.C.; Tagliati, C.A. Preclinical toxicity of innovative molecules: In vitro, in vivo and metabolism prediction. Chem. Biol. Interact. 2020, 315, 108896. [Google Scholar] [CrossRef] [PubMed]
- Dongmo, O.L.; Epoh, N.J.; Tadjoua, H.T.; Yousuf, S.; Telefo, P.B.; Tapondjou, L.A.; Choudhary, M.I. Acute and sub-acute toxicity of the aqueous extract from the stem bark of Tetrapleura tetrapteura Taub. (Fabaceae) in mice and rats. J. Ethnopharmacol. 2019, 236, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S.; Morton, D.; Michael, B.; Roome, N.; Johnson, J.K.; Yano, B.L.; Perry, R.; Schafer, K. Society of Toxicologic Pathology position paper: Organ weight recommendations for toxicology studies. Toxicol. Pathol. 2007, 35, 751–755. [Google Scholar] [CrossRef] [Green Version]
- Issa, N.T.; Wathieu, H.; Ojo, A.; Byers, S.W.; Dakshanamurthy, S. Drug metabolism in preclinical drug development: A survey of the discovery process, toxicology, and computational tools. Curr. Drug Metab. 2017, 18, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Ramaiah, S.K. Preclinical safety assessment: Current gaps, challenges, and approaches in identifying translatable biomarkers of drug-induced liver injury. Clin. Lab. Med. 2011, 31, 161–172. [Google Scholar] [CrossRef]
- Pazhayattil, G.S.; Shirali, A.C. Drug-induced impairment of renal function. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, J.H. Overview of urea and creatinine. Lab. Med. 2014, 45, e19–e20. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Arika, W.N.; Nyamai, D.W.; Musila, M.N.; Ngugi, M.P.; Njagi, E.N. Hematological markers of in vivo toxicity. J. Hematol. Thrombo. Dis. 2016, 4, 236. [Google Scholar] [CrossRef]
- Santos, E.W.; Oliveira, D.C.; Hastreiter, A.; Silva, G.B.; Beltran, J.S.; Tsujita, M.; Crisma, A.R.; Neves, S.M.; Fock, R.A.; Borelli, P. Hematological and biochemical reference values for C57BL/6, Swiss Webster and BALB/c mice. Braz. J. Vet. Res. Anim. Sci. 2016, 53, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, B.S.; Praxedes, É.A.; Lima, M.A.; Pimentel, M.M.; Santos, F.A.; Brito, P.D.; Lelis, I.C.; Macedo, M.F.; Bezerra, M.B. Haematological and biochemical profile of Balb-c mice. Acta Sci. Vet. 2017, 45, 1477. [Google Scholar] [CrossRef] [Green Version]
- Spellberg, B.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 2005, 192, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lim, J.K.; Lee, C.C.; Murphy, P.M. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J. Innate Immun. 2011, 3, 180–199. [Google Scholar] [CrossRef] [Green Version]
- Jae-Chen, S.; Young-Joo, J.; Seon-Min, P.; Seok, S.K.; Jung-Hyun, S.; Jung-Il, C. Mechanism underlying renal failure caused by pathogenic Candida albicans infection. Biomed. Rep. 2015, 3, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Antachopoulos, C.; Roilides, E. Cytokines and fungal infections. Br. J. Haematol. 2005, 129, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; Tran, D.Q.; Schaal, J.B.; Tran, P.; Eriguchi, Y.; Ngole, D.; Cabebe, A.E.; Park, A.Y.; Beringer, P.M.; Ouellette, A.J.; et al. Rhesus theta defensin 1 promotes long term survival in systemic candidiasis by host directed mechanisms. Sci. Rep. 2019, 9, 16905. [Google Scholar] [CrossRef]
- Yarrow, D. Methods for the isolation, maintenance and identification of yeasts. In The Yeasts, A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 1998; pp. 77–100. [Google Scholar] [CrossRef]
- Cassagne, C.; Normand, A.C.; L′Ollivier, C.; Ranque, S.; Piarroux, R. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses 2016, 59, 678–690. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; 4th Informational Supplement; CLSI Document M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Klepser, M.E.; Wolfe, E.J.; Jones, R.N.; Nightingale, C.H.; Pfaller, M.A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 1997, 41, 1392–1395. [Google Scholar] [CrossRef] [Green Version]
- Scorneaux, B.; Angulo, D.; Borroto-Esoda, K.; Ghannoum, M.; Peel, M.; Wring, S. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob. Agents Chemother. 2017, 61, e01961-16. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Malik, A.; Ahmad, I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med. Mycol. 2012, 50, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, V.; Rella, A.; Farnoud, A.M.; Singh, A.; Munshi, M.; Bryan, A.; Naseem, S.; Konopka, J.B.; Ojima, I.; Bullesbach, E.; et al. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio 2015, 6, e00647-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini-Valerio, F.K.; Bonfim-Mendonça, P.S.; Rosseto, H.C.; Bruschi, M.L.; Henriques, M.; Negri, M.; Silva, S.; Svidzinski, T.I. Propolis: A potential natural product to fight Candida species infections. Future Microbiol. 2016, 11, 1035–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, A.G.; Spago, F.R.; Simionato, A.S.; Navarro, M.O.; da Silva, C.S.; Barazetti, A.R.; Cely, M.V.; Tischer, C.A.; San Martin, J.A.; Andrade, C.G.; et al. Bioactive organocopper compound from Pseudomonas aeruginosa inhibits the growth of Xanthomonas citri subsp. citri. Front. Microbiol. 2016, 7, 113. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (ANVISA). Guia Para a Condução de Estudos não Clínicos de Toxicologia e Segurança Farmacológica Necessários ao Desenvolvimento de Medicamentos. 2013. Available online: https://bit.ly/2OA6uWr (accessed on 5 February 2020).
- Kifayatullah, M.; Mustafa, M.S.; Sengupta, P.; Sarker, M.M.; Das, A.; Das, S.K. Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. J. Acute. Dis. 2015, 4, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Briggs, C.; Bain, B.J. Basic haematological techniques. In Dacie and Lewis Practical Haematology; Elsevier: London, UK, 2017; pp. 18–49. [Google Scholar]
- Araujo, A.V. Estudos Pré-Clínicos de Toxicidade Aguda e de Doses Repetidas da Fosfoetanolamina Sintética. Master′s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2017. Available online: https://www.teses.usp.br/teses/disponiveis/5/5160/tde-15032018-092543/publico/AlineVieiraPinheiroDeAraujo.pdf (accessed on 1 June 2020).
- Brecher, G.; Cronkite, E.P. Morphology and enumeration of human blood platelets. J. Appl. Physiol. 1950, 3, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, E.J.; Zijlstra, W.G. Determination of hemoglobin and its derivatives. Adv. Clin. Chem. 1965, 8, 141–187. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Kao, R.Y.; Yuen, K.Y.; Wang, Y.; Yang, D.; Samaranayake, L.P.; Seneviratne, C.J. In vitro and in vivo activity of a novel antifungal small molecule against Candida infections. PLoS ONE 2014, 9, e85836. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| MIC (µg/mL) | Interpretation † N (%) | |||||
|---|---|---|---|---|---|---|
| Antifungal Drugs | Range | MIC50 | MIC90 | Susceptible | SDD | Resistent |
| Amphotericin B | 0.03–0.5 | 0.125 | 0.25 | 31 (100) | 0 (0) | 0 (0) |
| Caspofungin | 0.03–0.25 | 0.125 | 0.25 | 31 (100) | 0 (0) | 0 (0) |
| Fluconazole | 0.06 > 64 | 0.25 | 0.25 | 30 (96.8) | 0 (0) | 1 (3.2) |
| Itraconazole | 0.03 > 16 | 0.125 | 0.25 | 24 (77.4) | 6 (19.4) | 1 (3.2) |
| LMM6 | 8.0–32 | 16 | 32 | - | - | - |
| Strains | Combinations | FICA | FICB | FIC Index | Interpretation |
|---|---|---|---|---|---|
| Reference strain | AMB/LMM6 | 0.5 | 0.031 | 0.531 | Synergistic |
| CAS/LMM6 | 0.5 | 0.063 | 0.563 | Synergistic | |
| FLC/LMM6 | 1 | 1 | 2 | No effect | |
| ITC/LMM6 | 1 | 1 | 2 | No effect | |
| SangHUMCa7 | AMB/LMM6 | 0.5 | 0.25 | 0.75 | Synergistic |
| CAS/LMM6 | 0.5 | 0.063 | 0.563 | Synergistic | |
| FLC/LMM6 | 1 | 0.5 | 1.5 | No effect | |
| ITC/LMM6 | 0.5 | 1 | 1.5 | No effect |
| Hematological Parameters | Analyzed Groups | ||||
|---|---|---|---|---|---|
| Healthy | IP Control | IV Control | IP LMM6 | IV LMM6 | |
| Total RBC (106/mm3) | 8.39 ± 0.53 | 8.96 ± 0.92 | 9.13 ± 0.32 | 8.96 ± 0.58 | 8.31 ± 1.28 |
| Haematocrit (%) | 43.66 ± 0.58 | 45 ± 1 | 45.5 ± 1 | 44.43 ± 0.53 | 46 ± 0 |
| Hemoglobin (g/dL) | 21.48 ± 0.64 | 20.31 ± 0.40 | 20.90 ± 0.69 | 20.65 ± 0.58 | 21.01 ± 0.21 |
| MCV (fL) | 52.15 ± 3.08 | 50.31 ± 5.11 | 49.86 ± 0.98 | 49.77 ± 3.67 | 53.46 ± 3.40 |
| MCH (pg) | 25.64 ± 1.15 | 22.83 ± 2.06 | 22.89 ± 0.46 | 23.05 ± 1.49 | 24.02 ± 1.44 |
| MCHC (%) | 49.21 ± 1.83 | 44.88 ± 0.50 * | 45.92 ± 0.73 * | 46.49 ± 1.37 * | 45.27 ± 1.03 * |
| Platelet (103/mm3) | 355.66 ± 71.28 | 570.00 ± 125.79 * | 406.00 ± 42.68 | 402.71 ± 48.33 | 394.00 ± 77.24 |
| Groups | Leukogram | |||||
|---|---|---|---|---|---|---|
| Leukocytes | Neutrophils | Monocytes | Lymphocytes | Eosinophils | Basophils | |
| 103/mm3 | % (103/mm3) | |||||
| Healthy | 4.5 ± 1.9 | 18 ± 4.36 (0.83 ± 0.62) | 1 ± 1 (0.05 ± 0.05) | 78.66 ± 5.14 (3.52 ± 1.47) | 2.33 ± 0.58 (0.11 ± 0.06) | - |
| IP control | 7.9 ± 2.0 | 17.25 ± 1.70 (1.36 ± 0.35) | 0.5 ± 1 (0.04 ± 0.09) | 80 ± 3.74 (6.37 ± 1.82) | 2.25 ± 1.5 (0.16 ± 0.12) | - |
| IV control | 10.0 ± 2.6 * | 20 ± 3.91 (2.05 ±0.77) | 0.5 ± 0.58 (0.05 ± 0.06) | 77.75 ± 4.19 (7.77 ± 2.02) | 1.75 ± 1.5 (0.15 ± 0.12) | - |
| IP LMM6 | 5.4 ± 2.4 | 18.14 ± 4.45 (1.06 ± 0.62) | 1 ± 0.63 (0.06 ± 0.06) | 78.86 ± 4.88 (4.21 ± 1.70) | 2.14 ± 1.21 (0.11 ± 0.11) | - |
| IV LMM6 | 6.0 ± 1.9 | 19.66 ± 2.94 (1.14 ± 0.25) | 0.66 ± 0.51 (0.05 ± 0.04) | 77.66 ± 2.16 (4.71 ± 1.65) | 2 ± 0.82 (0.13 ± 0.08) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, D.R.; Melo, R.C.; Arita, G.S.; Sakita, K.M.; Rodrigues-Vendramini, F.A.V.; Capoci, I.R.G.; Becker, T.C.A.; Bonfim-Mendonça, P.d.S.; Felipe, M.S.S.; Svidzinski, T.I.E.; et al. Fungicidal Activity of a Safe 1,3,4-Oxadiazole Derivative Against Candida albicans. Pathogens 2021, 10, 314. https://doi.org/10.3390/pathogens10030314
Faria DR, Melo RC, Arita GS, Sakita KM, Rodrigues-Vendramini FAV, Capoci IRG, Becker TCA, Bonfim-Mendonça PdS, Felipe MSS, Svidzinski TIE, et al. Fungicidal Activity of a Safe 1,3,4-Oxadiazole Derivative Against Candida albicans. Pathogens. 2021; 10(3):314. https://doi.org/10.3390/pathogens10030314
Chicago/Turabian StyleFaria, Daniella Renata, Raquel Cabral Melo, Glaucia Sayuri Arita, Karina Mayumi Sakita, Franciele Abigail Vilugron Rodrigues-Vendramini, Isis Regina Grenier Capoci, Tania Cristina Alexandrino Becker, Patrícia de Souza Bonfim-Mendonça, Maria Sueli Soares Felipe, Terezinha Inez Estivalet Svidzinski, and et al. 2021. "Fungicidal Activity of a Safe 1,3,4-Oxadiazole Derivative Against Candida albicans" Pathogens 10, no. 3: 314. https://doi.org/10.3390/pathogens10030314
APA StyleFaria, D. R., Melo, R. C., Arita, G. S., Sakita, K. M., Rodrigues-Vendramini, F. A. V., Capoci, I. R. G., Becker, T. C. A., Bonfim-Mendonça, P. d. S., Felipe, M. S. S., Svidzinski, T. I. E., & Kioshima, E. S. (2021). Fungicidal Activity of a Safe 1,3,4-Oxadiazole Derivative Against Candida albicans. Pathogens, 10(3), 314. https://doi.org/10.3390/pathogens10030314







