Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment
Abstract
1. Introduction
2. Pathogenesis
3. Risk Factors
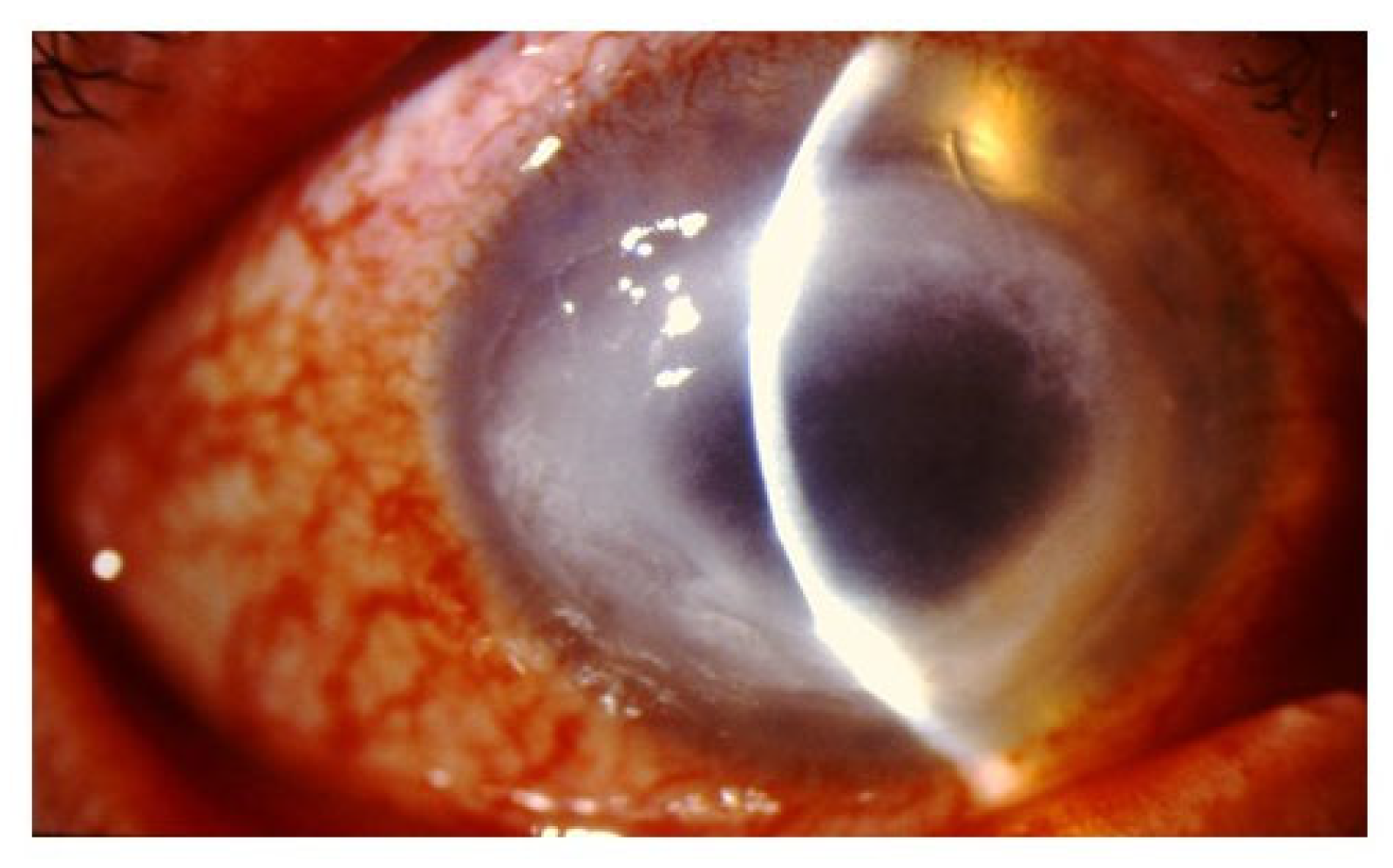
4. Signs/Symptoms
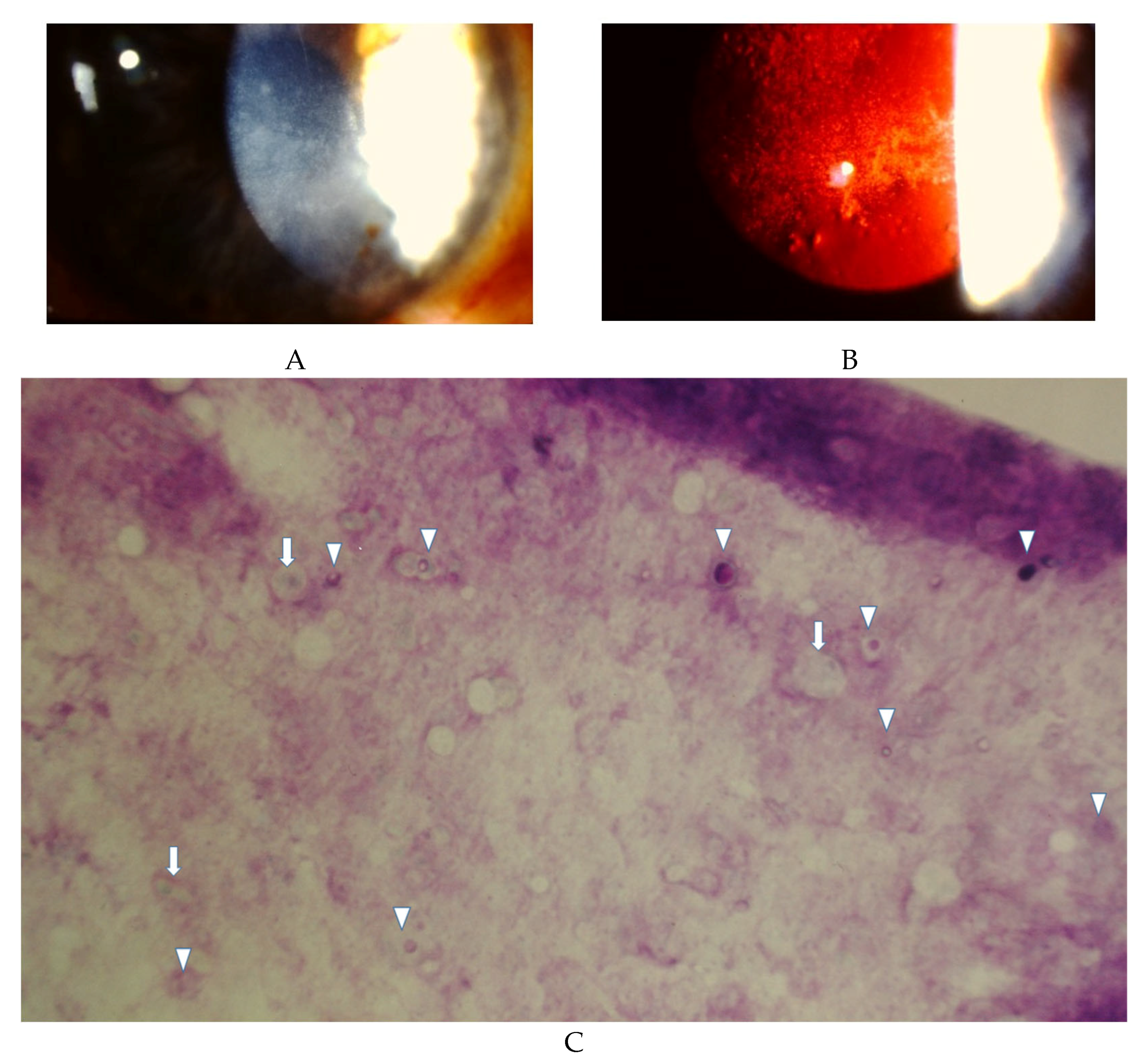
5. Diagnosis
5.1. Corneal Scraping
5.2. Polymerase Chain Reaction
5.3. In Vivo Confocal Microscopy
5.4. Impression Cytology
6. Treatment
6.1. Biguanides
6.2. Aromatic Diamidines
6.3. Antibiotics
6.4. Steroids
6.5. Surgery
6.6. New Treatment Approaches
7. Prevention
8. Summary/Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef]
- Ocampo, A.J.; Moreira, R.L.; Sandi, A. In vitro effecto of environmental isolates of Acanthamoeba T4 and T5 over human erythrocytes and platelets. Exp. Parasitol. 2020, 210, 7842. [Google Scholar]
- Oddo, D. Infecciones por amebas de vida libre. Comentarios históricos, taxonomía y nomenclatura, protozoología y cuadros anátomo-clínicos. Rev. Chil. Infectol. 2006, 23, 200–214. [Google Scholar] [CrossRef]
- Sharma, G.; Kalra, S.K.; Tejan, N.; Ghoshal, U. Nanoparticles based therapeutic efficacy against Acanthamoeba: Updates and future prospect. Exp. Parasitol. 2020, 218, 8008. [Google Scholar] [CrossRef] [PubMed]
- Nwachuku, N.; Gerba, C.P. Health Effects of Acanthamoeba spp. and Its Potential for Waterborne Transmission. Rev. Environ. Contam. Toxicol. 2004, 180, 93–131. [Google Scholar] [CrossRef]
- Pacella, N.; Torre, L.G.; De Giusti, M.; Brillante, C.; Lonbardi, A.M.; Smaldone, G.; Lenzi, T.; Pacella, F. Results of case-control studies support the association between contact lens use and Acanthamoeba keratitis. Clin. Ophthalmol. 2013, 7, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.S.; Verani, J.; Heidman, N.; Bauer, H.J.; Alfonso, E.C.; Miller, D.; Jones, D.B.; Bruckner, D.; Langston, R.; Jeng, B.H.; et al. Acanthamoeba keratitis: The Persistence of Cases Following a Multistate Outbreak. Ophthalmic Epidemiol. 2012, 19, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Carnt, N.; Hoffman, J.J.; Verma, S.; Hau, S.; Radford, C.F.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis: Confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br. J. Ophthalmol. 2018, 102, 1621–1628. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Ivarsen, A.; Hjortdal, J. Increasing incidence of Acanthmoeba keratitis in a large tertiary ophthalmology department for year 1994. Acta Ophthalmol. 2020, 98, 445–448. [Google Scholar] [CrossRef]
- Randag, A.C.; Van Rooij, J.; Van Goor, A.T.; Verkerk, S.; Wisse, R.P.L.; Saelens, I.E.Y.; Stoutenbeek, R.; Van Dooren, B.T.H.; Cheng, Y.Y.Y.; Eggink, C.A. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE 2019, 14, 2092. [Google Scholar] [CrossRef]
- Graffi, S.; Peretz, A.; Jabaly, H.; Joiefman, A.; Naftali, M. Acanthamoeba keratitis: Study of the 5-year incidence in Israel. Br. J. Ophthalmol. 2013, 97, 1382–1383. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Snow, K.K.; Dana, M.R. The epidemic of Acanthamoeba keratitis: Where do we stand? Cornea 1998, 17, 3–10. [Google Scholar] [CrossRef]
- Iovieno, A.; Gore, D.M.; Carnt, N.; Dart, J.K. Acanthamoeba sclerokeratitis: Epidemiology, clinical features, and treatment outcomes. Opthalmology 2014, 121, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Kulsoom, H.; Baig, A.M.; Siddiqui, R.; Khan, N.A. Combined drug therapy in the management of granulomatous amoebic encephalitis due to Acanthamoeba spp.; and Balamuthia mandrillaris. Exp. Parasitol. 2014, 145, S115–S120. [Google Scholar] [CrossRef]
- Kot, K.; Lanocha-Arendarczyk, N.A.; Kosik-Bogacka, D.I. Amoebas from the genera Acanthamoeba and their pathogenic properties. Ann. Parasitol. 2018, 64, 299–308. [Google Scholar] [PubMed]
- Velázquez, C.D.; Olivares, S.A.; Muñozledo, C.F.; Jiménez, L.L.F.; Anaya, R.L.Z.; Shibayama, M.; Luna, S.J. Acanthamoeba mauritaniensis genotype T4D: An environmental isolate displays pathogenic behavior. Parasitol. Int. 2020, 74, 2002. [Google Scholar] [CrossRef]
- Montiel, A.F.; Fernández, L.B.; Navarro, M.C.M.; Valladares, B.; Velez, L.R.; Laiz, M.R.; Morales, L.J. Influence of Acanthamoeba Genotype on Clinical Course and Outcomes for Patients with Acanthamoeba Keratitis in Spain. J. Clin. Microbiol. 2014, 52, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Stothard, D.R.; Diedrich, S.J.M.; Awwad, M.H.; Gast, R.J.; Ledee, D.R.; Zaragoza, R.S.; Dean, C.L.; Fuerst, P.A.; Byers, T.J. The Evolutionary History of the Genus Acanthamoeba and the Identification of Eight New 18S rRNA Gene Sequence Types. J. Eukaryot. Microbiol. 1998, 45, 45–54. [Google Scholar] [CrossRef]
- De Lacerda, A.G.; Lira, M. Acanthamoeba keratitis: A review of biology, pathophysiology and epidemiology. Ophthalmic Physiol. Opt. 2021, 41, 116–135. [Google Scholar] [CrossRef]
- Brown, A.C.; Ross, J.; Jones, D.B.; Collier, S.A.; Ayers, T.L.; Hoekstra, R.M.; Backensen, B.; Roy, S.L.; Beach, M.J.; Yoder, J.S.; et al. Risk Factors for Acanthamoeba Keratitis-A Multistate Case-Control Study, 2008. Eye Contact Lens. 2018, 44, S173–S178. [Google Scholar] [CrossRef]
- Niederkorn, J.Y.; Alizadeh, H.; Leher, H.; McCulley, J.P. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1999, 1, 437–443. [Google Scholar] [CrossRef]
- Panjwani, N. Pathogenesis of Acanthamoeba Keratitis. Ocul. Surf. 2010, 8, 70–79. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasit Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Radford, C.F.; Bacon, A.S.; Dart, J.K.G.; Minassian, D.C. Risk factors for acanthamoeba keratitis in contact lens users: A case-control study. BMJ 1995, 310, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.W.; Boase, D.L.; Cree, I.A. Factors Affecting the Epidemiology ofAcanthamoebaKeratitis. Ophthalmic Epidemiol. 2007, 14, 53–60. [Google Scholar] [CrossRef]
- Morales, L.J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.P.; Sriram, R.; Qvarnstrom, Y.; Roy, S.; Verani, J.; Yoder, J.; Lorick, S.; Roberts, J.; Beach, M.J.; Visversvara, G. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J. Clin. Microbiol. 2009, 47, 2040–2045. [Google Scholar] [CrossRef]
- Illingworth, C.D.; Cook, S.D. Acanthamoeba Keratitis. Surv. Ophthalmol. 1998, 42, 493–508. [Google Scholar] [CrossRef]
- Morales, L.J.; Navarro, M.C.M.; Arencibia, L.A.; Montiel, A.F.; Piñero, J.E.; Valladares, B. Acanthamoeba keratitis: An emerging disease gathering importance worldwide? Trends Parasitol. 2013, 29, 181–187. [Google Scholar] [CrossRef]
- Szentmáry, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba keratitis–Clinical signs, differential diagnosis and treatment. J. Curr. Ophthalmol. 2019, 31, 16–23. [Google Scholar] [CrossRef]
- Somani, N.S.; Ronquillo, Y.; Moshirfar, M. Acanthamoeba Keratitis; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Perry, H.D.; Donnenfeld, E.D.; Foulks, G.N.; Moadel, K.; Kanellopoulos, A.J. Decreased Corneal Sensation as an Initial Feature of Acanthamoeba Keratitis. Ophthalmology 1995, 102, 1565–1568. [Google Scholar] [CrossRef]
- Bacon, A.S.; Dart, J.K.; Ficker, L.A.; Matheson, M.M.; Wright, P. Acanthamoeba keratitis. The value of early diagnosis. Ophthalmology 1993, 100, 1238–1243. [Google Scholar] [CrossRef]
- Singh, A.; Sahu, S.K.; Sharma, S.; Das, S. Acanthamoeba Keratitis Versus Mixed Acanthamoeba and Bacterial Keratitis: Comparison of Clinical and Mi-crobiological Profiles. Cornea 2020, 39, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, A.; Vaidwal, S.; Venkatapathy, N.; Rammohan, R. The Acanthamoeba-Fungal Keratitis Study. Am. J. Ophthalmol. 2019, 201, 31–36. [Google Scholar] [CrossRef]
- Yera, H.; Ok, V.; Kuet, L.K.F.; Dahane, N.; Ariey, F.; Hasseine, L.; Delaunay, P.; Martiano, D.; Marty, P.; Bourges, J.L. PCR and culture for diagnosis of Acanthamoeba keratitis. Br. J. Ophthalmol. 2020, 2020, 6730. [Google Scholar] [CrossRef]
- Goh, J.W.Y.; Harrison, R.; Hau, S.; Alexander, C.L.; Tole, D.M.; Avadhanam, V.S. Comparison of In Vivo Confocal Microscopy, PCR and Culture of Corneal Scrapes in the Diagnosis of Acanthamoeba Keratitis. Cornea 2018, 37, 480–485. [Google Scholar] [CrossRef]
- Wilhelmus, K.R.; Osato, M.S.; Font, R.L.; Robinson, N.M.; Jones, D.B. Rapid Diagnosis of Acanthamoeba Keratitis Using Calcofluor White. Arch. Ophthalmol. 1986, 104, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Marines, M.H.; Osatp, M.S.; Font, R.L. The value of calcofluor white in the diagnosis of myotic and Acanthamoebic infections of the eye and ocular adnexa. Opthalmology 1987, 94, 23–26. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Waring, G.O.; Akor, C.; Sanchez, C.A.A.; Bennett, K. Evaluation of hematoxylin and eosin and special stains for the detection of acanthamoeba keratitis in penetrating keratoplasties. Am. J. Ophthalmol. 2003, 136, 520–526. [Google Scholar] [CrossRef]
- Wang, Y.E.; Tepelus, T.C.; Vickers, L.A.; Baghdasaryan, E.; Gui, W.; Huang, P.; Irvine, J.A.; Sadda, S.; Hsu, H.Y.; Lee, O.L. Role of in vivo confocal microscopy in the diagnosis of infectious keratitis. Int. Ophthalmol. 2019, 39, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Alkatan, H.M.; Essa, A.R.S. Challenges in the diagnosis of microbial keratitis: A detailed review with update and general guidelines. Saudi J. Ophthalmol. 2019, 33, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Alsoudi, A.F.; Golen, J.R.; Seitzman, G.D.; Lietman, T.M.; Keenan, J.D. Comparison of two confocal microscopes for diagnosis of acanthamoeba keratitis. Eye 2020, 1–3. [Google Scholar] [CrossRef]
- Sawada, Y.; Yuan, C.; Huang, A.J.W. Impression cytology in the diagnosis of acanthamoeba keratitis with surface involvement. Am. J. Ophthalmol. 2004, 137, 323–328. [Google Scholar] [CrossRef]
- Patel, S.P.; Schaefer, J.L.; Jaber, R.; Paterson, J.; Liu, W.; Fernandez, G.F. The volue of cytology smears for Acanthamoeba keratitis. Case Rep. Ophthalmol. Med. 2016, 2016, 8968. [Google Scholar]
- Wang, Z.Q.; Li, R.; Zhang, C.; Luo, S.Y.; Sun, X.G.; Jin, X.Y. Morphological characteristics in corneal smear of acanthamoeba keratitis. J. Ophthalmol. 2010, 46, 432–436. [Google Scholar]
- Niyyati, M.; Dodangeh, S.; Morales, L.J. A Review of the Current Research Trends in the Application of Medicinal Plants as a Source for Novel Therapeutic Agents Against Acanthamoeba Infections. Iran. J. Pharm. Res. IJPR 2016, 15, 893–900. [Google Scholar] [PubMed]
- Hadaś, E.; Derda, M.; Cholewiński, M. Evaluation of the effectiveness of tea tree oil in treatment of Acanthamoeba infection. Parasitol. Res. 2017, 116, 997–1001. [Google Scholar] [CrossRef][Green Version]
- Elkadery, A.A.S.; Elsherif, E.A.; Eldin, H.M.E.; Fahmy, I.A.F.; Mohammad, O.S. Efficient therapeutic effect of Nigella sativa aqueous extract and chitosan nanoparticles against ex-perimentally induced Acanthamoeba keratitis. Parasitol. Res. 2019, 118, 2243–2454. [Google Scholar] [CrossRef] [PubMed]
- Larkin, D.; Kilvington, S.; Dart, J. Treatment of Acanthamoeba Keratitis with Polyhexamethylene Biguanide. Ophthalmology 1992, 99, 185–191. [Google Scholar] [CrossRef]
- Seal, D.; Hay, J. Acanthamoeba keratitis: Early diagnosis, rational drug intervention and prevention. Hong Kong J. Ophthalmol. 1997, 1, 46–52. [Google Scholar]
- Cohen, E.J.; Parlato, C.J.; Arentsen, J.J.; Genvert, G.I.; Eagle, R.C.; Wieland, M.R.; Laibson, P.R. Medical and Surgical Treatment of Acanthamoeba Keratitis. Am. J. Ophthalmol. 1987, 103, 615–625. [Google Scholar] [CrossRef]
- Hay, J.; Kirkness, C.M.; Seal, D.V.; Wright, P. Drug resistance and Acanthamoeba Keratitis: The quest for alternative antiprotozoal chemotherapy. Eye 1994, 8, 555–563. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsumoto, Y.; Kabata, T.; Watanabe, R.; Hommura, S.; Yasuraoka, K.; Ishii, K. Oral Itraconazole and Topical Miconazole With Debridement for Acanthamoeba Keratitis. Am. J. Ophthalmol. 1990, 109, 121–126. [Google Scholar] [CrossRef]
- Varga, J.H.; Wolf, T.C.; Jensen, H.G.; Parmley, V.C.; Rowsey, J.J. Combined treatment of Acanthamoeba keratitis with pro-pamidine, neomycin, and polyhexamethylene biguanide. Am. J. Ophthalmol. 1993, 115, 466–470. [Google Scholar] [CrossRef]
- Berger, S.T.; Katsev, D.A.; Mondino, B.J.; Petit, T.H. Successful medical management of Acanthamoeba keratitis. Am. J. Ophthalmol. 1990, 110, 395–403. [Google Scholar] [CrossRef]
- Carnt, N.; Robaei, D.; Watson, S.L.; Minassian, D.C.; Dart, J.K. The Impact of Topical Corticosteroids Used in Conjunction with Antiamoebic Therapy on the Outcome of Acanthamoeba Keratitis. Ophthalmology 2016, 123, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Seal, D.V. Acanthamoeba keratitis update—Incidence, molecular epidemiology and new drugs for treatment. Eye 2003, 17, 893–905. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanselow, N.; Sirajuddin, N.; Yin, X.-T.; Huang, A.J.W.; Stuart, P.M. Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment. Pathogens 2021, 10, 323. https://doi.org/10.3390/pathogens10030323
Fanselow N, Sirajuddin N, Yin X-T, Huang AJW, Stuart PM. Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment. Pathogens. 2021; 10(3):323. https://doi.org/10.3390/pathogens10030323
Chicago/Turabian StyleFanselow, Nicholas, Nadia Sirajuddin, Xiao-Tang Yin, Andrew J. W. Huang, and Patrick M. Stuart. 2021. "Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment" Pathogens 10, no. 3: 323. https://doi.org/10.3390/pathogens10030323
APA StyleFanselow, N., Sirajuddin, N., Yin, X.-T., Huang, A. J. W., & Stuart, P. M. (2021). Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment. Pathogens, 10(3), 323. https://doi.org/10.3390/pathogens10030323






