Current Status and Potential of RNA Interference for the Management of Tomato Spotted Wilt Virus and Thrips Vectors
Abstract
1. Introduction
2. Thrips as a Vector of TSWV
3. Current Management Strategies
4. RNA Interference and Its Role in Plant–Virus Interactions
5. RNAi and TSWV
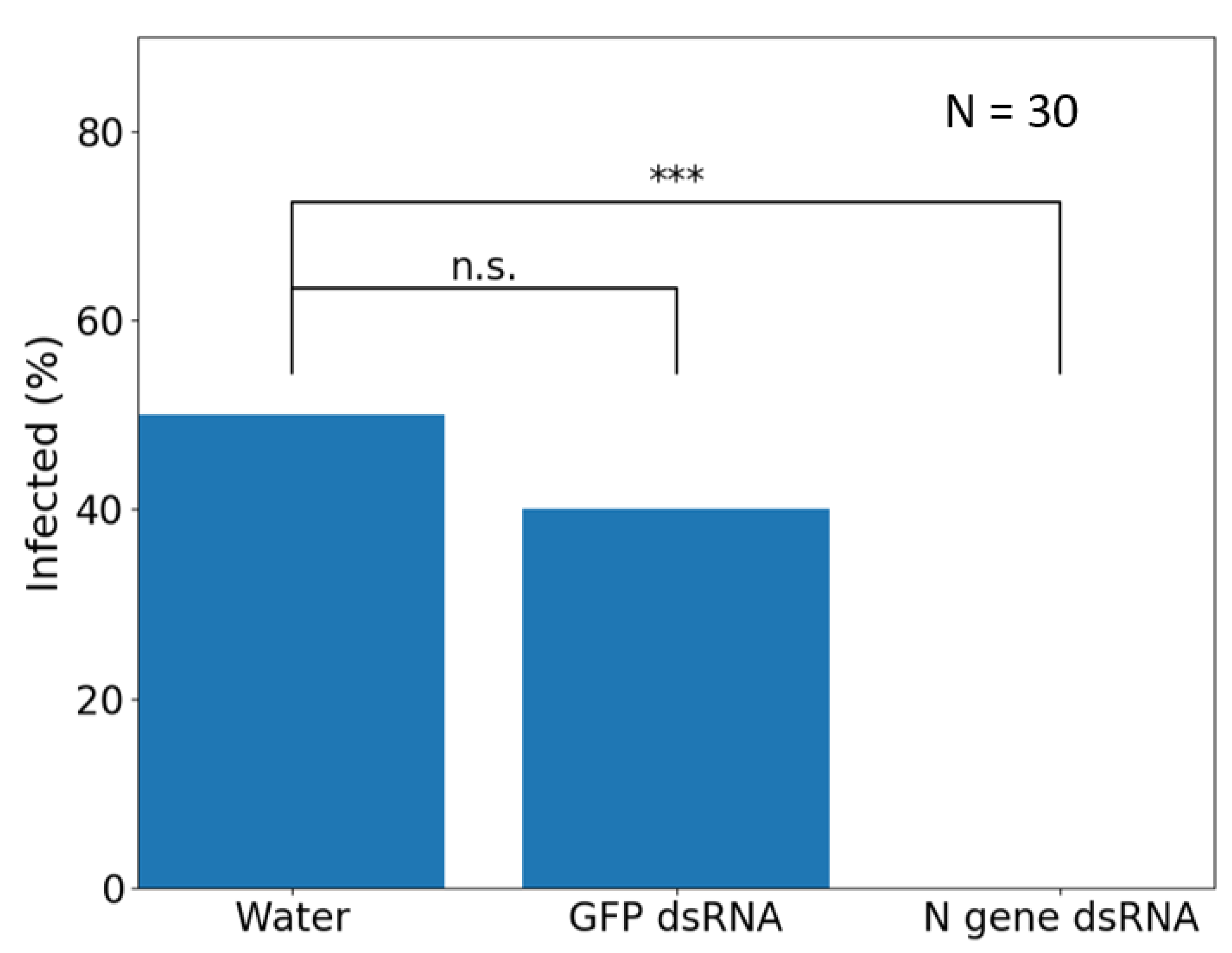
6. Transgenic RNAi for Induced Viral Resistance
7. RNAi in Thrips
8. Future Potential for RNAi against TSWV
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 Plant Viruses in Molecular Plant Pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Parrella, G.; Gognalons, P.; Gebre-Selassiè, K.; Vovlas, C.; Marchoux, G. An Update of the Host Range of Tomato Spotted Wilt Virus. J. Plant Pathol. 2003, 85, 227–264. [Google Scholar]
- Sevik, M.A.; Arli-Sokmen, M. Estimation of the Effect of Tomato Spotted Wilt Virus (TSWV) Infection on Some Yield Components of Tomato. Phytoparasitica 2012, 40, 87–93. [Google Scholar] [CrossRef]
- Resende, R.; Pappu, H. Orthotospoviruses (Tospoviridae). In Encylopedia of Virology; Elsevier Press: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kirk, W.D.J. Feeding. In Thrips as Crop Pests; CAB International: Cambridge, UK, 1997; pp. 119–174. [Google Scholar]
- Chisholm, I.F.; Lewis, T. A New Look at Thrips (Thysanoptera) Mouthparts, Their Action and Effects of Feeding on Plant Tissue. Bull. Entomol. Res. 1984, 74, 663–675. [Google Scholar] [CrossRef]
- Nagata, T.; Inoue-Nagata, A.K.; Smid, H.M.; Goldbach, R.; Peters, D. Tissue Tropism Related to Vector Competence of Frankliniella Occidentalis for Tomato Spotted Wilt Tospovirus. J. Gen. Virol. 1999, 80, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Wijkamp, I.; Wetering, F.V.D.; Goldbach, R.; Peters, D. Transmission of Tomato Spotted Wilt Virus by Frankliniella Occidentalism Median Acquisition and Inoculation Access Period. Ann. Appl. Biol. 1996, 129, 303–313. [Google Scholar] [CrossRef]
- Teulon, D.A.J.; Penman, D.R. Effects of Temperature and Diet on Oviposition Rate and Development Time of the New Zealand Flower Thrips, Thrips Obscuratus. Entomol. Exp. Appl. 1991, 60, 143–155. [Google Scholar] [CrossRef]
- Van de Wetering, F.; Goldbach, R.; Peters, D. Tomato Spotted Wilt Tospovirus Ingestion by First Instar Larvae of Frankliniella Occidentalis Is a Prerequisite for Transmission. Phytopathology 1996, 86, 900–905. [Google Scholar] [CrossRef]
- De Assis Filho, F.M.; Deom, C.M.; Sherwood, J.L. Acquisition of Tomato Spotted Wilt Virus by Adults of Two Thrips Species. Phytopathology 2004, 94, 333–336. [Google Scholar] [CrossRef]
- Montero-Astúa, M.; Stafford-Banks, C.A.; Badillo-Vargas, I.E.; Rotenberg, D.; Ullman, D.E.; Whitfield, A.E. Tospovirus-Thrips Biology. In Vector-Mediated Transmission of Plant Pathogens; Montero-Astúa, M., Stafford-Banks, C., Badillo-Vargas, I.E., Rotenberg, D., Ullman, D.E., Whitfield, A.E., Eds.; General Plant Pathology; The American Phytopathological Society: St. Paul, MN, USA, 2016; pp. 289–308. ISBN 978-0-89054-535-5. [Google Scholar]
- Pappu, H.; Jones, R.A.C.; Jain, R.K. Global Status of Tospovirus Epidemics in Diverse Cropping Systems: Successes Achieved and Challenges Ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Whitfield, A.E. The Genus Tospovirus: Emerging Bunyaviruses That Threaten Food Security. Annu. Rev. Virol. 2016, 3, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Mound, L.A. The thysanoptera vector species of tospoviruses. Acta Hortic. 1996, 298–309. [Google Scholar] [CrossRef]
- Riley, D.G.; Pappu, H.R. Tactics for Management of Thrips (Thysanoptera: Thripidae) and Tomato Spotted Wilt Virus in Tomato. J. Econ. Entomol. 2004, 97, 1648–1658. [Google Scholar] [CrossRef]
- Pappu, H.; Whitfield, A.; Oliveira, A.S. de Tomato Spotted Wilt Virus (Tospoviridae). In Encylopedia of Virology; Elsevier Press: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Gao, Y.; Lei, Z.; Reitz, S.R. Western Flower Thrips Resistance to Insecticides: Detection, Mechanisms and Management Strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef]
- Zhao, X.; Reitz, S.R.; Yuan, H.; Lei, Z.; Paini, D.R.; Gao, Y. Pesticide-Mediated Interspecific Competition between Local and Invasive Thrips Pests. Sci. Rep. 2017, 7, 40512. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, R.A.; Sadof, C.S. Effects of Spinosad and Acephate on Western Flower Thrips Inside and Outside a Greenhouse. HortTechnology 2000, 10, 359–362. [Google Scholar] [CrossRef]
- Li, D.; Shang, X.; Reitz, S.; Nauen, R.; Lei, Z.; Lee, S.H.; Gao, Y. Field Resistance to Spinosad in Western Flower Thrips Frankliniella Occidentalis (Thysanoptera: Thripidae). J. Integr. Agric. 2016, 15, 2803–2808. [Google Scholar] [CrossRef]
- Jensen, S.E. Insecticide Resistance in the Western Flower Thrips, Frankliniella Occidentalis. Integr. Pest Manag. Rev. 2000, 5, 131–146. [Google Scholar] [CrossRef]
- Nolte, P.; Miller, J.; Duellman, K.M.; Gevens, A.J.; Banks, E. Disease Management. In Potato Production Systems; Stark, J.C., Thornton, M., Nolte, P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 203–257. ISBN 978-3-030-39157-7. [Google Scholar]
- Coutts, B.A.; Thomas-Carroll, M.L.; Jones, R.a.C. Patterns of Spread of Tomato Spotted Wilt Virus in Field Crops of Lettuce and Pepper: Spatial Dynamics and Validation of Control Measures. Ann. Appl. Biol. 2004, 145, 231–245. [Google Scholar] [CrossRef]
- Nault, B.A.; Speese III, J.; Jolly, D.; Groves, R.L. Seasonal Patterns of Adult Thrips Dispersal and Implications for Management in Eastern Virginia Tomato Fields. Crop Prot. 2003, 22, 505–512. [Google Scholar] [CrossRef]
- Riley, D.G.; Joseph, S.V.; Srinivasan, R. Reflective Mulch and Acibenzolar-S-Methyl Treatments Relative to Thrips (Thysanoptera: Thripidae) and Tomato Spotted Wilt Virus Incidence in Tomato. J. Econ. Entomol. 2012, 105, 1302–1310. [Google Scholar] [CrossRef][Green Version]
- Brown, S.L.; Culbreath, A.K.; Todd, J.W.; Gorbet, D.W.; Baldwin, J.A.; Beasley, J.P., Jr. Development of a Method of Risk Assessment to Facilitate Integrated Management of Spotted Wilt of Peanut. Plant Dis. 2005, 89, 348–356. [Google Scholar] [CrossRef]
- Srinivasan, R.; Abney, M.R.; Culbreath, A.K.; Kemerait, R.C.; Tubbs, R.S.; Monfort, W.S.; Pappu, H.R. Three Decades of Managing Tomato Spotted Wilt Virus in Peanut in Southeastern United States. Virus Res. 2017, 5, 16. [Google Scholar] [CrossRef]
- Mirnezhad, M.; Romero-González, R.R.; Leiss, K.A.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Metabolomic Analysis of Host Plant Resistance to Thrips in Wild and Cultivated Tomatoes. Phytochem. Anal. 2010, 21, 110–117. [Google Scholar] [CrossRef]
- Maris, P.C.; Joosten, N.N.; Goldbach, R.W.; Peters, D. Restricted Spread of Tomato Spotted Wilt Virus in Thrips-Resistant Pepper. Phytopathology 2003, 93, 1223–1227. [Google Scholar] [CrossRef]
- Batuman, O.; Turini, T.A.; Oliveira, P.V.; Rojas, M.R.; Macedo, M.; Mellinger, H.C.; Adkins, S.; Gilbertson, R.L. First Report of a Resistance-Breaking Strain of Tomato Spotted Wilt Virus Infecting Tomatoes With the Sw-5 Tospovirus-Resistance Gene in California. Plant Dis. 2016, 101, 637. [Google Scholar] [CrossRef]
- Ferrand, L.; Almeida, M.M.S.; Orílio, A.F.; Bó, E.D.; Resende, R.O.; García, M.L. Biological and Molecular Characterization of Tomato Spotted Wilt Virus (TSWV) Resistance-Breaking Isolates from Argentina. Plant Pathol. 2019, 68, 1587–1601. [Google Scholar] [CrossRef]
- Debreczeni, D.E.; López, C.; Aramburu, J.; Darós, J.A.; Soler, S.; Galipienso, L.; Falk, B.W.; Rubio, L. Complete Sequence of Three Different Biotypes of Tomato Spotted Wilt Virus (Wild Type, Tomato Sw-5 Resistance-Breaking and Pepper Tsw Resistance-Breaking) from Spain. Arch. Virol. 2015, 160, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, Y.; Sun, L.; Wang, B.; Zhu, M.; Li, J.; Huang, C.; Liu, Y.; Li, F.; Liu, Y.; et al. Occurrence and Diversity of Tomato Spotted Wilt Virus Isolates Breaking the Tsw Resistance Gene of Capsicum Chinense in Yunnan, Southwest China. Plant Pathol. 2017, 66, 980–989. [Google Scholar] [CrossRef]
- Bese, G.; Krizbai, L.; Horváth, J.; Takács, A. Resistance Breaking Strain of Tomato Spotted Wilt Virus (TSWV) on Resistant Pepper Cultivars in Hungary. In International Symposium: Current Trends in Plant Protection-Proceedings; Institute for Plant Protection and Environment: Belgrade, Serbia, 2012. [Google Scholar]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological Control Using Invertebrates and Microorganisms: Plenty of New Opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Bosco, L.; Giacometto, E.; Tavella, L. Colonization and Predation of Thrips (Thysanoptera: Thripidae) by Orius Spp. (Heteroptera: Anthocoridae) in Sweet Pepper Greenhouses in Northwest Italy. Biol. Control 2008, 44, 331–340. [Google Scholar] [CrossRef]
- Broughton, S.; Harrison, J.; Rahman, T. Effect of New and Old Pesticides on Orius Armatus (Gross)—An Australian Predator of Western Flower Thrips, Frankliniella Occidentalis (Pergande). Pest. Manag. Sci. 2014, 70, 389–397. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Weinstein, E.G.; Rhoades, M.W.; Bartel, B.; Bartel, D.P. MicroRNAs in Plants. Genes Dev. 2002, 16, 1616–1626. [Google Scholar] [CrossRef]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Small RNAs in Plants: Recent Development and Application for Crop Improvement. Front. Plant Sci. 2015, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, Y. Dissection of RNAi-Based Antiviral Immunity in Plants. Curr. Opin. Virol. 2018, 32, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.L.; Blau, H.M. A Brief History of RNAi: The Silence of the Genes. FASEB J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef]
- Poethig, R.S.; Peragine, A.; Yoshikawa, M.; Hunter, C.; Willmann, M.; Wu, G. The Function of RNAi in Plant Development. Cold Spring Harb. Symp. Quant. Biol. 2006, 71, 165–170. [Google Scholar] [CrossRef][Green Version]
- Buchon, N.; Vaury, C. RNAi: A Defensive RNA-Silencing against Viruses and Transposable Elements. Heredity 2006, 96, 195. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, V.; Scholthof, H.B. Plant Responses against Invasive Nucleic Acids: RNA Silencing and Its Suppression by Plant Viral Pathogens. Semin. Cell Dev. Biol. 2009, 20, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.W.; van Rij, R.P. The Long and Short of Antiviral Defense: Small RNA-Based Immunity in Insects. Curr. Opin. Virol. 2014, 7, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-W.; Voinnet, O. Antiviral Immunity Directed by Small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef]
- Molnár, A.; Csorba, T.; Lakatos, L.; Várallyay, É.; Lacomme, C.; Burgyán, J. Plant Virus-Derived Small Interfering RNAs Originate Predominantly from Highly Structured Single-Stranded Viral RNAs. J. Virol. 2005, 79, 7812–7818. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, V.; Szittya, G.; Burgyán, J. Molecular Bases of Viral RNA Targeting by Viral Small Interfering RNA-Programmed RISC. J. Virol. 2007, 81, 3797–3806. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Koundal, V.; Williams, S.; Pappu, H. Differential Expression of Tomato Spotted Wilt Virus-Derived Viral Small RNAs in Infected Commercial and Experimental Host Plants. PLoS ONE 2013, 8, e76276. [Google Scholar] [CrossRef]
- Olaya, C.; Fletcher, S.J.; Zhai, Y.; Peters, J.; Margaria, P.; Winter, S.; Mitter, N.; Pappu, H.R. The Tomato Spotted Wilt Virus (TSWV) Genome Is Differentially Targeted in TSWV-Infected Tomato (Solanum Lycopersicum) with or without Sw-5 Gene. Viruses 2020, 12, 363. [Google Scholar] [CrossRef]
- Csorba, T.; Kontra, L.; Burgyán, J. Viral Silencing Suppressors: Tools Forged to Fine-Tune Host-Pathogen Coexistence. Virology 2015, 479–480, 85–103. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H.; Gabriel Peralta, S.M.; Harte-Maxwell, P.A. Tomato Spotted Wilt Virus NSs Protein Supports Infection and Systemic Movement of a Potyvirus and Is a Symptom Determinant. Viruses 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Sugiyama, K.; Nagano, H.; Mori, M.; Kaido, M.; Mise, K.; Tsuda, S.; Okuno, T. Identification of a Novel RNA Silencing Suppressor, NSs Protein of Tomato Spotted Wilt Virus. FEBS Lett. 2002, 532, 75–79. [Google Scholar] [CrossRef]
- Zhai, Y.; Bag, S.; Mitter, N.; Turina, M.; Pappu, H.R. Mutational Analysis of Two Highly Conserved Motifs in the Silencing Suppressor Encoded by Tomato Spotted Wilt Virus (Genus Tospovirus, Family Bunyaviridae). Arch. Virol. 2014, 159, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Olaya, C.; Adhikari, B.; Raikhy, G.; Cheng, J.; Pappu, H.R. Identification and Localization of Tospovirus Genus-Wide Conserved Residues in 3D Models of the Nucleocapsid and the Silencing Suppressor Proteins. Virol. J. 2019, 16, 7. [Google Scholar] [CrossRef]
- Schnettler, E.; Hemmes, H.; Huismann, R.; Goldbach, R.; Prins, M.; Kormelink, R. Diverging Affinity of Tospovirus RNA Silencing Suppressor Proteins, NSs, for Various RNA Duplex Molecules. J. Virol. 2010, 84, 11542–11554. [Google Scholar] [CrossRef]
- Ocampo, T.O.; Peralta, S.M.G.; Bacheller, N.; Uiterwaal, S.; Knapp, A.; Hennen, A.; Ochoa-Martinez, D.L.; Garcia-Ruiz, H. Antiviral RNA Silencing Suppression Activity of Tomato Spotted Wilt Virus NSs Protein. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Hedil, M.; Sterken, M.G.; de Ronde, D.; Lohuis, D.; Kormelink, R. Analysis of Tospovirus NSs Proteins in Suppression of Systemic Silencing. PLoS ONE 2015, 10, e0134517. [Google Scholar] [CrossRef]
- Wu, X.; Xu, S.; Zhao, P.; Zhang, X.; Yao, X.; Sun, Y.; Fang, R.; Ye, J. The Orthotospovirus Nonstructural Protein NSs Suppresses Plant MYC-Regulated Jasmonate Signaling Leading to Enhanced Vector Attraction and Performance. PLoS Pathog. 2019, 15, e1007897. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Ciuffo, M.; Pacifico, D.; Turina, M. Evidence That the Nonstructural Protein of Tomato Spotted Wilt Virus Is the Avirulence Determinant in the Interaction with Resistant Pepper Carrying the Tsw Gene. MPMI 2007, 20, 547–558. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, D.; Butterbach, P.; Lohuis, D.; Hedil, M.; van Lent, J.W.M.; Kormelink, R. Tsw Gene-Based Resistance Is Triggered by a Functional RNA Silencing Suppressor Protein of the Tomato Spotted Wilt Virus. Mol. Plant Pathol. 2013, 14, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Almási, A.; Nemes, K.; Csömör, Z.; Tóbiás, I.; Palkovics, L.; Salánki, K. A Single Point Mutation in Tomato Spotted Wilt Virus NSs Protein Is Sufficient to Overcome Tsw-Gene-Mediated Resistance in Pepper. J. Gen. Virol. 2017, 98, 1521–1525. [Google Scholar] [CrossRef]
- De Ronde, D.; Pasquier, A.; Ying, S.; Butterbach, P.; Lohuis, D.; Kormelink, R. Analysis of Tomato Spotted Wilt Virus NSs Protein Indicates the Importance of the N-Terminal Domain for Avirulence and RNA Silencing Suppression. Mol. Plant Pathol. 2014, 15, 185–195. [Google Scholar] [CrossRef]
- Margaria, P.; Bosco, L.; Vallino, M.; Ciuffo, M.; Mautino, G.C.; Tavella, L.; Turina, M. The NSs Protein of Tomato Spotted Wilt Virus Is Required for Persistent Infection and Transmission by Frankliniella Occidentalis. J. Virol. 2014, 88, 5788–5802. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.R.; Monsanto, C.; Sammons, B.; Kaniewski, W.; Haley, L.; Layton, J.; La Valle, B.J.; Delannay, X.; Turner, N.E. Field Resistance of Transgenic Tomatoes Expressing the Tobacco Mosaic Virus or Tomato Mosaic Virus Coat Protein Genes. Phytopathology 1992, 82, 683–690. [Google Scholar] [CrossRef]
- Swaney, S.; Powers, H.; Goodwin, J.; Rosales, L.S.; Dougherty, W.G. RNA-Mediated Resistance with Nonstructural Genes from the Tobacco Etch Virus Genome. Mol. Plant Microbe Interact. 1995, 8, 1004–1011. [Google Scholar] [CrossRef]
- Kumar, S.; Tanti, B.; Patil, B.L.; Mukherjee, S.K.; Sahoo, L. RNAi-Derived Transgenic Resistance to Mungbean Yellow Mosaic India Virus in Cowpea. PLoS ONE 2017, 12, e0186786. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, C.A.; Waterhouse, P.M. Constructs and Methods for Hairpin RNA-Mediated Gene Silencing in Plants. In Methods in Enzymology; RNA Interference; Academic Press: Cambridge, MA, USA, 2005; Volume 392, pp. 24–35. [Google Scholar]
- Ammara, U.e.; Mansoor, S.; Saeed, M.; Amin, I.; Briddon, R.W.; Al-Sadi, A.M. RNA Interference-Based Resistance in Transgenic Tomato Plants against Tomato Yellow Leaf Curl Virus-Oman (TYLCV-OM) and Its Associated Betasatellite. Virol. J. 2015, 12, 38. [Google Scholar] [CrossRef]
- Smith, N.A.; Singh, S.P.; Wang, M.-B.; Stoutjesdijk, P.A.; Green, A.G.; Waterhouse, P.M. Total Silencing by Intron-Spliced Hairpin RNAs. Nature 2000, 407, 319–320. [Google Scholar] [CrossRef]
- Gielen, J.J.L.; de Haan, P.; Kool, A.J.; Peters, D.; van Grinsven, M.Q.J.M.; Goldbach, R.W. Engineered Resistance to Tomato Spotted Wilt Virus, a Negative–Strand RNA Virus. Nat. Biotechnol. 1991, 9, 1363. [Google Scholar] [CrossRef]
- De Haan, P.; Gielen, J.J.L.; Prins, M.; Wijkamp, I.G.; van Schepen, A.; Peters, D.; van Grinsven, M.Q.J.M.; Goldbach, R. Characterization of RNA–Mediated Resistance to Tomato Spotted Wilt Virus in Transgenic Tobacco Plants. Nat. Biotechnol. 1992, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.C.; Johnston, S.A. The Concept of Parasite-Derived Resistance—Deriving Resistance Genes from the Parasite’s Own Genome. J. Theor. Biol. 1985, 113, 395–405. [Google Scholar] [CrossRef]
- Stam, M.; Mol, J.N.M.; Kooter, J.M. Review Article: The Silence of Genes in Transgenic Plants. Ann. Bot. 1997, 79, 3–12. [Google Scholar] [CrossRef]
- Ratcliff, F.; Harrison, B.D.; Baulcombe, D.C. A Similarity Between Viral Defense and Gene Silencing in Plants. Science 1997, 276, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, P.M.; Graham, M.W.; Wang, M.-B. Virus Resistance and Gene Silencing in Plants Can Be Induced by Simultaneous Expression of Sense and Antisense RNA. Proc. Natl. Acad. Sci. USA 1998, 95, 13959–13964. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Resende, R.d.O.; Anker, C.; van Schepen, A.; de Haan, P.; Goldbach, R. Engineered RNA-Mediated Resistance to Tomato Spotted Wilt Virus Is Sequence Specific. Mol. Plant Microbe Interact. 1996, 9, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Jan, F.J.; Fagoaga, C.; Pang, S.Z.; Gonsalves, D. A Minimum Length of N Gene Sequence in Transgenic Plants Is Required for RNA-Mediated Tospovirus Resistance. J. Gen. Virol. 2000, 81, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Tsumuki, H. Analysis of RNA-Mediated Virus Resistance by NSs and NSm Gene Sequences from Tomato Spotted Wilt Virus. Plant Sci. 2004, 166, 771–778. [Google Scholar] [CrossRef]
- Mitter, N.; Zhai, Y.; Bai, A.X.; Chua, K.; Eid, S.; Constantin, M.; Mitchell, R.; Pappu, H.R. Evaluation and Identification of Candidate Genes for Artificial MicroRNA-Mediated Resistance to Tomato Spotted Wilt Virus. Virus Res. 2016, 211, 151–158. [Google Scholar] [CrossRef]
- Bucher, E.; Lohuis, D.; van Poppel, P.M.J.A.; Geerts-Dimitriadou, C.; Goldbach, R.; Prins, M. Multiple Virus Resistance at a High Frequency Using a Single Transgene Construct. J. Gen. Virol. 2006, 87, 3697–3701. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Ku, H.-M.; Tsai, W.-S.; Green, S.K.; Jan, F.-J. Resistance to a DNA and a RNA Virus in Transgenic Plants by Using a Single Chimeric Transgene Construct. Transgenic Res. 2011, 20, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-C.; Chen, T.-C.; Raja, J.A.J.; Yang, C.-F.; Chien, W.-C.; Lin, C.-H.; Liu, F.-L.; Wu, H.-W.; Yeh, S.-D. Broad-Spectrum Transgenic Resistance against Distinct Tospovirus Species at the Genus Level. PLoS ONE 2014, 9, e96073. [Google Scholar] [CrossRef]
- Dzitoyeva, S.; Dimitrijevic, N.; Manev, H. Intra-Abdominal Injection of Double-Stranded RNA into Anesthetized Adult Drosophila Triggers RNA Interference in the Central Nervous System. Mol. Psychiatry 2001, 6, 665. [Google Scholar] [CrossRef][Green Version]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the Elements of Successful Insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef]
- Leggewie, M.; Schnettler, E. RNAi-Mediated Antiviral Immunity in Insects and Their Possible Application. Curr. Opin. Virol. 2018, 32, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Aliyari, R.; Wu, Q.; Li, H.-W.; Wang, X.-H.; Li, F.; Green, L.D.; Han, C.S.; Li, W.-X.; Ding, S.-W. Mechanism of Induction and Suppression of Antiviral Immunity Directed by Virus-Derived Small RNAs in Drosophila. Cell Host Microbe 2008, 4, 387–397. [Google Scholar] [CrossRef]
- Goic, B.; Stapleford, K.A.; Frangeul, L.; Doucet, A.J.; Gausson, V.; Blanc, H.; Schemmel-Jofre, N.; Cristofari, G.; Lambrechts, L.; Vignuzzi, M.; et al. Virus-Derived DNA Drives Mosquito Vector Tolerance to Arboviral Infection. Nat. Commun. 2016, 7, 12410. [Google Scholar] [CrossRef] [PubMed]
- Poirier, E.Z.; Goic, B.; Tomé-Poderti, L.; Frangeul, L.; Boussier, J.; Gausson, V.; Blanc, H.; Vallet, T.; Loyd, H.; Levi, L.I.; et al. Dicer-2-Dependent Generation of Viral DNA from Defective Genomes of RNA Viruses Modulates Antiviral Immunity in Insects. Cell Host Microbe 2018, 23, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, H.; Smagghe, G. Mechanisms of DsRNA Uptake in Insects and Potential of RNAi for Pest Control: A Review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, Y.; Miller, S.C.; Tomita, S.; Schoppmeier, M.; Grossmann, D.; Bucher, G. Exploring Systemic RNA Interference in Insects: A Genome-Wide Survey for RNAi Genes in Tribolium. Genome Biol. 2008, 9, R10. [Google Scholar] [CrossRef]
- Lycett, G.J.; McLaughlin, L.A.; Ranson, H.; Hemingway, J.; Kafatos, F.C.; Loukeris, T.G.; Paine, M.J.I. Anopheles Gambiae P450 Reductase Is Highly Expressed in Oenocytes and in Vivo Knockdown Increases Permethrin Susceptibility. Insect Mol. Biol. 2006, 15, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.-L.; Barthel, A.; et al. RNA Interference in Lepidoptera: An Overview of Successful and Unsuccessful Studies and Implications for Experimental Design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.N.; Santos, A.; Pinto, F.S.; Gontijo, N.F.; Lehane, M.J.; Pereira, M.H. RNA Interference of the Salivary Gland Nitrophorin 2 in the Triatomine Bug Rhodnius Prolixus (Hemiptera: Reduviidae) by DsRNA Ingestion or Injection. Insect Biochem. Mol. Biol. 2006, 36, 683–693. [Google Scholar] [CrossRef]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested Double-Stranded RNAs Can Act as Species-Specific Insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of Coleopteran Insect Pests through RNA Interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Mao, Y.-B.; Tao, X.-Y.; Xue, X.-Y.; Wang, L.-J.; Chen, X.-Y. Cotton Plants Expressing CYP6AE14 Double-Stranded RNA Show Enhanced Resistance to Bollworms. Transgenic Res. 2011, 20, 665–673. [Google Scholar] [CrossRef]
- Badillo-Vargas, I.E.; Rotenberg, D.; Schneweis, B.A.; Whitfield, A.E. RNA Interference Tools for the Western Flower Thrips, Frankliniella Occidentalis. J. Insect Physiol. 2015, 76, 36–46. [Google Scholar] [CrossRef]
- Nelson, N.; Perzov, N.; Cohen, A.; Hagai, K.; Padler, V.; Nelson, H. The Cellular Biology of Proton-Motive Force Generation by V-ATPases. J. Exp. Biol. 2000, 203, 89–95. [Google Scholar] [PubMed]
- Whitten, M.M.A.; Facey, P.D.; Sol, R.D.; Fernández-Martínez, L.T.; Evans, M.C.; Mitchell, J.J.; Bodger, O.G.; Dyson, P.J. Symbiont-Mediated RNA Interference in Insects. Proc. R. Soc. B 2016, 283, 20160042. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, J.H.; Kim, K.; Lee, S.H. Selection of Lethal Genes for Ingestion RNA Interference against Western Flower Thrips, Frankliniella Occidentalis, via Leaf Disc-Mediated DsRNA Delivery. Pestic. Biochem. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Andongma, A.A.; Greig, C.; Dyson, P.J.; Flynn, N.; Whitten, M.M.A. Optimization of Dietary RNA Interference Delivery to Western Flower Thrips Frankliniella Occidentalis and Onion Thrips Thrips Tabaci. Arch. Insect Biochem. Physiol. 2020, 103, e21645. [Google Scholar] [CrossRef]
- Qiu, W.; Moyer, J.W. Tomato Spotted Wilt Tospovirus Adapts to the TSWV N Gene-Derived Resistance by Genome Reassortment. Phytopathology 1999, 89, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Hassani-Mehraban, A.; Brenkman, A.B.; van den Broek, N.J.F.; Goldbach, R.; Kormelink, R. RNAi-Mediated Transgenic Tospovirus Resistance Broken by IntraspeciesSilencing Suppressor Protein Complementation. MPMI 2009, 22, 1250–1257. [Google Scholar] [CrossRef][Green Version]
- Ishii, T.; Araki, M. Consumer Acceptance of Food Crops Developed by Genome Editing. Plant Cell Rep. 2016, 35, 1507–1518. [Google Scholar] [CrossRef]
- Ludlow, K. Regulation of Genome Editing in Plant Biotechnology: Australia. In Regulation of Genome Editing in Plant Biotechnology: A Comparative Analysis of Regulatory Frameworks of Selected Countries and the EU; Dederer, H.-G., Hamburger, D., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–110. ISBN 978-3-030-17119-3. [Google Scholar]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; dos Santos, E.Á.; Smagghe, G.; Zotti, M.J. Management of Pest Insects and Plant Diseases by Non-Transformative RNAi. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. DsRNA Uptake in Plant Pests and Pathogens: Insights into RNAi-Based Insect and Fungal Control Technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Aalto, A.P.; Sarin, L.P.; van Dijk, A.A.; Saarma, M.; Poranen, M.M.; Arumäe, U.; Bamford, D.H. Large-Scale Production of DsRNA and SiRNA Pools for RNA Interference Utilizing Bacteriophage Φ6 RNA-Dependent RNA Polymerase. RNA 2007, 13, 422–429. [Google Scholar] [CrossRef]
- Tenllado, F.; Martínez-García, B.; Vargas, M.; Díaz-Ruíz, J.R. Crude Extracts of Bacterially Expressed DsRNA Can Be Used to Protect Plants against Virus Infections. BMC Biotechnol. 2003, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Tenllado, F.; Díaz-Ruíz, J.R. Double-Stranded RNA-Mediated Interference with Plant Virus Infection. J. Virol. 2001, 75, 12288–12297. [Google Scholar] [CrossRef]
- Lau, S.E.; Mazumdar, P.; Hee, T.W.; Song, A.L.A.; Othman, R.Y.; Harikrishna, J.A. Crude Extracts of Bacterially-Expressed DsRNA Protect Orchid Plants against Cymbidium Mosaic Virus during Transplantation from in Vitro Culture. J. Hortic. Sci. Biotechnol. 2014, 89, 569–576. [Google Scholar] [CrossRef]
- Gan, D.; Zhang, J.; Jiang, H.; Jiang, T.; Zhu, S.; Cheng, B. Bacterially Expressed DsRNA Protects Maize against SCMV Infection. Plant Cell Rep. 2010, 29, 1261–1268. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, C.; Cheng, F.; He, M.; Zhang, Z.; Liu, Y. Crude Extracts of Bacterially Expressed DsRNA Can Be Used to Protect Tobaccos against CMV Infections. Acta Phytopathol. Sin. 2008, 3. [Google Scholar] [CrossRef]
- Tabein, S.; Jansen, M.; Noris, E.; Vaira, A.M.; Marian, D.; Behjatnia, S.A.A.; Accotto, G.P.; Miozzi, L. The Induction of an Effective DsRNA-Mediated Resistance Against Tomato Spotted Wilt Virus by Exogenous Application of Double-Stranded RNA Largely Depends on the Selection of the Viral RNA Target Region. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay Nanosheets for Topical Delivery of RNAi for Sustained Protection against Plant Viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous Application of RNAi-Inducing Double-Stranded RNA Inhibits Aphid-Mediated Transmission of a Plant Virus. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Lardeux, F.; Torrico, G.; Aliaga, C. Calculation of the ELISA’s Cut-off Based on the Change-Point Analysis Method for Detection of Trypanosoma Cruzi Infection in Bolivian Dogs in the Absence of Controls. Memórias Inst. Oswaldo Cruz 2016, 111, 501–504. [Google Scholar] [CrossRef]
- San Miguel, K.; Scott, J.G. The next Generation of Insecticides: DsRNA Is Stable as a Foliar-Applied Insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef]
- Palli, S.R. RNA Interference in Colorado Potato Beetle: Steps toward Development of DsRNA as a Commercial Insecticide. Curr. Opin. Insect Sci. 2014, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA Interference for Managing the Populations of the Colorado Potato Beetle, Leptinotarsa Decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilon, A.; Robinson, K.; Pappu, H.R.; Mitter, N. Current Status and Potential of RNA Interference for the Management of Tomato Spotted Wilt Virus and Thrips Vectors. Pathogens 2021, 10, 320. https://doi.org/10.3390/pathogens10030320
Nilon A, Robinson K, Pappu HR, Mitter N. Current Status and Potential of RNA Interference for the Management of Tomato Spotted Wilt Virus and Thrips Vectors. Pathogens. 2021; 10(3):320. https://doi.org/10.3390/pathogens10030320
Chicago/Turabian StyleNilon, Alexander, Karl Robinson, Hanu R. Pappu, and Neena Mitter. 2021. "Current Status and Potential of RNA Interference for the Management of Tomato Spotted Wilt Virus and Thrips Vectors" Pathogens 10, no. 3: 320. https://doi.org/10.3390/pathogens10030320
APA StyleNilon, A., Robinson, K., Pappu, H. R., & Mitter, N. (2021). Current Status and Potential of RNA Interference for the Management of Tomato Spotted Wilt Virus and Thrips Vectors. Pathogens, 10(3), 320. https://doi.org/10.3390/pathogens10030320






