P-Glycoprotein Inhibitors Differently Affect Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti Proliferation in Bovine Primary Endothelial Cells
Abstract
:1. Introduction
2. Results
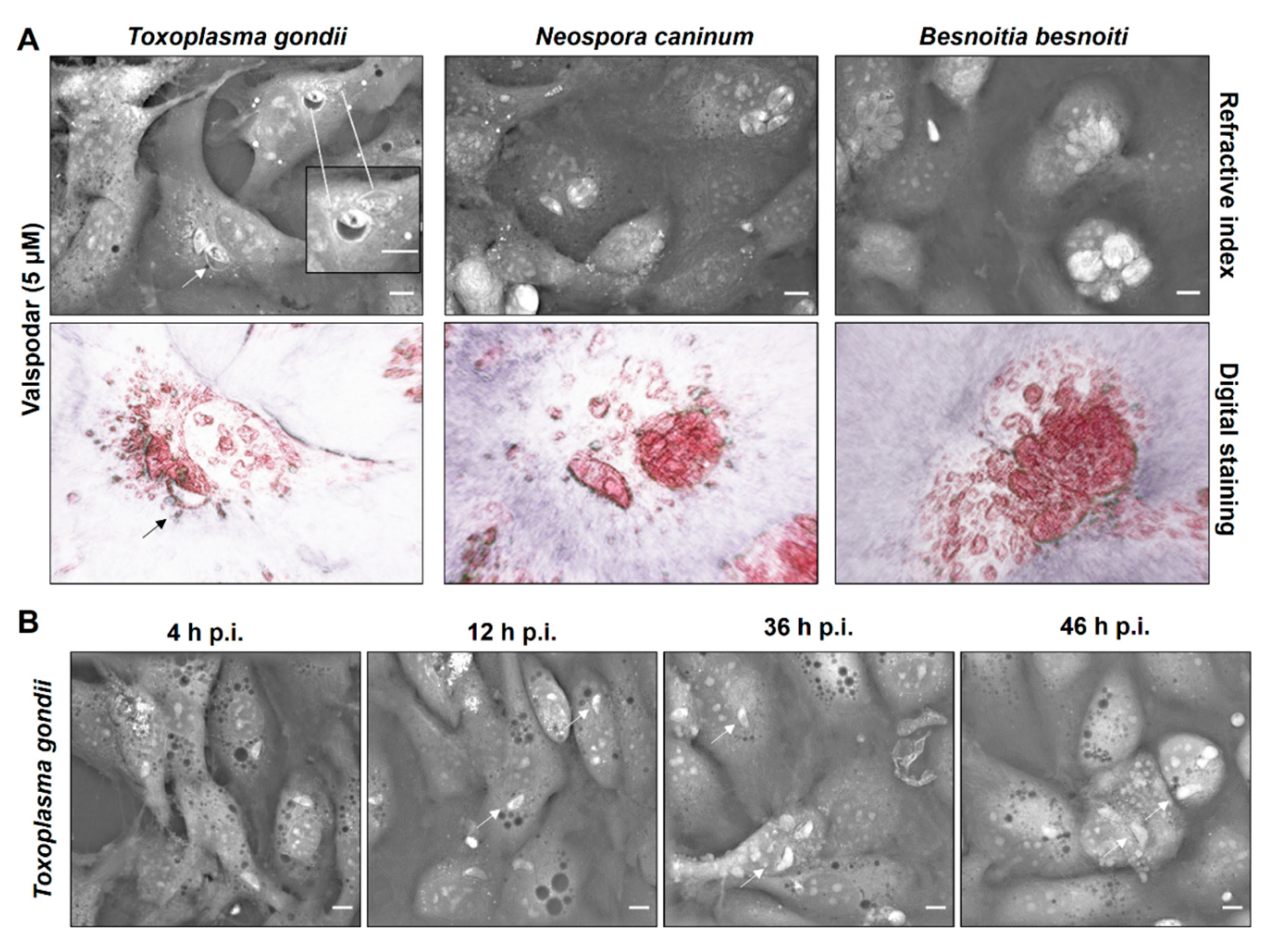
2.1. Different Generations of P-Gp Inhibitors Vary in Their Impact on Tachyzoite Replication
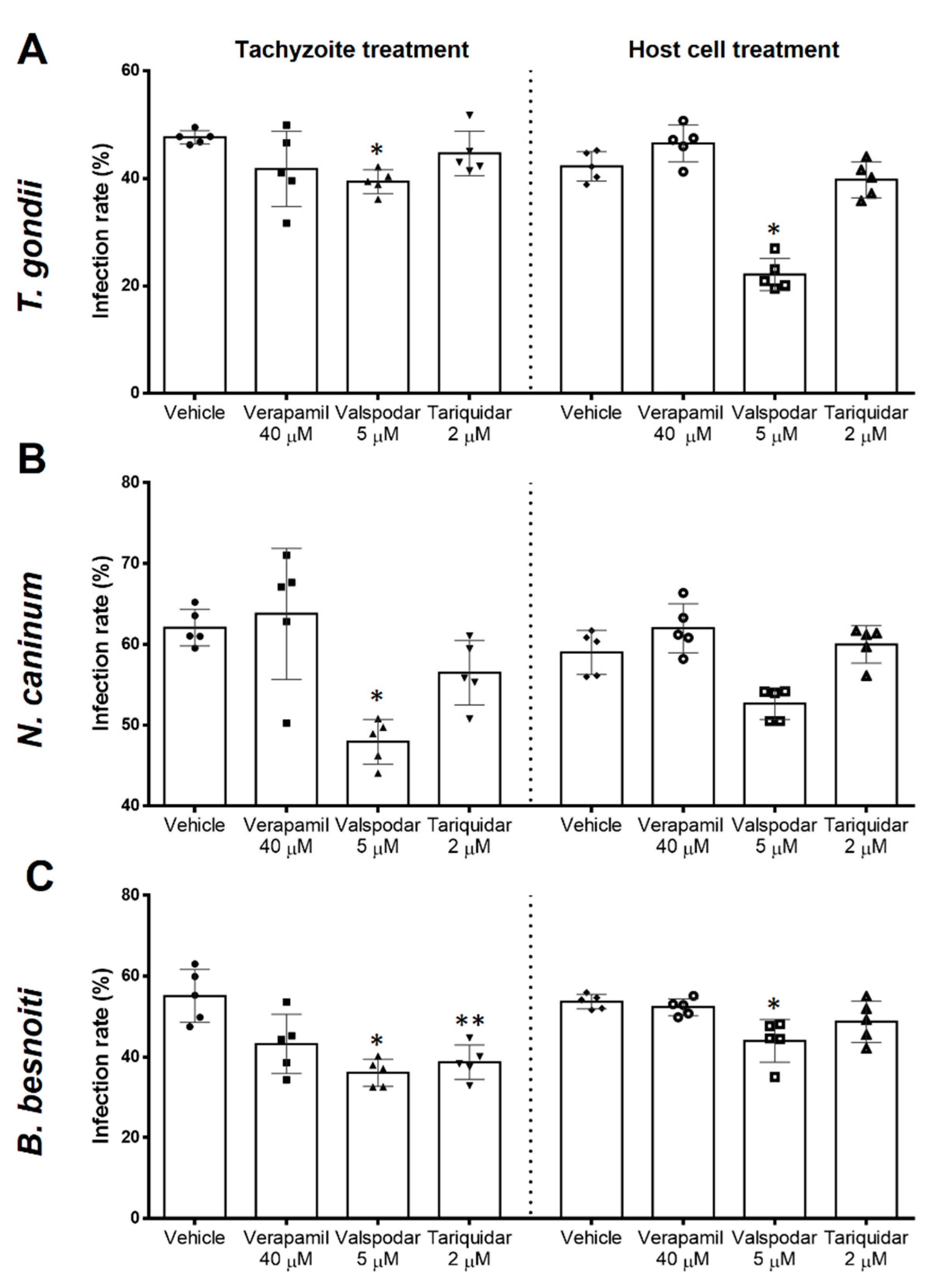
2.2. Infection Rates Are Differentially Influenced by Different P-Gp Inhibitors
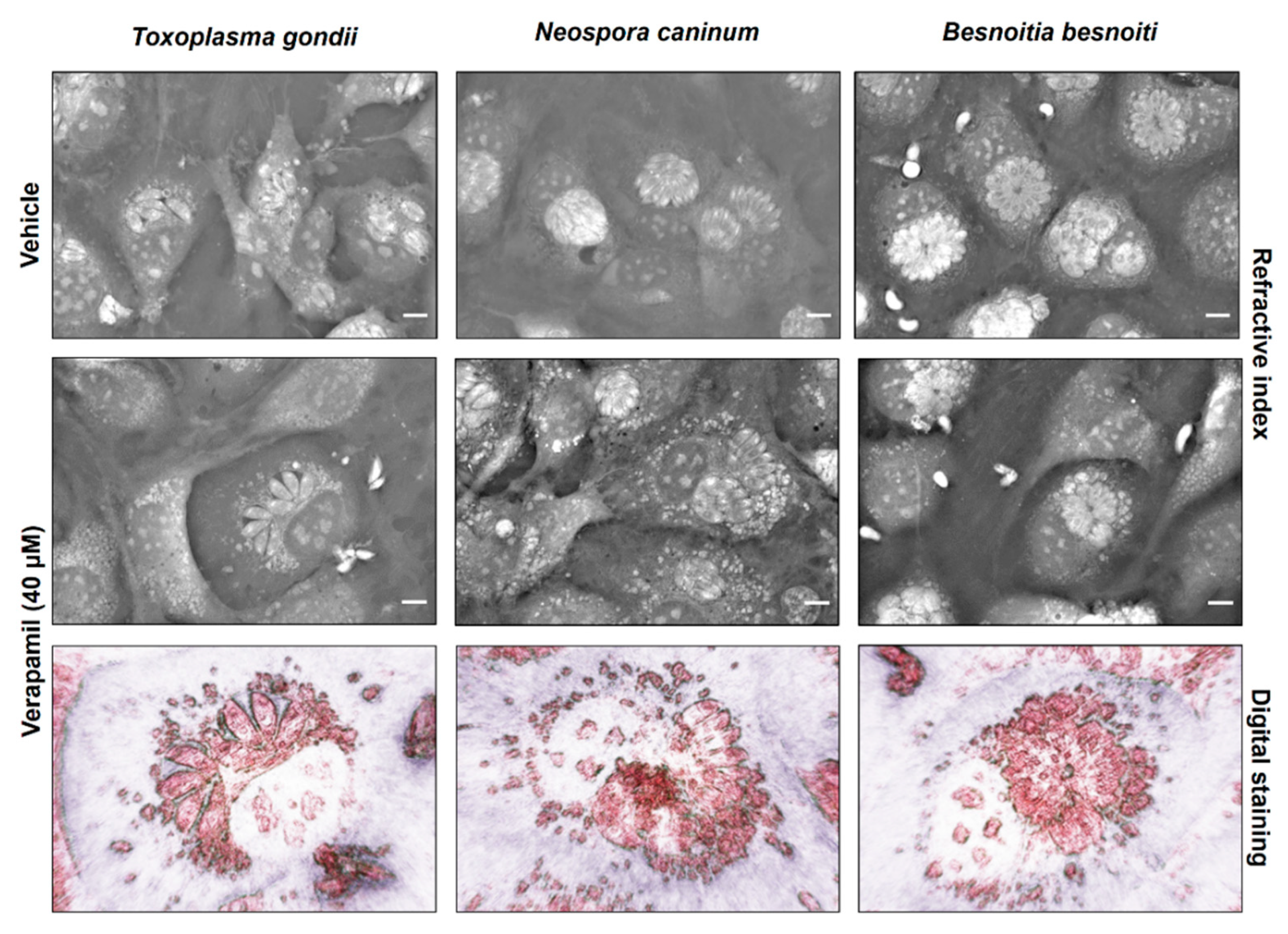
2.3. P-Gp Inhibitor-Driven Morphological Alterations in Primary Bovine Endothelial Cells
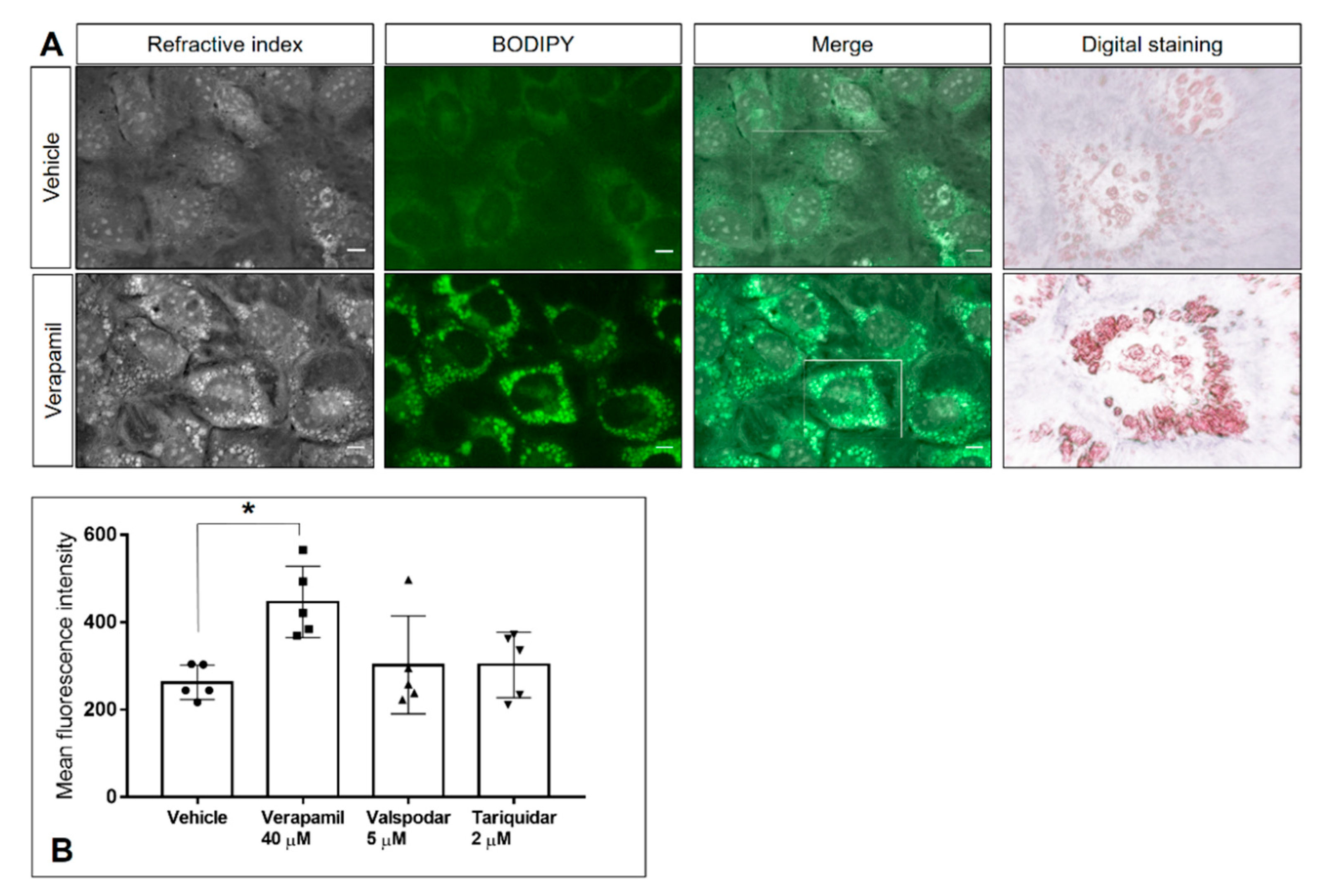
2.4. P-Gp Inhibitor Treatment Does Not Cause Cytotoxic Damage to Host Cells or Tachyzoites
3. Discussion
4. Materials and Methods
4.1. Host Cell Culture
4.2. Parasite Cultures
4.3. Inhibitor Treatment
4.4. Flow Cytometry Analysis
4.5. Live Cell 3D-Holotomographic Microscopy
4.6. Cell Toxicity Assays
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Votýpka, J.; Modrý, D.; Oborník, M.; Šlapeta, J.; Lukeš, J. Apicomplexa. In Handbook of the Protists; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 567–624. [Google Scholar] [CrossRef]
- Innes, E.A. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 2010, 57, 1–7. [Google Scholar] [CrossRef]
- Benavides, J.; Fernandez, M.; Castano, P.; Ferreras, M.C.; Ortega-Mora, L.; Perez, V. Ovine Toxoplasmosis: A New Look at its Pathogenesis. J. Comp. Pathol. 2017, 157, 34–38. [Google Scholar] [CrossRef]
- Nayeri, T.; Sarvi, S.; Moosazadeh, M.; Amouei, A.; Hosseininejad, Z.; Daryani, A. The global seroprevalence of anti-Toxoplasma gondii antibodies in women who had spontaneous abortion: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008103. [Google Scholar] [CrossRef]
- Lagomarsino, H.; Scioli, A.; Rodríguez, A.; Armendano, J.; Fiorani, F.; Bence, Á.; García, J.; Hecker, Y.; Gual, I.; Cantón, G.; et al. Controlling Endemic Neospora caninum-Related Abortions in a Dairy Herd From Argentina. Front. Vet. Sci. 2019, 6, 446. [Google Scholar] [CrossRef]
- Reichel, M.P.; Alejandra Ayanegui-Alcérreca, M.; Gondim, L.F.; Ellis, J.T. What is the global economic impact of Neospora caninum in cattle-the billion dollar question. Int. J. Parasitol. 2013, 43, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, G.; Frey, C.F.; Mora, L.M.; Schares, G. A century of bovine besnoitiosis: An unknown disease re-emerging in Europe. Trends Parasitol. 2013, 29, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.R.; Lutjohann, D.; Hamid, P.; Velasquez, Z.D.; Kerner, K.; Larrazabal, C.; Failing, K.; Hermosilla, C.; Taubert, A. Besnoitia besnoiti infection alters both endogenous cholesterol de novo synthesis and exogenous LDL uptake in host endothelial cells. Sci. Rep. 2019, 9, 6650. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A.; Zahner, H.; Hermosilla, C. Dynamics of transcription of immunomodulatory genes in endothelial cells infected with different coccidian parasites. Vet. Parasitol. 2006, 142, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A.; Krüll, M.; Zahner, H.; Hermosilla, C. Toxoplasma gondii and Neospora caninum infections of bovine endothelial cells induce endothelial adhesion molecule gene transcription and subsequent PMN adhesion. Vet. Immunol. Immunopathol. 2006, 112, 272–283. [Google Scholar] [CrossRef]
- Velásquez, Z.D.; Lopez-Osorio, S.; Pervizaj-Oruqaj, L.; Herold, S.; Hermosilla, C.; Taubert, A. Besnoitia besnoiti-driven endothelial host cell cycle alteration. Parasitol. Res. 2020, 119, 2563–2577. [Google Scholar] [CrossRef]
- Velasquez, Z.D.; Conejeros, I.; Larrazabal, C.; Kerner, K.; Hermosilla, C.; Taubert, A. Toxoplasma gondii-induced host cellular cell cycle dysregulation is linked to chromosome missegregation and cytokinesis failure in primary endothelial host cells. Sci. Rep. 2019, 9, 12496. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.J.; Romano, J.D.; Luechtefeld, T.; Coppens, I. Neospora caninum Recruits Host Cell Structures to Its Parasitophorous Vacuole and Salvages Lipids from Organelles. Eukaryot. Cell 2015, 14, 454–473. [Google Scholar] [CrossRef] [PubMed]
- Coppens, I.; Sinai, A.P.; Joiner, K.A. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 2000, 149, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ehrenman, K.; Wanyiri, J.W.; Bhat, N.; Ward, H.D.; Coppens, I. Cryptosporidium parvum scavenges LDL-derived cholesterol and micellar cholesterol internalized into enterocytes. Cell. Microbiol. 2013, 15, 1182–1197. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Ibrahim, H.M.; Kameyama, K.; Shiga, I.; Hiasa, J.; Xuan, X. Host cholesterol synthesis contributes to growth of intracellular Toxoplasma gondii in macrophages. J. Vet. Med. Sci. 2011, 73, 633–639. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. How cells handle cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Rogers, M.A. Sterol Metabolism and Transport in Atherosclerosis and Cancer. Front. Endocrinol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Chang, T.Y.; Li, B.L.; Chang, C.C.; Urano, Y. Acyl-coenzyme A:cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1–E9. [Google Scholar] [CrossRef]
- Hu, X.; Binns, D.; Reese, M.L. The Coccidian Parasites Toxoplasma and Neospora Dysregulate Mammalian Lipid Droplet Biogenesis. J. Biol. Chem. 2017, 292, 11009–11020. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Ehrenman, K.; Sehgal, A.; Lige, B.; Stedman, T.T.; Joiner, K.A.; Coppens, I. Novel roles for ATP-binding cassette G transporters in lipid redistribution in Toxoplasma. Mol. Microbiol. 2010, 76, 1232–1249. [Google Scholar] [CrossRef]
- Satyamoorthy, K.; Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. PLoS ONE 2014, 4, 41. [Google Scholar] [CrossRef]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Sauvage, V.; Escotte-Binet, S.; Aubert, D.; Terryn, C.; Garnotel, R.; Villena, I. Molecular characterization and expression analysis of a P-glycoprotein homologue in Toxoplasma gondii. Mol. Biochem. Parasitol. 2009, 163, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Muñoz, E.; Pérez-Victoria, J.M.; Gamarro, F.; Castanys, S. Characterization of an ABCG-like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob. Agents Chemother. 2008, 52, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Deuchars, K.; Kartner, N.; Alon, N.; Trent, J.; Ling, V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature 1985, 316, 817–819. [Google Scholar] [CrossRef]
- Kasinathan, R.S.; Morgan, W.M.; Greenberg, R.M. Genetic knockdown and pharmacological inhibition of parasite multidrug resistance transporters disrupts egg production in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2011, 5, e1425. [Google Scholar] [CrossRef]
- Bartley, D.J.; McAllister, H.; Bartley, Y.; Dupuy, J.; Ménez, C.; Alvinerie, M.; Jackson, F.; Lespine, A. P-glycoprotein interfering agents potentiate ivermectin susceptibility in ivermectin sensitive and resistant isolates of Teladorsagia circumcincta and Haemonchus contortus. Parasitology 2009, 136, 1081–1088. [Google Scholar] [CrossRef]
- Nicolao, M.C.; Denegri, G.M.; Cárcamo, J.G.; Cumino, A.C. P-glycoprotein expression and pharmacological modulation in larval stages of Echinococcus granulosus. Parasitol. Int. 2014, 63, 1–8. [Google Scholar] [CrossRef]
- Bottova, I.; Hehl, A.B.; Stefanic, S.; Fabrias, G.; Casas, J.; Schraner, E.; Pieters, J.; Sonda, S. Host cell P-glycoprotein is essential for cholesterol uptake and replication of Toxoplasma gondii. J. Biol. Chem. 2009, 284, 17438–17448. [Google Scholar] [CrossRef] [PubMed]
- Leitch, G.J.; Scanlon, M.; Shaw, A.; Visvesvara, G.S. Role of P glycoprotein in the course and treatment of Encephalitozoon microsporidiosis. Antimicrob. Agents Chemother. 2001, 45, 73–78. [Google Scholar] [CrossRef]
- Palmeira, A.; Sousa, E.; Vasconcelos, M.H.; Pinto, M.M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef]
- Tsuruo, T.; Iida, H.; Tsukagoshi, S.; Sakurai, Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981, 41, 1967–1972. [Google Scholar]
- Archinal-Mattheis, A.; Rzepka, R.W.; Watanabe, T.; Kokubu, N.; Itoh, Y.; Combates, N.J.; Bair, K.W.; Cohen, D. Analysis of the interactions of SDZ PSC 833 ([3’-keto-Bmt1]-Val2]-Cyclosporine), a multidrug resistance modulator, with P-glycoprotein. Oncol. Res. 1995, 7, 603–610. [Google Scholar]
- Atadja, P.; Watanabe, T.; Xu, H.; Cohen, D. PSC-833, a frontier in modulation of P-glycoprotein mediated multidrug resistance. Cancer Metastasis Rev. 1998, 17, 163–168. [Google Scholar] [CrossRef]
- Martin, C.; Berridge, G.; Mistry, P.; Higgins, C.; Charlton, P.; Callaghan, R. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br. J. Pharmacol. 1999, 128, 403–411. [Google Scholar] [CrossRef]
- Roe, M.; Folkes, A.; Ashworth, P.; Brumwell, J.; Chima, L.; Hunjan, S.; Pretswell, I.; Dangerfield, W.; Ryder, H.; Charlton, P. Reversal of P-glycoprotein mediated multidrug resistance by novel anthranilamide derivatives. Bioorg. Med. Chem. Lett. 1999, 9, 595–600. [Google Scholar] [CrossRef]
- Coppens, I. Targeting lipid biosynthesis and salvage in apicomplexan parasites for improved chemotherapies. Nat. Rev. Microbiol. 2013, 11, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Bottova, I.; Sauder, U.; Olivieri, V.; Hehl, A.B.; Sonda, S. The P-glycoprotein inhibitor GF120918 modulates Ca2+-dependent processes and lipid metabolism in Toxoplasma gondii. PLoS ONE 2010, 5, e10062. [Google Scholar] [CrossRef]
- Holfels, E.; McAuley, J.; Mack, D.; Milhous, W.K.; McLeod, R. In vitro effects of artemisinin ether, cycloguanil hydrochloride (alone and in combination with sulfadiazine), quinine sulfate, mefloquine, primaquine phosphate, trifluoperazine hydrochloride, and verapamil on Toxoplasma gondii. Antimicrob. Agents Chemother. 1994, 38, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Adovelande, J.; Deleze, J.; Schrevel, J. Synergy between two calcium channel blockers, verapamil and fantofarone (SR33557), in reversing chloroquine resistance in Plasmodium falciparum. Biochem. Pharmacol. 1998, 55, 433–440. [Google Scholar] [CrossRef]
- Martiney, J.A.; Cerami, A.; Slater, A.F. Verapamil reversal of chloroquine resistance in the malaria parasite Plasmodium falciparum is specific for resistant parasites and independent of the weak base effect. J. Biol. Chem. 1995, 270, 22393–22398. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Tertov, V.V.; Kudryashov, S.A.; Khashimov Kh, A.; Smirnov, V.N. Primary culture of human aortic intima cells as a model for testing antiatherosclerotic drugs. Effects of cyclic AMP, prostaglandins, calcium antagonists, antioxidants, and lipid-lowering agents. Atherosclerosis 1986, 60, 101–110. [Google Scholar] [CrossRef]
- Rokosova, B.; Bentley, J.P. Effect of calcium on cell proliferation and extracellular matrix synthesis in arterial smooth muscle cells and dermal fibroblasts. Exp. Mol. Pathol. 1986, 44, 307–317. [Google Scholar] [CrossRef]
- Stein, O.; Halperin, G.; Stein, Y. Long-term effects of verapamil on aortic smooth muscle cells cultured in the presence of hypercholesterolemic serum. Arteriosclerosis 1987, 7, 585–592. [Google Scholar] [CrossRef]
- Hupe, D.J.; Pfefferkorn, E.R.; Behrens, N.D.; Peters, K. L-651,582 inhibition of intracellular parasitic protozoal growth correlates with host-cell directed effects. J. Pharmacol. Exp. Ther. 1991, 256, 462–467. [Google Scholar]
- Polin, R.A.; Fox, W.W.; Abman, S.H. Fetal and Neonatal Physiology, 3rd ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 2004. [Google Scholar]
- Louters, L.L.; Stehouwer, N.; Rekman, J.; Tidball, A.; Cok, A.; Holstege, C.P. Verapamil inhibits the glucose transport activity of GLUT1. J. Med. Toxicol. Off. J. Am. Coll. Med Toxicol. 2010, 6, 100–105. [Google Scholar] [CrossRef]
- Silverman, J.A.; Hayes, M.L.; Luft, B.J.; Joiner, K.A. Characterization of anti-Toxoplasma activity of SDZ 215-918, a cyclosporin derivative lacking immunosuppressive and peptidyl-prolyl-isomerase-inhibiting activity: Possible role of a P glycoprotein in Toxoplasma physiology. Antimicrob. Agents Chemother. 1997, 41, 1859–1866. [Google Scholar] [CrossRef]
- Bell, A.; Wernli, B.; Franklin, R.M. Roles of peptidyl-prolyl cis-trans isomerase and calcineurin in the mechanisms of antimalarial action of cyclosporin A, FK506, and rapamycin. Biochem. Pharmacol. 1994, 48, 495–503. [Google Scholar] [CrossRef]
- Perkins, M.E.; Wu, T.W.; Le Blancq, S.M. Cyclosporin analogs inhibit in vitro growth of Cryptosporidium parvum. Antimicrob. Agents Chemother. 1998, 42, 843–848. [Google Scholar] [CrossRef]
- Black, M.W.; Boothroyd, J.C. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. MMBR 2000, 64, 607–623. [Google Scholar] [CrossRef]
- Caldas, L.A.; Attias, M.; de Souza, W. Dynamin inhibitor impairs Toxoplasma gondii invasion. FEMS Microbiol. Lett. 2009, 301, 103–108. [Google Scholar] [CrossRef]
- Le Goff, W.; Settle, M.; Greene, D.J.; Morton, R.E.; Smith, J.D. Reevaluation of the role of the multidrug-resistant P-glycoprotein in cellular cholesterol homeostasis. J. Lipid Res. 2006, 47, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Maeda, M.; Manucat, N.B.; Ueda, K. Cyclosporine A and PSC833 inhibit ABCA1 function via direct binding. Biochim. Biophys. Acta 2013, 1831, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nishimaki-Mogami, T.; Tamehiro, N.; Inoue, K.; Arakawa, R.; Abe-Dohmae, S.; Tanaka, A.R.; Ueda, K.; Yokoyama, S. Verapamil increases the apolipoprotein-mediated release of cellular cholesterol by induction of ABCA1 expression via Liver X receptor-independent mechanism. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Saetersdal, T.; Røli, J.; Engedal, H.; Jodalen, H.; Rotevatn, S. Protective effects of verapamil against isoprenaline- induced mobilization of mitochondrial calcium and cellular lipid droplets in the myocardium. Res. Exp. Med. Z. Fur Die Gesamte Exp. Med. Einschl. Exp. Chir. 1982, 181, 39–47. [Google Scholar] [CrossRef]
- Munoz-Caro, T.; Silva, L.M.; Ritter, C.; Taubert, A.; Hermosilla, C. Besnoitia besnoiti tachyzoites induce monocyte extracellular trap formation. Parasitol. Res. 2014, 113, 4189–4197. [Google Scholar] [CrossRef]
- Cervantes-Valencia, M.E.; Hermosilla, C.; Alcalá-Canto, Y.; Tapia, G.; Taubert, A.; Silva, L.M.R. Antiparasitic Efficacy of Curcumin Against Besnoitia besnoiti Tachyzoites in vitro. Front. Vet. Sci. 2018, 5, 333. [Google Scholar] [CrossRef] [PubMed]



| Effect | Verapamil | Valspodar | Tariquidar |
|---|---|---|---|
| Reduced tachyzoite proliferation | yes | yes | only B. besnoiti |
| Diminished meront size at 24 h p. i. | no | yes | no |
| Reduced infection rate | no | yes | only B. besnoiti |
| Decreased host cell permissiveness | no | only T. gondii and B. besnoiti | no |
| Neutral lipid accumulation | yes | no | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larrazabal, C.; Silva, L.M.R.; Pervizaj-Oruqaj, L.; Herold, S.; Hermosilla, C.; Taubert, A. P-Glycoprotein Inhibitors Differently Affect Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti Proliferation in Bovine Primary Endothelial Cells. Pathogens 2021, 10, 395. https://doi.org/10.3390/pathogens10040395
Larrazabal C, Silva LMR, Pervizaj-Oruqaj L, Herold S, Hermosilla C, Taubert A. P-Glycoprotein Inhibitors Differently Affect Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti Proliferation in Bovine Primary Endothelial Cells. Pathogens. 2021; 10(4):395. https://doi.org/10.3390/pathogens10040395
Chicago/Turabian StyleLarrazabal, Camilo, Liliana M. R. Silva, Learta Pervizaj-Oruqaj, Susanne Herold, Carlos Hermosilla, and Anja Taubert. 2021. "P-Glycoprotein Inhibitors Differently Affect Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti Proliferation in Bovine Primary Endothelial Cells" Pathogens 10, no. 4: 395. https://doi.org/10.3390/pathogens10040395






