In Vitro Evaluation of the Combination of Melaleuca alternifolia (Tea Tree) Oil and Dimethyl Sulfoxide (DMSO) against Trophozoites and Cysts of Acanthamoeba Strains. Oxygen Consumption Rate (OCR) Assay as a Method for Drug Screening
Abstract
:1. Introduction
2. Results
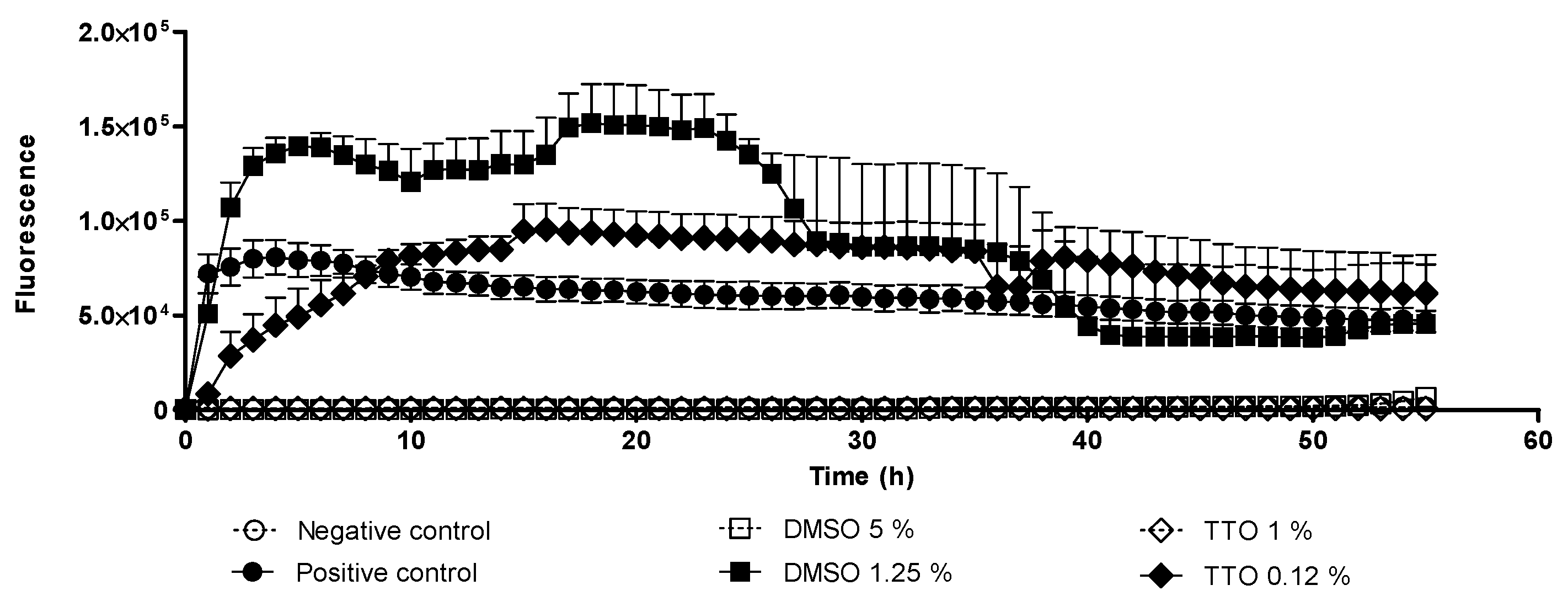
2.1. Trophocidal Properties of Tea Tree Oil (TTO) and Dimethyl Sulfoxide (DMSO)
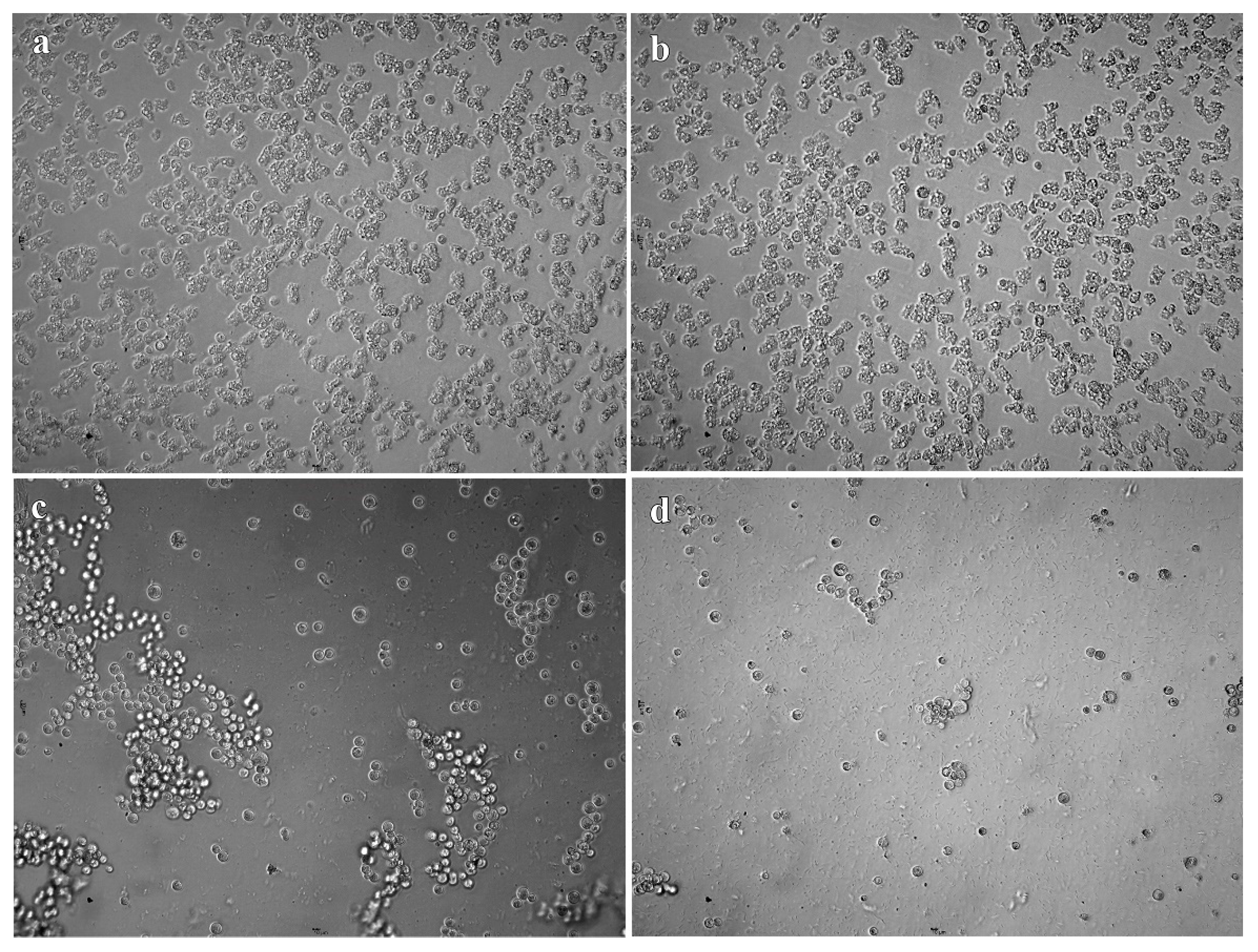
2.2. In Vitro Cysticidal Assays
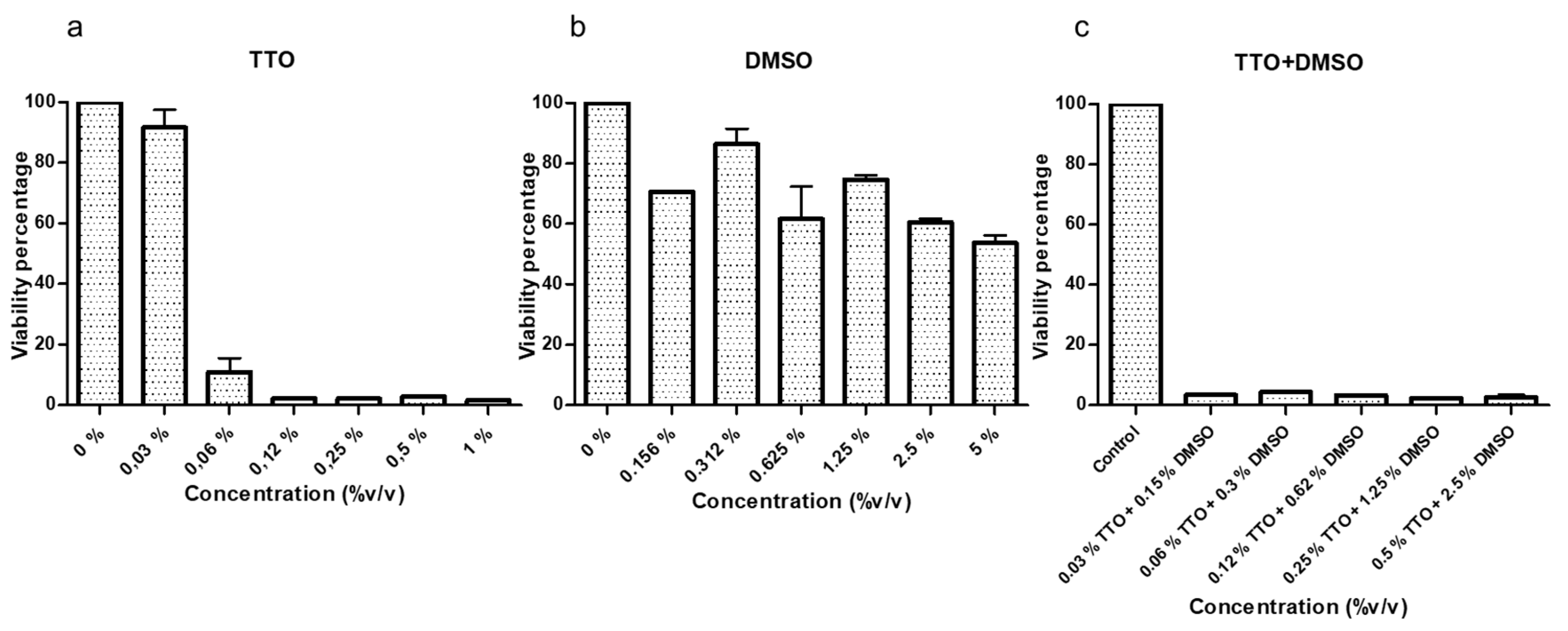
2.3. Cytotoxicity Test on Human Corneal Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Acanthamoeba Strains
4.2. In Vitro Amebicide Assays
4.3. In Vitro Cysticidal Assays
4.4. Cytotoxicity Test on Human Corneal Epithelial Cells
4.5. Calculation of In Vitro Fractional Inhibitory Concentration Index (FICI)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, A.J.; Visvesvara, G.S. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997, 7, 583–598. [Google Scholar] [CrossRef] [PubMed]
- de Lacerda, A.G.; Lira, M. Acanthamoeba keratitis: A review of biology, pathophysiology and epidemiology. Ophthalmic Physiol. Opt. 2020, 41, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Angelici, M.C.; Walochnik, J.; Calderaro, A.; Saxinger, L.; Dacks, J.B. Free-living amoebae and other neglected protistan pathogens: Health emergency signals? Eur. J. Protistol. 2020, 77, 125760. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, Y.; Siddiqui, R.; Iftikhar, H.; Khan, N.A. The effect of different environmental conditions on the encystation of Acanthamoeba castellanii belonging to the T4 genotype. Exp. Parasitol. 2013, 135, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D. Encystment in Acanthamoeba castellanii: A review. Exp. Parasitol. 2014, 145, S20–S27. [Google Scholar] [CrossRef]
- Khan, N.A. Acanthamoeba: Biology and Pathogenesis; Caister Academic Press: Norfolk, UK, 2009; pp. 1–209. [Google Scholar]
- Siddiqui, R.; Aqeel, Y.; Khan, N.A. The use of dimethyl sulfoxide in contact lens disinfectants is a potential preventative strategy against contracting Acanthamoeba keratitis. Contact Lens Anterior Eye 2016, 39, 389–393. [Google Scholar] [CrossRef]
- Siddiqui, R.; Lakhundi, S.; Iqbal, J.; Khan, N.A. Effect of non-steroidal anti-inflammatory drugs on biological properties of Acanthamoeba castellanii belonging to the T4 genotype. Exp. Parasitol. 2016, 168, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, C.E.; Acharya, N.R.; Tu, E.Y.; Zegans, M.E.; Mannis, M.J.; Gaynor, B.D.; Keenan, J.D. Practice patterns and opinions in the treatment of acanthamoeba keratitis. Cornea 2011, 30, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of microbial keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Moon, E.K.; Park, H.R.; Quan, F.S.; Kong, H.H. Efficacy of Korean Multipurpose Contact Lens Disinfecting Solutions against Acanthamoeba castellanii. Korean J. Parasitol. 2016, 54, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Tzanetou, K.; Miltsakakis, D.; Droutsas, D.; Alimisi, S.; Petropoulou, D.; Ganteris, G.; Dolapsaki, E.; Markomichelakis, N.; Mallias, I.; Malamou-Lada, E. Acanthamoeba keratitis and contact lens disinfecting solutions. Ophthalmologica 2006, 220, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.W.; Boase, D.L.; Cree, I.A. How Could Contact Lens Wearers Be at Risk of Acanthamoeba Infection? A Review. J. Optom. 2009, 2, 60–66. [Google Scholar] [CrossRef] [Green Version]
- de Aguiar, A.P.C.; Oliveira-Silveira, C.; Todero-Winck, M.A.; Brittes-Rott, M. Susceptibility of Acanthamoeba to multipurpose lens-cleaning solutions. Acta Parasitol. 2013, 58, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Üstüntürk, M.; Zeybek, Z. Amoebicidal efficacy of a novel multi-purpose disinfecting solution: First findings. Exp. Parasitol. 2014, 145, S93–S97. [Google Scholar] [CrossRef]
- Siddiqui, R.; Lakhundi, S.; Khan, N.A. Status of the effectiveness of contact lens solutions against keratitis-causing pathogens. Contact Lens Anterior Eye 2015, 38, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Shoff, M.E.; Joslin, C.E.; Tu, E.Y.; Kubatko, L.; Fuerst, P.A. Efficacy of contact lens systems against recent clinical and tap water Acanthamoeba isolates. Cornea 2008, 27, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Kilvington, S.; Lonnen, J.A. Comparison of regimen methods for the removal and inactivation of bacteria, fungi and Acanthamoeba from two types of silicone hydrogel lenses. Contact Lens Anterior Eye 2009, 32, 73–77. [Google Scholar] [CrossRef]
- Wright, P.; Warhurst, D.; Jones, B.R. Acanthamoeba keratitis successfully treated medically. Br. J. Ophthalmol. 1985, 69, 778–782. [Google Scholar] [CrossRef]
- Arnalich-Montiel, F.; Martín-Navarro, C.M.; Alió, J.L.; López-Vélez, R.; Martínez-Carretero, E.; Valladares, B.; Lorenzo-Morales, J. Successful monitoring and treatment of intraocular dissemination of Acanthamoeba. AMA Arch. Ophthalmol. 2012, 130, 1474–1475. [Google Scholar] [CrossRef] [Green Version]
- Heredero-Bermejo, I.; Sanchez-Nieves, J.; Soliveri, J.; Gomez, R.; de la Mata, F.J.; Copa-Patino, J.L.; Perez-Serrano, J. In Vitro anti-Acanthamoeba synergistic effect of chlorhexidine and cationic carbosilane dendrimers against both trophozoite and cyst forms. Int. J. Pharm. 2016, 509, 1–7. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Hernández-Ros, J.M.; Sánchez-García, L.; Maly, M.; Verdú-Expósito, C.; Soliveri, J.; Copa-Patiño, J.L.; Perez-Serrano, J.; de la Mata, F.J.; Gómez, R. Ammonium and guanidine carbosilane dendrimers and dendrons as microbicides. Eur. Polym. J. 2018, 101, 159–168. [Google Scholar] [CrossRef]
- Hadaś, E.; Derda, M.; Cholewiński, M. Evaluation of the effectiveness of tea tree oil in treatment of Acanthamoeba infection. Parasitol. Res. 2017, 116, 997–1001. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.E.; Yeng, C.Y.; Mahboob, T.; Ling, L.C.; Raju, C.S.; Barusrux, S.; Nissapatron, V. Natural products: Alternative therapeutic compounds against Acanthamoeba spp. Asian J. Pharmacogn. 2019, 3, 29–38. [Google Scholar]
- Arnalich-Montiel, F.; Lumbreras-Fernández, B.; Martín-Navarro, C.M.; Valladares, B.; Lopez-Velez, R.; Morcillo-Laiz, R.; Lorenzo-Morales, J. Influence of Acanthamoeba genotype on clinical course and outcomes for patients with Acanthamoeba keratitis in Spain. J. Clin. Microbiol. 2014, 52, 1213–1216. [Google Scholar] [CrossRef] [Green Version]
- Heredero-Bermejo, I.; Criado-Fornelio, A.; Soliveri, J.; Díaz-Martín, J.A.; Matilla-Fuentes, J.; Sánchez-Arias, J.A.; Pérez-Serrano, J. Development of a new oxygen consumption rate assay in cultures of Acanthamoeba (Protozoa: Lobosea) and its application to evaluate viability and amoebicidal activity in vitro. Exp. Parasitol. 2015, 155, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Kliescikova, J.; Kulda, J.; Nohynkova, E. Stress-induced pseudocyst formation-a newly identified mechanism of protection against organic solvents in Acanthamoebae of the T4 genotype. Protist 2011, 162, 58–69. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol. 2006, 44, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.F.; Myerscough, M.R.; Currie, B.J. Studies In Vitro on the relative efficacy of current acaricides for Sarcoptes scabiei var. hominis. R. Soc. Trop. Med. Hyg. 2000, 94, 92–96. [Google Scholar] [CrossRef]
- Iori, A.; Grazioli, D.; Gentile, E.; Marano, G.; Salvatore, G. Acaricidal properties of the essential oil of Melaleuca alternifolia Cheel (tea tree oil) against nymphs of Ixodes ricinus. Vet. Parasitol. 2005, 129, 173–176. [Google Scholar] [CrossRef]
- Albouchi, F.; Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Piñero, J.E.; Lorenzo-Morales, J.; Abderrabba, M. Chemical composition and anti-Acanthamoeba activity of Melaleuca styphelioides essential oil. Exp. Parasitol. 2017, 183, 104–108. [Google Scholar] [CrossRef]
- Brand, C.; Ferrante, A.; Prager, R.H.; Riley, T.V.; Carson, C.F.; Finlay-Jones, J.J.; Hart, P.H. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 2001, 50, 213–219. [Google Scholar] [CrossRef]
- Jacob, S.W.; Herschler, R. Pharmacology of DMSO. Cryobiology 1986, 23, 14–27. [Google Scholar] [CrossRef]
- Dudley, R.; Matin, A.; Alsam, S.; Sissons, J.; Maghsood, A.H.; Khan, N.A. Acanthamoeba isolates belonging to T1, T2, T3, T4 but not T7 encyst in response to increased osmolarity and cysts do not bind to human corneal epithelial cells. Acta Trop. 2005, 95, 100–108. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Desai, D.; Sahm, D. Adherence of Acanthamoeba castellanii cysts and trophozoites to unworn soft contact lenses. Am. J. Ophthalmol. 1989, 108, 658–664. [Google Scholar] [CrossRef]
- Kilvington, S.; Larkin, D.F.P. Acanthamoeba adherence to contact lenses and removal by cleaning agents. Eye 1990, 4, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Verani, J.R.; Lorick, S.A.; Yoder, J.S.; Beach, M.J.; Braden, C.R.; Roberts, J.M.; Roy, S.L. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg. Infect. Dis. 2009, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Heredero-Bermejo, I.; Criado-Fornelio, A.; De Fuentes, I.; Soliveri, J.; Copa-Patiño, J.L.; Pérez-Serrano, J. Characterization of a human-pathogenic Acanthamoeba griffini isolated from a contact lens-wearing keratitis patient in Spain. Parasitology 2015, 142, 363. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Martin, C.S.J.; de Carranza, J.S.; Copa-Patiño, J.L.; Pérez-Serrano, J. Acanthamoeba castellanii: In Vitro UAH-T17c3 trophozoite growth study in different culture media. Parasitol. Res. 2012, 110, 2563–2567. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Copa-Patiño, J.L.; Soliveri, J.; Fuentes-Paniagua, E.; de la Mata, F.J.; Gómez, R.; Pérez-Serrano, J. Evaluation of the activity of carbosilane dendrimers on trophozoites and cysts of Acanthamoeba polyphaga. Parasitology 2015, 114, 473–486. [Google Scholar] [CrossRef]
- Martín-Pérez, T.; Criado-Fornelio, A.; Ávila-Blanco, M.; Pérez-Serrano, J. Development and optimization of new culture media for Acanthamoeba spp. (Protozoa: Amoebozoa). Eur. J. Protistol. 2018, 64, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Elder, M.J.; Kilvington, S.; Dart, J.K. A clinicopathologic study of In Vitro sensitivity testing and Acanthamoeba keratitis. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1059–1064. [Google Scholar]
- Kohno, Y.; Ohno, H.; Miyazaki, Y.; Higashiyama, Y.; Yanagihara, K.; Hirakata, Y.; Kohno, S. In Vitro and in vivo activities of novel fluoroquinolones alone and in combination with clarithromycin against clinically isolated Mycobacterium avium complex strains in Japan. Antimicrob. Agents Chemother. 2007, 51, 4071–4076. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| A. griffini MYP2004 | A. polyphaga 2961 | |||
|---|---|---|---|---|
| MTAC | MCC | MTAC | MCC | |
| TTO | 0.5 | >1 | 0.5 | >1 |
| DMSO | >1.25 | >1.25 | >1.25 | >1.25 |
| TTO+DMSO | 0.25 + 1.25 | >0.5 + 2.5 | 0.12 + 0.625 | >0.5 + 2.5 |
| A. griffini MYP2004 (Genotype T3) MTAC (% v/v) | FICI | Interpretation | |||
| TTO (Alone) | DMSO (Alone) | TTO (Combination) | DMSO (Combination) | ||
| 0.5 | 1.25 | 0.25 | 1.25 | 1.5 | Indifference |
| A. polyphaga 2961 (Genotype T4) MTAC (% v/v) | FICI | Interpretation | |||
| TTO (Alone) | DMSO (Alone) | TTO (Combination) | DMSO (Combination) | ||
| 0.5 | 1.25 | 0.12 | 0.625 | 0.74 | Indifference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Pérez, T.; Heredero-Bermejo, I.; Verdú-Expósito, C.; Pérez-Serrano, J. In Vitro Evaluation of the Combination of Melaleuca alternifolia (Tea Tree) Oil and Dimethyl Sulfoxide (DMSO) against Trophozoites and Cysts of Acanthamoeba Strains. Oxygen Consumption Rate (OCR) Assay as a Method for Drug Screening. Pathogens 2021, 10, 491. https://doi.org/10.3390/pathogens10040491
Martín-Pérez T, Heredero-Bermejo I, Verdú-Expósito C, Pérez-Serrano J. In Vitro Evaluation of the Combination of Melaleuca alternifolia (Tea Tree) Oil and Dimethyl Sulfoxide (DMSO) against Trophozoites and Cysts of Acanthamoeba Strains. Oxygen Consumption Rate (OCR) Assay as a Method for Drug Screening. Pathogens. 2021; 10(4):491. https://doi.org/10.3390/pathogens10040491
Chicago/Turabian StyleMartín-Pérez, Tania, Irene Heredero-Bermejo, Cristina Verdú-Expósito, and Jorge Pérez-Serrano. 2021. "In Vitro Evaluation of the Combination of Melaleuca alternifolia (Tea Tree) Oil and Dimethyl Sulfoxide (DMSO) against Trophozoites and Cysts of Acanthamoeba Strains. Oxygen Consumption Rate (OCR) Assay as a Method for Drug Screening" Pathogens 10, no. 4: 491. https://doi.org/10.3390/pathogens10040491
APA StyleMartín-Pérez, T., Heredero-Bermejo, I., Verdú-Expósito, C., & Pérez-Serrano, J. (2021). In Vitro Evaluation of the Combination of Melaleuca alternifolia (Tea Tree) Oil and Dimethyl Sulfoxide (DMSO) against Trophozoites and Cysts of Acanthamoeba Strains. Oxygen Consumption Rate (OCR) Assay as a Method for Drug Screening. Pathogens, 10(4), 491. https://doi.org/10.3390/pathogens10040491






