Response of Resistant and Susceptible Bayberry Cultivars to Infection of Twig Blight Pathogen by Histological Observation and Gibberellin Related Genes Expression
Abstract
:1. Introduction
2. Results
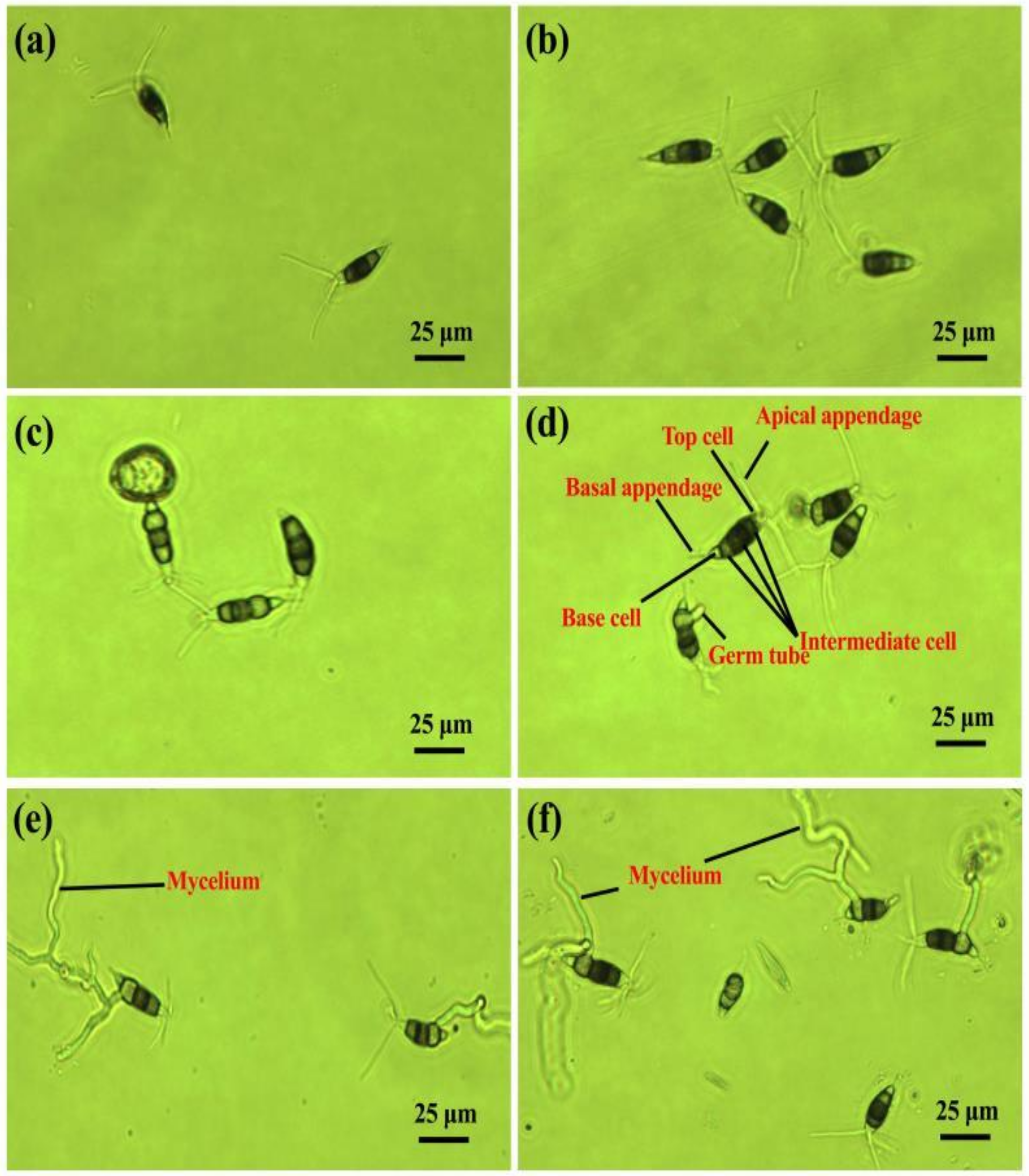
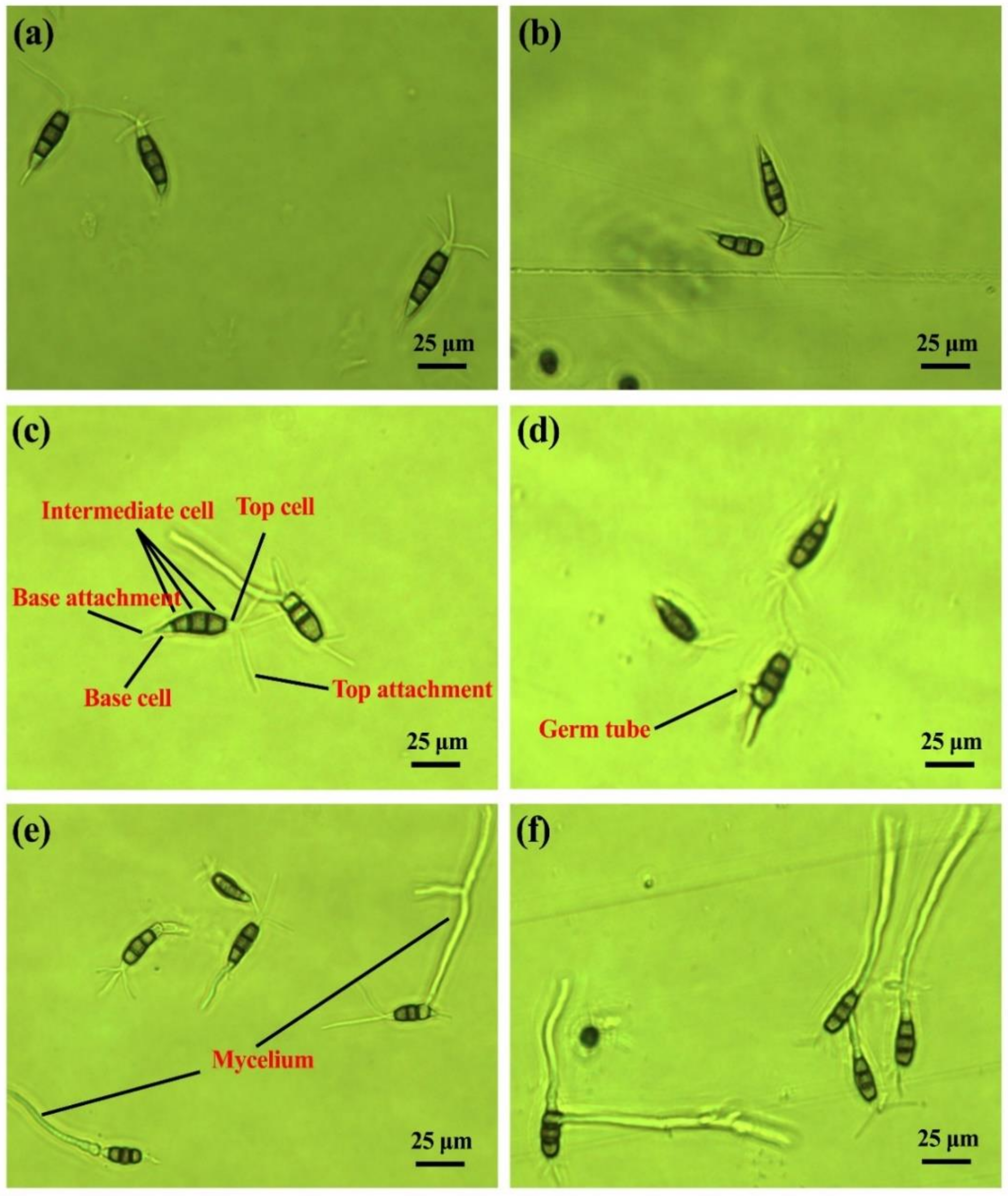
2.1. Conidia Germination and Morphology
2.2. Bayberry–Pestalotiopsis Interactions
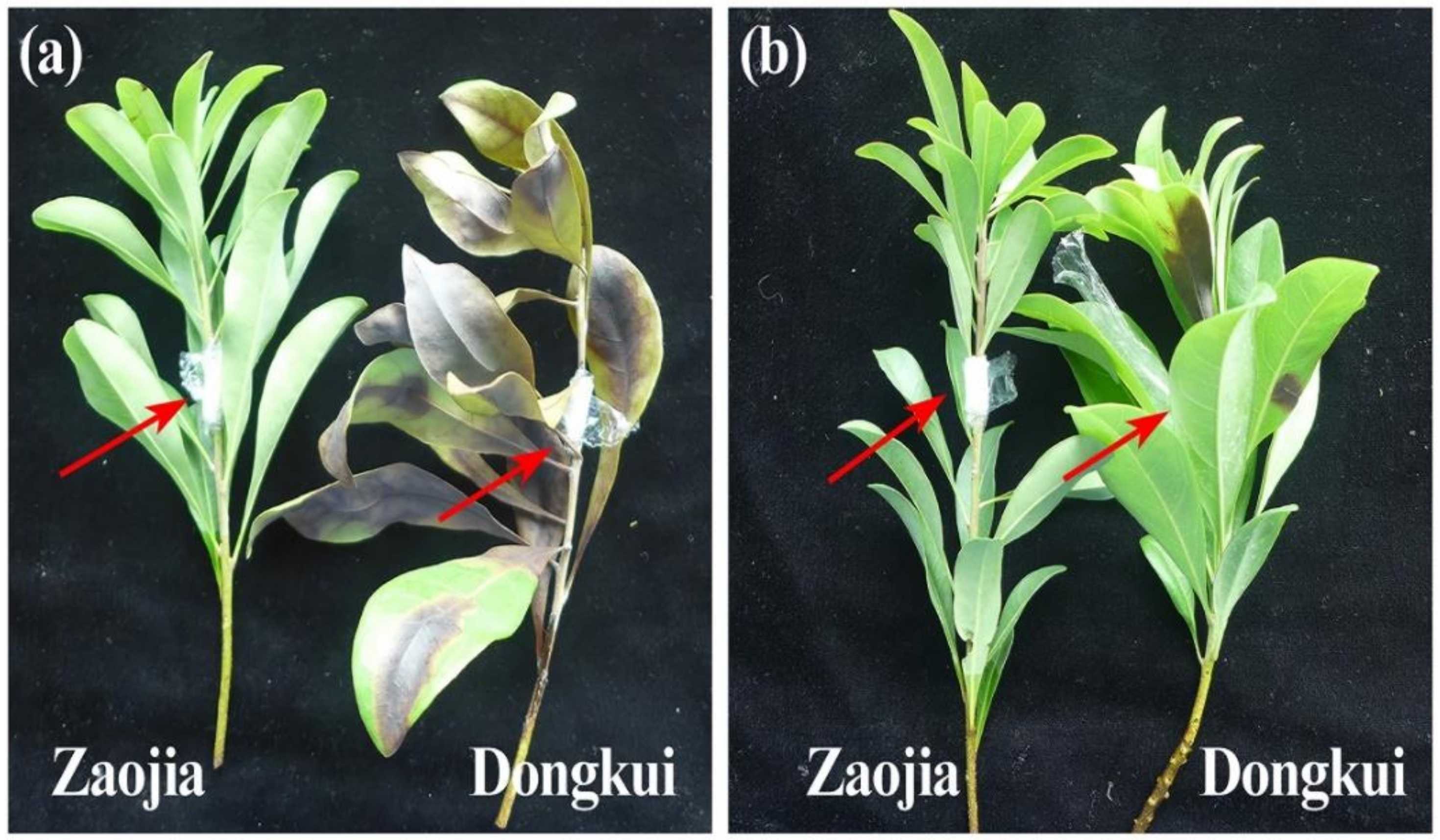
2.2.1. Symptoms Development
2.2.2. Effects on Conidia and Mycelia
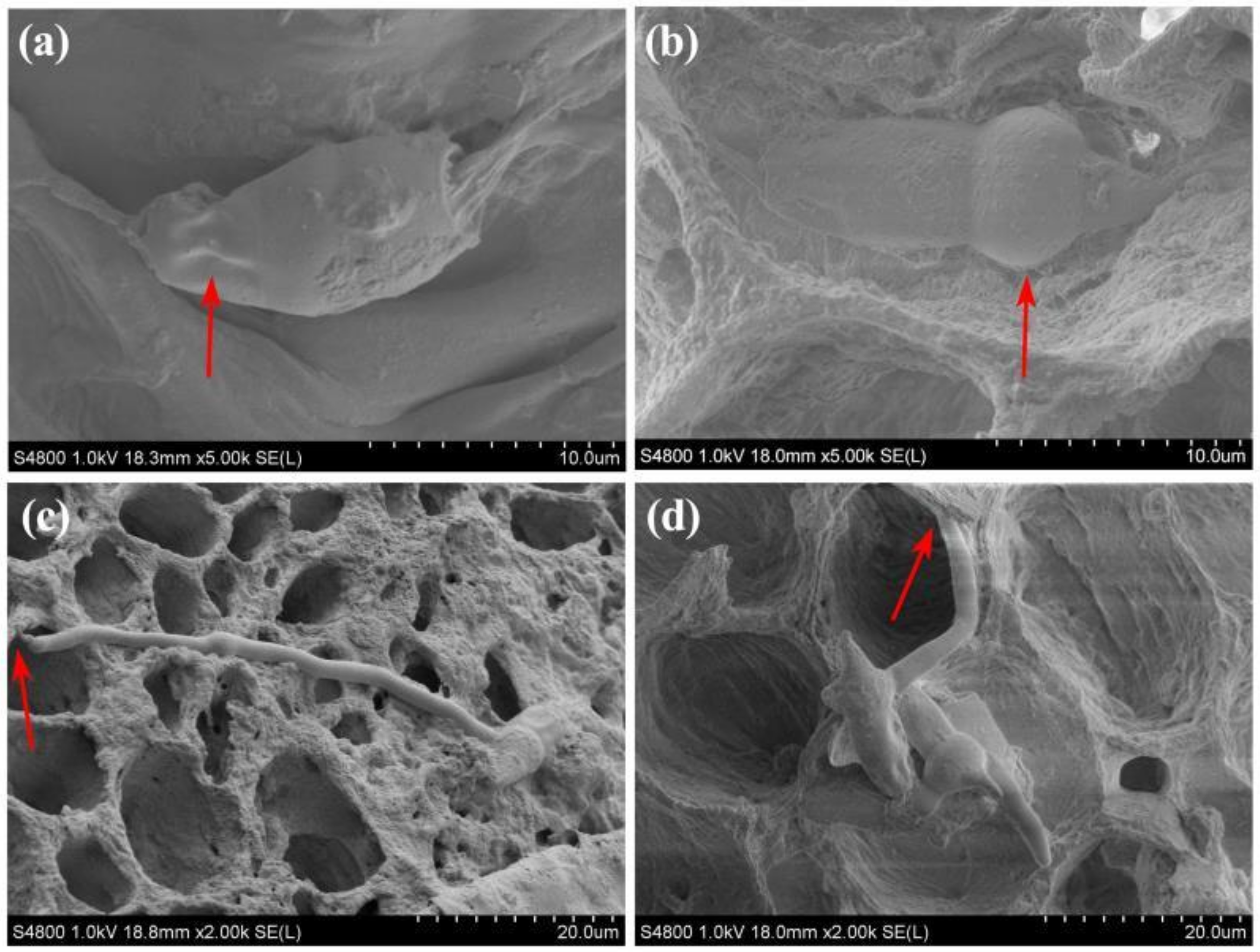
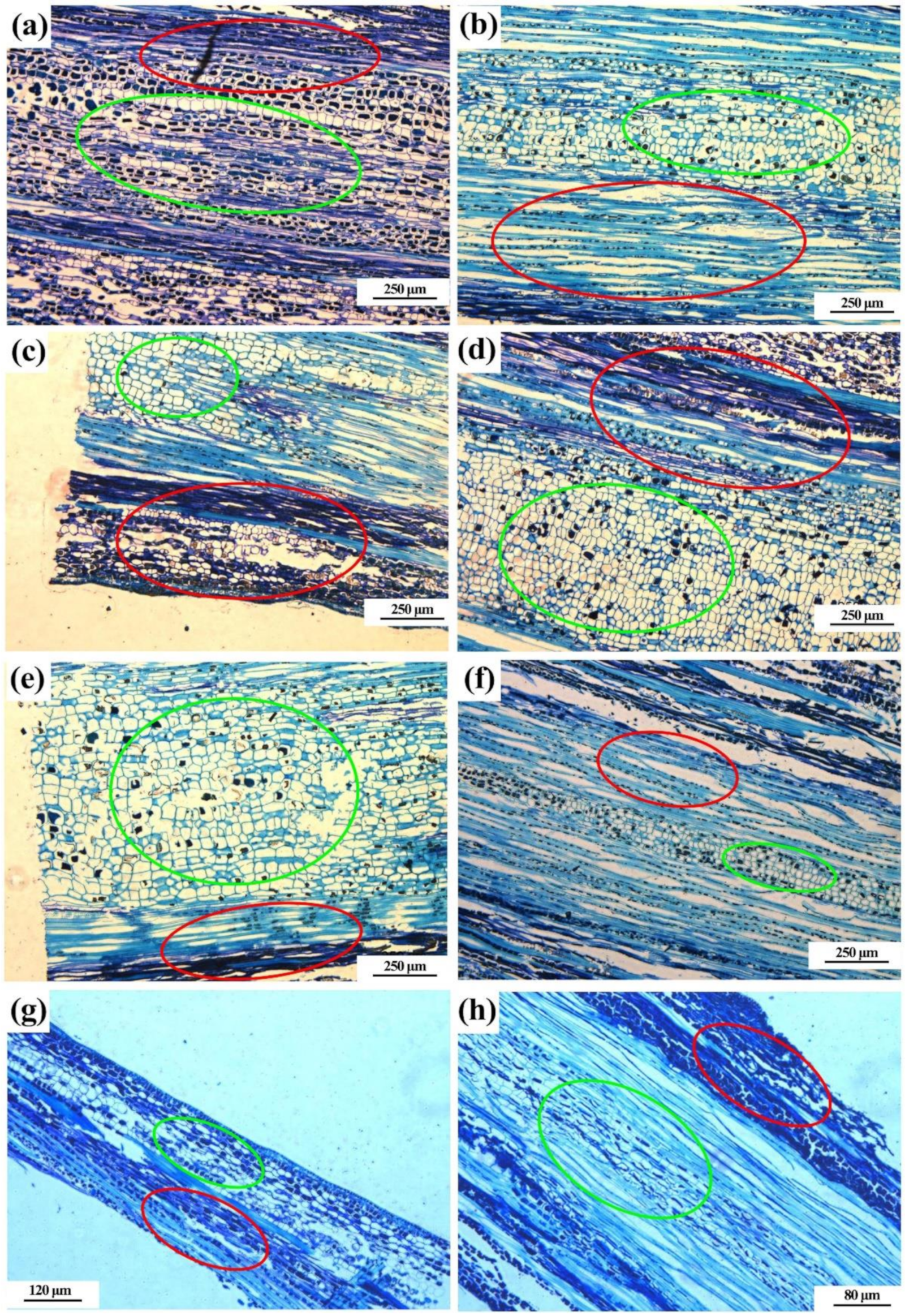
2.2.3. Effects on Phloem, Xylem and Medulla
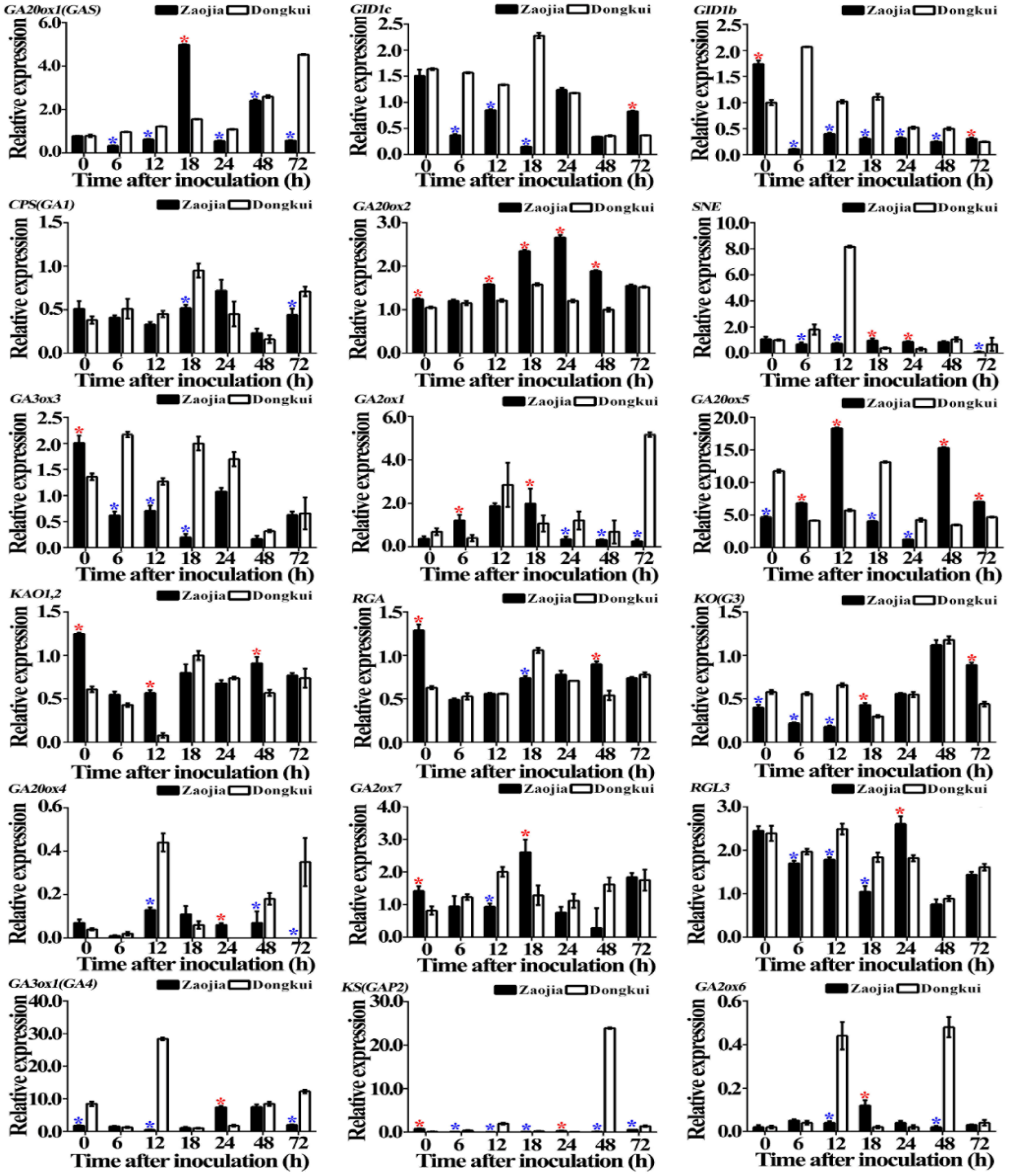
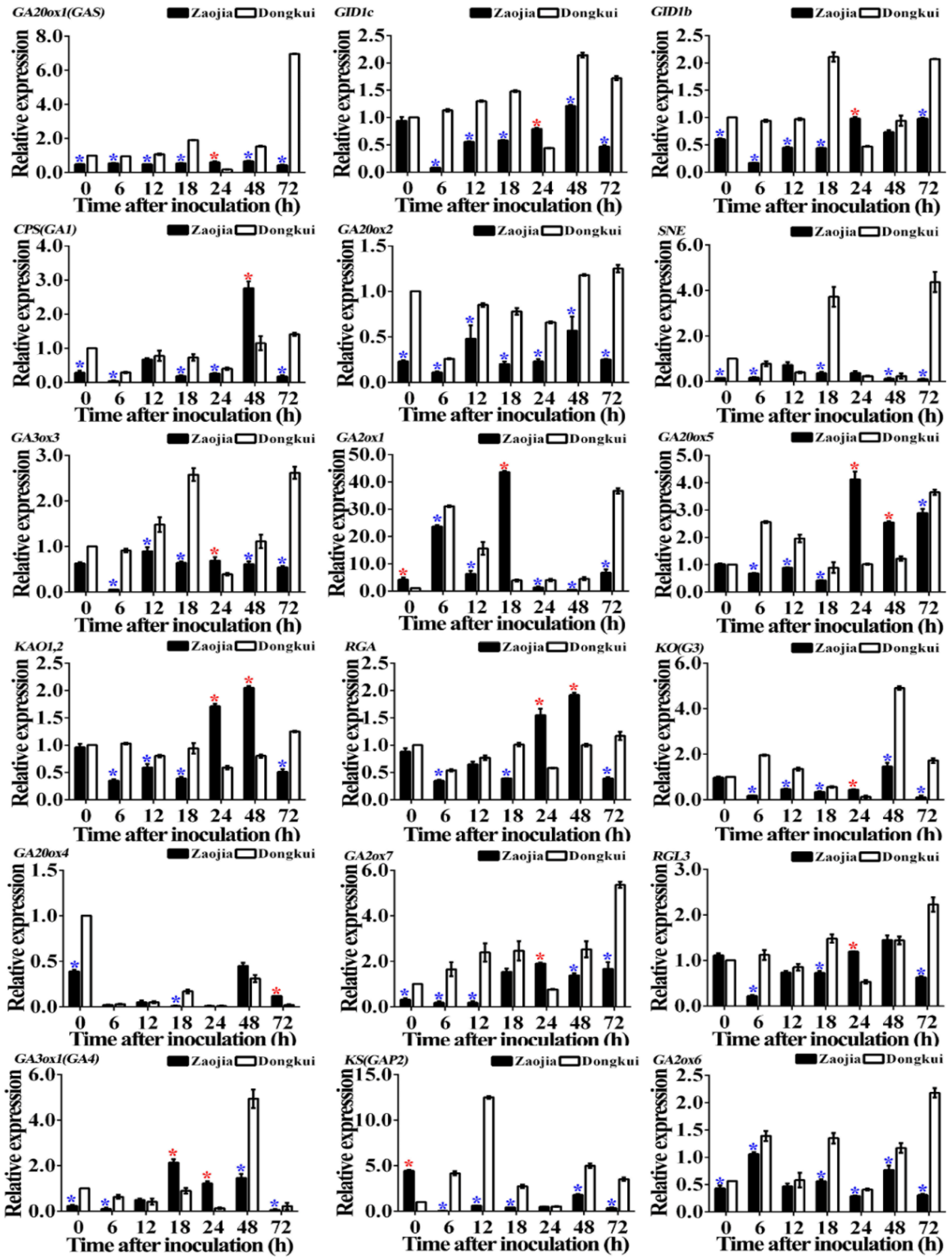
2.3. Gibberellin Genes Relative Expression
2.3.1. In Leaves
2.3.2. In Stems
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Fungal Strains
4.3. Conidia Germination and Morphology
4.4. Bayberry–Pestalotiopsis Interactions
4.4.1. Inoculation and Sampling
4.4.2. Symptoms Development and Disease Investigation
4.4.3. Effects on Conidia and Mycelia
4.4.4. Effects on Phloem, Xylem and Medulla
4.5. Gibberellin Genes Relative Expression
4.5.1. Genes Selection and Primers Design
4.5.2. Relative Expression Analyses
4.6. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Z.; Zhang, M.; Tao, G.; Sun, Y.; Sun, J. Chemical composition of clarified bayberry (Myrica rubra Sieb. et Zucc.) juice sediment. J. Agric. Food Chem. 2006, 54, 7710–7716. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Cao, S.; Chen, X.; Chen, W.; Zheng, Y.; Yang, Z. Proanthocyanidin synthesis in Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruits. Front. Plant Sci. 2018, 9, 212. [Google Scholar] [CrossRef]
- Ren, H.Y.; Li, G.; Qi, X.J.; Fang, L.; Wang, H.R.; Wei, J.G.; Zhong, S. Identification and characterization of Pestalotiopsis spp. causing twig blight disease of bayberry (Myrica rubra Sieb. & Zucc) in China. Eur. J. Plant Pathol. 2013, 137, 451–461. [Google Scholar]
- Chen, F.; Lu, L.; Ni, H.; Wang, Y.; Wang, Y.; Li, G. First report of Pestalotiopsis mangiferae and P. vismiae causing twig dieback of Myrica rubra in China. Plant Dis. 2012, 96, 588. [Google Scholar] [CrossRef]
- Li, W.; Hu, M.; Xue, Y.; Li, Z.; Zhang, Y.; Zheng, D.; Lu, G.; Wang, J.; Zhou, J. Five fungal pathogens are responsible for bayberry twig blight and fungicides were screened for disease control. Microorganisms 2020, 8, 689. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.M.; Chen, G.Q.; Hu, X.R.; Du, D.C.; Pu, Z.X.; Peng, A.T.; Cheng, B.P. Identification of Pestalotiopsis clavispora causing brown leaf spot on Chinese bayberry in China. Can. J. Plant Pathol. 2015, 37, 397–402. [Google Scholar] [CrossRef]
- Keith, L.M.; Velasquez, M.E.; Zee, F.T. Identification and characterization of Pestalotiopsis spp. causing scab disease of guava, Psidium guajava, in Hawaii. Plant Dis. 2006, 90, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, J.G.; Briceño, E.X.; Keith, L.M.; Latorre, B.A. Canker and twig dieback of blueberry caused by Pestalotiopsis spp. and a Truncatella sp. in Chile. Plant Dis. 2008, 92, 1407–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valencia, A.; Torres, R.; Latorre, B. First report of Pestalotiopsis clavispora and Pestalotiopsis spp. causing postharvest stem end rot of avocado in Chile. Plant Dis. 2011, 95, 492. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Yao, K.; Chen, C.; Lin, C. First report of gray leaf spot of mango (Mangifera indica) caused by Pestalotiopsis mangiferae in Taiwan. Plant Dis. 2007, 91, 1684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, W.X.; Zhang, A.F.; Yang, X.; Xu, Y.L. First report of Pestalotiopsis theae on Loquat (Eriobotrya japonica) in Anhui province of China. Plant Dis. 2013, 97, 558. [Google Scholar] [CrossRef]
- Fang, L.; Wang, H.; Feng, J. Branch blight of loquat caused by Pestalotiopsis sydowiana in China. Plant Dis. 2013, 97, 990. [Google Scholar] [CrossRef]
- Yang, J.; Ji, J.Y.; Zhang, B.W.; Chen, Y.Z.; Wang, S.R.; Zhang, G.C.; Zhang, J. Transcriptome and cell wall degrading enzyme-related gene analysis of Pestalotiopsis neglecta in response to sodium pheophorbide a. Pestic. Biochem. Physiol. 2020, 169, 104639. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, P.; Lu, S.; Liu, X.; Zhai, J.; Xu, J.; Wang, Y.; Zhang, G.; Liu, X.; Wan, Z. Occurrence and risk evaluation of organophosphorus pesticides in typical water bodies of Beijing, China. Environ. Sci. Poll. Res. 2021, 28, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Baibakova, E.V.; Nefedjeva, E.E.; Suska-Malawska, M.; Wilk, M.; Sevriukova, G.A.; Zheltobriukhov, V.F. Modern fungicides: Mechanisms of action, fungal resistance and phytotoxic effects. Annu. Res. Rev. 2019, 32, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.; Ren, H.; Noman, M.; Shahid, M.; Liu, M.; Ali, M.A.; Zhang, J.; Tian, Y.; Qi, X.; Li, B. Green synthesis and characterization of zirconium oxide nanoparticles by using a native Enterobacter sp. and its antifungal activity against bayberry twig blight disease pathogen Pestalotiopsis versicolor. NanoImpact 2021, 21, 100281. [Google Scholar] [CrossRef]
- Ali, M.; Ren, H.; Ahmed, T.; Luo, J.; An, Q.; Qi, X.; Li, B. Antifungal effects of rhizospheric Bacillus species against bayberry twig blight pathogen Pestalotiopsis versicolor. Agronomy 2020, 10, 1811. [Google Scholar] [CrossRef]
- Ren, H.; Yu, H.; Zhang, S.; Liang, S.; Zheng, X.; Zhang, S.; Yao, P.; Zheng, H.; Qi, X. Genome sequencing provides insights into the evolution and antioxidant activity of Chinese bayberry. BMC Genom. 2019, 20, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Liu, J.H.; Zhao, W.S.; Chen, X.J.; Guo, Z.J.; Peng, Y.L. Gibberellin 20-oxidase gene OsGA20ox3 regulates plant stature and disease development in rice. Mol. Plant Microbe Interact. 2013, 26, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, C.; Yan, Y.; Jia, H.; Guo, X. Overexpression of cotton GhMPK11 decreases disease resistance through the gibberellin signaling pathway in transgenic Nicotiana benthamiana. Front. Plant Sci. 2016, 7, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhao, D.; Dong, J.; Kong, X.; Zhang, Q.; Li, T.; Meng, Y.; Shan, W. AtRTP5 negatively regulates plant resistance to Phytophthora pathogens by modulating the biosynthesis of endogenous jasmonic acid and salicylic acid. Mol. Plant Pathol. 2020, 21, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vleesschauwer, D.; Seifi, H.S.; Filipe, O.; Haeck, A.; Huu, S.N.; Demeestere, K.; Höfte, M. The DELLA protein SLR1 integrates and amplifies salicylic acid-and jasmonic acid-dependent innate immunity in rice. Plant Physiol. 2016, 170, 1831–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef]
- Huang, X.; Sun, M.; Lu, X.; Li, S. Serial passage through resistant and susceptible cucumber cultivars affects the virulence of Fusarium oxysporum f. sp. cucumerinum. MicrobiologyOpen 2019, 8, e00641. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; González-Torres, R.; Álvarez, J. Plant tissue colonization by the fungus race 1.2 of Fusarium oxysporum f. sp. melonis in resistant melon genotypes. Commun. Agric. Appl. Biol. Sci. 2009, 74, 711–713. [Google Scholar] [PubMed]
- Zhou, X.; Everts, K. Quantification of root and stem colonization of watermelon by Fusarium oxysporum f. sp. niveum and its use in evaluating resistance. Phytopathology 2004, 94, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Vallad, G.; Subbarao, K. Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology 2008, 98, 871–885. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, J.; Henderson, M.; Schweizer, P.; Burton, R.A.; Fincher, G.B.; Little, A. Differential accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp. hordei. New Phytol. 2014, 204, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Wang, X.; Wang, W.; Li, Z.; Wu, J.; Wang, G.; Wu, L.; Zhang, G.; Ma, Z. HyPRP1 performs a role in negatively regulating cotton resistance to V. dahliae via the thickening of cell walls and ROS accumulation. BMC Plant Biol. 2018, 18, 339. [Google Scholar] [CrossRef]
- Wang, G.; Praphat, K.; Xie, G.; Zhu, B.; Li, B.; Liu, B.; Zhou, Q. Bacterial wilt of mulberry (Morus alba) caused by Enterobacter cloacae in China. Plant Dis. 2008, 92, 483. [Google Scholar] [CrossRef] [PubMed]
- Chatelet, D.S.; Wistrom, C.M.; Purcell, A.H.; Rost, T.L.; Matthews, M.A. Xylem structure of four grape varieties and 12 alternative hosts to the xylem-limited bacterium Xylella fastidious. Ann. Bot. 2011, 108, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Itoh, H.; Tatsumi, T.; Sakamoto, T.; Otomo, K.; Toyomasu, T.; Kitano, H.; Ashikari, M.; Ichihara, S.; Matsuoka, M. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol. Biol. 2004, 54, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Foo, E.; Ross, J.J.; Reid, J.B. Relationship between gibberellin, ethylene and nodulation in Pisum sativum. New Phytol. 2011, 189, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi-Tanaka, M.; Nakajima, M.; Motoyuki, A.; Matsuoka, M. Gibberellin receptor and its role in gibberellin signaling in plants. Annu. Rev. Plant Biol. 2007, 58, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Pérez-García, B.; Mendoza-Ruiz, A.; Espinosa-Matías, S.; Gómez-Pignataro, L.D. Gametophyte morphology of Platycerium andinum Baker and Platycerium wandae Racif. Micron 2010, 41, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhu, B. Evolutionary analysis of three gibberellin oxidase genesin rice, Arabidopsis, and soybean. Gene 2011, 473, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; An, H.; Luo, J.; Wang, X.; Shi, C.; Xu, F. Comparative analysis of transcriptomes to identify genes associated with fruit size in the early stage of fruit development in Pyrus pyrifolia. Int. J. Mol. Sci. 2018, 19, 2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cultivars | Latent Period (d) | Disease Incidence (%) | Disease Index | |||
|---|---|---|---|---|---|---|
| XJ27 | YS26 | XJ27 | YS26 | XJ27 | YS26 | |
| Dongkui | 5 | 5 | 100 a | 100 a | 100 a | 87.7 a |
| Zaojia | / | / | 0 b | 0 b | 0 b | 0 b |
| Primer Name | Gene Name | Nucleotide Sequence (5′–3′) | Putative Function of Target Gene | PCR Product |
|---|---|---|---|---|
| 015554-F | GA20ox1(GAS) | CACGATCCCCACGATTGACA | 2OG-Fe(II) oxygenase superfamily | 144 bp |
| 015554-R | GCCCTATGCTCCACGCTATT | |||
| 007568-F | GID1c- | CGTCGCCCTGATGGTACTTT | Gibberellin receptor GID1C GN | 88 bp |
| 007568-R | AAACTCCATCCACCGGCTTT | |||
| 013954-F | GID1b | AAATGAGGCTCAATGGGGCA | Gibberellin receptor GID1B GN | 142 bp |
| 013954-R | GCGGCGACAAAAAGTGTCAT | |||
| 017149-F | CPS(GA1) | ATGACACGGCATGGGTTTCT | Ent-copalyl diphosphate synthase | 117 bp |
| 017149-R | TATCGCCCCATGAACCATCG | |||
| 016673-F | GA20ox2 | CGTGCGGTGAGGAAATCTCT | Gibberellin 20 oxidase 1 GN | 123 bp |
| 016673-R | AGCTGGCCATCTGAAGTCTG | |||
| 010912-F | SNE | AGCGGCTCTACATGGTATGC | F-box protein SNE GN | 150 bp |
| 010912-R | ACGCATCACCGAGTCTTCTC | |||
| 010100-F | GA3ox3 | ACCGGGCGATGTGAAGATTT | Gibberellin 3-beta-dioxygenase 1 GN | 85 bp |
| 010100-R | TTCCTTCCATGTTACCGGGC | |||
| 019537-F | GA2ox1 | AATGGGAGGTTCCAAAGCGT | Gibberellin 2-beta-dioxygenase 1 GN | 98 bp |
| 019537-R | TTCTCACTCAAAGGCGGTCC | |||
| 003769-F | GA20ox5 | AGTCTCATTCGCGCTGCATA | Gibberellin 20 oxidase 2 OS | 127 bp |
| 003769-R | AATAACGGTCTGCATGGGCA | |||
| 012730-F | KAO1,2 | TTTCCTACAGCAGCACCCAG | Ent-kaurenoic acid oxidase 2 GN | 99 bp |
| 012730-R | TTCAGCGTCAACCCACTCTG | |||
| 011275-F | RGA | TTTCCTACAGCAGCACCCAG | DELLA protein GAIP-B | 99 bp |
| 011275-R | TTCAGCGTCAACCCACTCTG | |||
| 002577-F | KO(G3) | GCTTTGGGACATGACCTGGA | Ent-kaurene oxidase | 103 bp |
| 002577-R | CACCTTCCATTACGTCGGCT | |||
| 019330-F | GA20ox4 | TTCGAACAGACAGGAGTGGC | Gibberellin 20 oxidase 2 OS | 101 bp |
| 019330-R | CGATCGACTCCCAAGCTGAT | |||
| 005597-F | GA2ox7 | TCCTCTGGTCGGAAGCCTTA | gibberellin 2-beta-dioxygenase 8 | 116 bp |
| 005597-R | TAAGCTTTGAGCCAGGTCGG | |||
| 004052-F | RGL3 | CGGACAACACTGACGCTTTG | DELLA protein GAI | 132 bp |
| 004052-R | TATCGAGCATGGACGGTTCG | |||
| 001863-F | GA3ox4 | ATGGGTCTAGCAGCGCATAC | gibberellin 3 oxidase 1 | 106 bp |
| 001863-R | GAACCATGATCCACCCCGTT | |||
| 009378-F | KS(GAP2) | CTCTGTGATGGCTCATGGGG | ent-kaurene synthase | 110 bp |
| 009378-R | TCACCAACACCCCATTGCTT | |||
| 025327-F | GA2ox6 | CCGGCTCGTCCTTTTGGTTA | Gibberellin 2-beta-dioxygenase 2 GN | 140 bp |
| 025327-R | AATTTCGTCGGGTCGTTGGA | |||
| 021433-F | MrUBQ1 | AAGGCGAAGATCCAAGACAA | used as the internal reference | 116 bp |
| 021433-R | GTGGAGCGTCGACTCTTTCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Wu, Y.; Ahmed, T.; Qi, X.; Li, B. Response of Resistant and Susceptible Bayberry Cultivars to Infection of Twig Blight Pathogen by Histological Observation and Gibberellin Related Genes Expression. Pathogens 2021, 10, 402. https://doi.org/10.3390/pathogens10040402
Ren H, Wu Y, Ahmed T, Qi X, Li B. Response of Resistant and Susceptible Bayberry Cultivars to Infection of Twig Blight Pathogen by Histological Observation and Gibberellin Related Genes Expression. Pathogens. 2021; 10(4):402. https://doi.org/10.3390/pathogens10040402
Chicago/Turabian StyleRen, Haiying, Yangchun Wu, Temoor Ahmed, Xingjiang Qi, and Bin Li. 2021. "Response of Resistant and Susceptible Bayberry Cultivars to Infection of Twig Blight Pathogen by Histological Observation and Gibberellin Related Genes Expression" Pathogens 10, no. 4: 402. https://doi.org/10.3390/pathogens10040402







