Abstract
Monitoring cases of insecticide resistance aggravation and the effect on the efficacy of control tools is crucial for successful malaria control. In this study, the resistance intensity of major malaria vectors from Uganda was characterised and its impact on the performance of various insecticide-treated nets elucidated. High intensity of resistance to the discriminating concentration (DC), 5× DC, and 10× DC of pyrethroids was observed in both Anopheles funestus and Anopheles gambiae in Mayuge and Busia leading to significant reduced performance of long-lasting insecticidal nets (LLINs) including the piperonyl butoxide (PBO)-based nets (Olyset Plus). Molecular analysis revealed significant over-expression of cytochrome P450 genes (CYP9K1 and CYP6P9a/b). However, the expression of these genes was not associated with resistance escalation as no difference was observed in the level of expression in mosquitoes resistant to 5× DC and 10× DC compared to 1× DC suggesting that other resistance mechanisms are involved. Such high intensity of pyrethroid resistance in Uganda could have terrible consequences on the effectiveness of insecticide-based interventions and urgent action should be taken to prevent the spread of super-resistance in malaria vectors.
1. Introduction
Malaria remains a major public health problem, with about 229 million cases and 409,000 deaths recorded in 2019 mainly in children under five years [1]. Uganda accounts for the sixth-highest malaria burden in Africa with approximately 14 million cases recorded in 2017 leading to 10,500 annual deaths [2]. Both long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) are the key prevention measures in this country as in many other African countries. Unfortunately, these interventions are facing the challenge of insecticide resistance occurring in the main malaria vectors.
Increasing reports of widespread resistance to pyrethroids are being made for the major malaria vectors Africa-wide [3,4,5,6]. Furthermore, increased resistance levels are also been reported and has been suggested to potentially present a greater risk of control failure [7]. For example, a tremendous loss of performance of pyrethroid-only and piperonyl butoxide (PBO)-based nets (nets that contains the synergist PBO and the insecticide) in a resistant population of An. funestus s.s. was shown recently in southern Mozambique (Southern Africa) [4,8]. Similarly, a study in Western Africa (Burkina Faso revealed that increased resistance in An. gambiae was associated with the low efficacy of pyrethroid-only nets [9]. In Chad (Central Africa), a study conducted in An. coluzzii pointed to similar reduced efficacy of standard nets and the second generation net Olyset plus due to a high level of resistance [6]. Therefore, unless such super-resistance is managed, recent gains in reducing malaria transmission could be lost with terrible consequences. Monitoring cases of aggravation of resistance intensity and its impact on the efficacy of control tools is therefore crucial to predict consequences of resistance and to implement suitable control measures.
However, standardised methods have not always been used to monitor the extent of resistance in the field preventing to accurately quantify the resistance intensity and to establish the molecular drivers of such aggravation. Lack of routine quantification of resistance aggravation has also prevented to establish the impact of resistance on the efficacy of insecticide-based tools such as long lasting insecticidal nets (LLINs).
In that order, to harmonise the estimation of resistance levels, WHO issued a protocol relying on the testing of field populations with different concentration of insecticides at (1×, 5× and 10× concentration) [10]. This system now enables to better quantify the extent of resistance escalation in the field populations of mosquitoes and also facilitates the exploration of mechanisms driving that aggravation. Furthermore, it could also allow to evaluate the influence of super-resistance on the performance of insecticide-treated tools. Pyrethroid-based insecticides have been used in the agricultural sector in Uganda since the early 1990s and from 2000 to 2010 in IRS programs [2]. In 2014, a universal LLIN coverage campaign (one LLIN per two people) was attempted in the country and more than 22 million LLINs were distributed. IRS was implemented later from 2007 to 2014 in 10 districts in northern Uganda supporting by USAID/PMI and the Department for International Development (DFID). This strategy was extended later in other new highly malaria-endemic districts (14 in total) in the northern and eastern parts of the country [2]. This control approach coupled with the massive use of agricultural pesticides exert insecticidal pressure on local malaria vectors, which may accelerate the development and spread of insecticide resistance. Such a situation offers an ideal opportunity to quantify resistance intensity and its impact on vector tools to help improve resistance management strategies.
Here we have used the WHO method for resistance intensity measurement, to thoroughly and comparatively measure the extent of resistance escalation in two major malaria vectors, explore the potential molecular drivers behind such aggravation and establish the effect of such super-resistance on the efficacy of LLINs. Our study reveals a high intensity of resistance to pyrethroids in both species from the two locations with an extensive reduced performance of all pyrethroid-based LLINs tested, including PBO-based LLINs. This resistance escalation was associated with a high expression of key cytochrome P450 genes in An. funestus s.s. and a fixation of the 1014S-Kdr-E resistance allele in An gambiae.
2. Results
2.1. Sampling
In total, 25 houses were surveyed in Busia (Bumanji) and 35 in Mayuge (Bubbalya) (Table 1). The predominant type of house was rectangular with a corrugated iron roof in Busia (52%) followed by both circular hut with a thatched roof (24%) and rectangular hut with a thatched roof (24%). However, in Mayuge, the predominant type was rectangular huts with thatched roof (48.6%) followed by rectangular huts with a corrugated iron roof (40%) with only few (11.4%) circular huts with a thatched roof (Table S1). The main vector control measure in the collection sites was impregnated bed net. Table 1 summarises the brand of bed nets used in both collection sites. Out of the 25 houses surveyed in Busia, 23 had at least one bed net corresponding to the coverage rate of 92%. Of the 89 inhabitants living in those houses, only 53 (59%) were really sleeping under a net. The bed net coverage rate was almost the same in Mayuge (86%) but the protection rate was lower (56%) although not significant. Concerning the brands of nets used, both standard pyrethroid-only and PBO-based nets from the mass campaign of 2016–2017 were found in these villages. The Proportion of PBO-based nets was significantly higher in Mayuge (69%) compared to Busia (17%) (Table 1).

Table 1.
Household indices and brand nets from the collection site.
2.2. Species Composition
The predominant malaria vector in both sites from indoor collection using electric aspirators was the member of the An. funestus s.l group followed by An. gambiae s.l (Table S2). The density of mosquitoes was higher in Mayuge (1200 for four (4) days of collection) compared to Busia (900 after seven (7) days of collection) despite the high proportion of PBO-based net used in this village. The oviposition rate was the same in both sites for An. funestus and An. gambiae.
Molecular identification of 99 F0 An. funestus s.l mosquitoes (60 oviposited and 39 non-oviposited) from Mayuge by cocktail PCR revealed that 94% (93/99) were An. funestus s.s. Three An. rivolurum (3%) were detected only among the oviposited females whereas 3 (3%) An. leesoni was detected only among the non-oviposited females. All the 110 An. funestus s.l (79 oviposited and 31 non-oviposited) analysed in Busia were An. funestus s.s. All these results confirm that An. funestus s.s. is the predominant malaria vector in both sites at the time of collection.
From the 17 An. gambiae s.l successfully identified in Mayuge using SINE PCR, 14 (82.3%) were An. gambiae whereas the remaining 3 (17.7%) were An. arabiensis. In Busia, out of the 36 oviposited An. gambiae s.l PCR tested, a majority was An. gambiae s.s at 94.4% (34/36) whereas 5.6% (2/36) were An. arabiensis.
2.3. Plasmodium Sporozoite Infection Rate
The Plasmodium sporozoite-infection rate in oviposited An. funestus s.s. from Busia was 3.7% (3/79) and 6% (2/32) in non-oviposited females. In Mayuge, 6.8% (4/59) oviposited An. funestus s.s. were infected with Plasmodium compared to 8.3% (3/36) in non oviposited females. All the An. funestus s.s.were infected only with P. falciparum. In An. gambiae s.l. from Busia, 11% (3/36) of oviposited females were infected with P. falciparum sporozoites and one (01) infected with P. ovale/vivax/malariae (OVM+) (2.7%, 1/36). In Mayuge, 6% An. gambiae (1/17) were infected with P. falciparum.
2.4. Insecticide Susceptibility Assays
2.4.1. Bioassays with the Discriminating Concentration 1× (DC)
F1 progeny from field-collected females An. funestus s.s. in both locations showed an extremely high resistance to permethrin and deltamethrin (Figure 1A). F1 females from Mayuge showed 17.5 ± 4.2% and 29.7 ± 9.08% mortality 24 h after exposure to permethrin 1× and deltamethrin 1× (DC) respectively (Figure 1A). In An funestus s.s from Busia, mortality rates of 37.5 ± 10.5% and 14.08 ± 3.7% were recorded 24 h post-exposure to both pyrethroids tested using the DC (permethrin 1× and deltamethrin 1×) (Figure 1A) while F1 females An. gambiae s.l showed 43.6 ± 6.8% and 18.7 ± 2.1% mortality to permethrin 1× in Busia and Mayuge respectively (Figure 1B). F1 females from both sites presented probable resistance to carbamate, bendiocarb 1× with mortalities of 93.2 ± 4.4%, and 98.3 ± 1.7% in Mayuge and Busia respectively (Figure 1A). Resistance was also noted for the organochlorine, dichlorodiphényltrichloroéthane (DDT) in both sites with mortalities of 78.4 ± 6.01% and 17.2 ± 10.5% in Mayuge and Busia respectively (Figure 1A). However, full susceptibility was observed with the organophosphate, pyrimiphos-methyl 1× with a 100% mortality rate. Mortality rates when exposed to the new insecticide clothianidin 24 h post-exposure were 66.05 ± 5.5% and 73.2 ± 7.7% for Mayuge and Busia respectively (Figure 1A). However, a full susceptibility was observed for this insecticide 72 h post-exposure in both sites with a mortality rate of 100%.
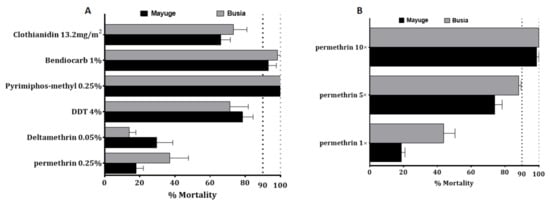
Figure 1.
Susceptibility profile to main insecticides of An. funestus and An. gambiae s.l populations from Busia and Mayuge: (A) susceptibility profile of An. funestus; (B) Susceptibility profile and intensity of An. gambiae s.l. Error bars represent standard error of the mean (SEM).
2.4.2. Bioassays with Pyrethroid 5× and 10× DC
Because of the high level of resistance observed to pyrethroids, intensity bioassays were carried out with 5× DC and 10× DC of permethrin (3.75% and 7.5%) and deltamethrin (0.25% and 0.5%). An funestus s.s from Mayuge exhibited a mortality rate of 62.4 ± 6.4% and 86.9 ± 3.7% to permethrin 5× and 10× respectively (Figure 2A) pointing a high intensity of resistance to permethrin in this locality. However, a significant increased mortality of An. funestus s.s. was observed from permethrin 1× to 5× (χ2 = 40.6, p ˂ 0.0001) and 10× (χ2 = 95.7, p ˂ 0.0001). A mortality of 54.7 ± 2.6% and 65.1 ± 8.6% was observed after exposure to deltamethrin 5× and 10× respectively indicating also a high intensity of resistance to deltamethrin in Mayuge (Figure 2A). Similar observations were made in Busia with mortality rates of 86.4 ± 5.2% and 90.9 ± 2.4% (for permethrin 5× and 10×) and 50.02 ± 3.01% and 60.1 ± 5.9% (for deltamethrin 5× and 10×) (Figure 2B). However, An. gambiae s.l displayed a moderate intensity of resistance to permethrin in both locations with mortality of 88.06 ± 1.4% and 100% to permethrin 5× and 10× respectively in Busia and 73.9 ± 4.3% for permethrin 5× and 98.5 ± 1.4% for permethrin 10× in Mayuge (Figure 1B).
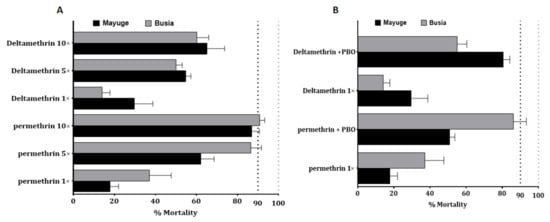
Figure 2.
Resistance intensity and synergist assay of An. funestus populations from Busia and Mayuge: (A) Determination of resistance intensity with 5× and 10× the diagnostic concentrations of permethrin and deltamethrin. Results are average of percentage mortalities ± SEM; (B) Effect of pre-exposure to synergist PBO against permethrin and deltamethrin. Results are average of percentage mortalities from four replicates each ± SEM.
2.4.3. Bioassays with the Synergist PBO
Bioassay using PBO as a synergist revealed a partial recovery of susceptibility to permethrin and deltamethrin. In Mayuge the mortality with permethrin + PBO was 50.9 ± 2.3% versus 17.8 ± 4.2% for permethrin without PBO pre-exposure (χ2 = 25.7 p < 0.0001). The same pattern was observed for deltamethrin: PBO pre-exposure 80.6 ± 3.5% vs. 29.7 ± 9.08% for no PBO pre-exposure (χ2 = 51.2 p < 0.0001) (Figure 2B). Synergist bioassay in Busia also revealed partial recovery of susceptibility to both pyrethroids [permethrin: PBO pre-exposure 86.2 ± 7.1% versus 37.1 ± 10.5% mortality without PBO pre-exposure (χ2 = 30.9 p < 0.0001); deltamethrin: PBO pre-exposure 55.07 ± 5.3% vs. 14.08 ± 3.7% mortality for no PBO pre-exposure (χ2 = 24.1 p < 0.0001)] (Figure 2B). These results show that cytochrome P450s are playing only a partial role in the escalation of resistance observed in Busia and Mayuge.
2.5. Bioefficacy of Insecticide-Treated Bed Nets
To evaluate the impact of super resistance on the efficacy of control tools, cone assays were conducted with various LLINs. In both localities, low efficacy of standard pyrethroid-only nets (Olyset and PermaNet 2.0) was observed against An. funestus s.s.: Olyset: 56.7 ± 3.1% and 36.7 ± 12.1% mortality for Busia and Mayuge respectively; PermaNet 2.0: 22.5 ± 13.1% and 11.4 ± 6.9% mortality for Mayuge and Busia respectively. However, PBO-based nets (Olyset Plus, and PermaNet 3.0) showed an increased efficacy (Olyset Plus: 90.8 ± 3.5% mortality; PermaNet 3.0-side: 24.7 ± 12.0%, PermaNet 3.0-roof: 100.0 ± 0.0%) in Busia (Figure 3). In contrast, in Mayuge increased susceptibility was noted only for PermaNet 3.0-roof (100.0 ± 0.0% mortality). A significantly reduced efficacy of the PBO-net Olyset plus (48.5 ± 1.5% mortality) was observed in this site confirming the very low mortality recorded with Permethrin + PBO in this site. The mortality with PermaNet 3.0 side did not differ significantly to that of PermaNet 2.0 (24.7 ± 12% vs. 22.5 ± 12% in Mayuge (χ2 = 0.07; p = 0.8) but in Busia (37.4 ± 11.5% vs. 11.4 ± 6.9%; χ2 = 9.07; p = 0.003)) indicating the high intensity of resistance in these locations particularly in Mayuge. Pyrethroid-only and PBO-nets used in this study induced total mortality against the control Kisumu susceptible An. gambiae mosquitoes (Figure 3).
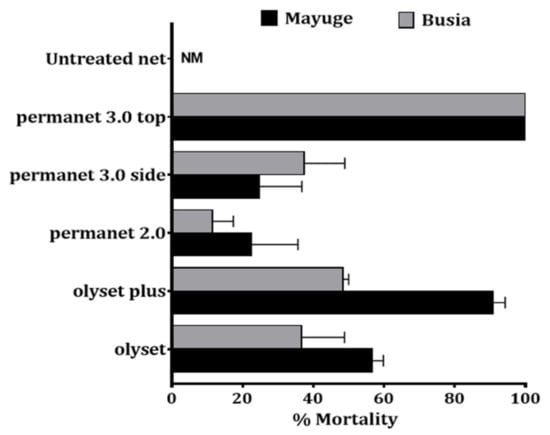
Figure 3.
Bio-efficacy of different commercial LLINs against An. funestus in Busia and Mayuge. Results of cone bioassays with PermaNet®3.0 (side and roof), PermaNet®2.0, Olyset®Plus and Olyset®Net. Results are average of percentage mortalities ± SEM of five replicates. NM = no mortality.
2.6. Distribution of Insecticide Resistance Markers in An. funestus
In total, 86 females An. funestus s.s (50 oviposited and 36 non-oviposited) from Mayuge and 82 (50 oviposited and 32 non-oviposited) from Busia were genotyped for different resistance markers. In Busia, out of 40 oviposited females which successfully amplified for the L119F mutation, only one (1) were homozygote resistant, six (06) were heterozygotes and 33 were homozygote susceptibles corresponding to the frequency of 10%. In non-oviposited, the frequency of this mutation was 9.6% (0 RR, 5 RS, and 21 SS) (Figure 4A). In Mayuge, the frequency of this mutation was 11.5% (0RR, 9RS, and 30SS) in oviposited females and 10.3% (2RR, 2RS, and 29 SS) in non-oviposited females (Figure 4A). A significant association was found in the ability of mosquitoes with the 119F resistant allele to survive exposure to deltamethrin 1× (OR = 2.7; confidence interval (CI) = 1.3–5.6; p = 0.004) but not the 5× (OR = 1.7; confidence interval (CI) = 0.9–3.4; p = 0.07) and 10× (OR = 0.5; confidence interval (CI) = 0.3–1.2; p = 0.08) (Figure 4B). This shows that the mutation is linked with deltamethrin resistance but not implicated in the escalation of resistance.
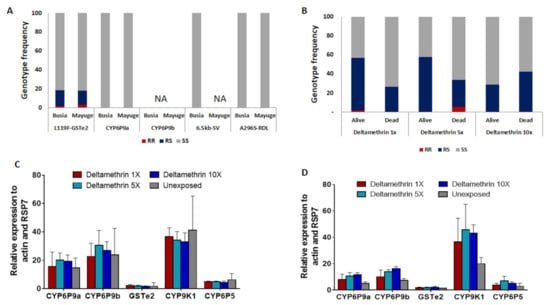
Figure 4.
Analysis of the molecular basis of the escalation of pyrethroid resistance in An. funestus: Distribution of the genotypes for key resistance markers in F0 female An. funestus from Eastern Uganda including CYP6P9a, CYP6P9b, L119F-GSTe2, and A296S-RDL showing low frequency of resistant genotypes (A) and Distribution of the genotypes at the L119F-GSTe2 locus between mosquitoes alive and dead after exposition to deltamethrin 1×, 5× and 10× (B). Differential gene expression of the P450 genes CYP6P9a, CYP6P9b, CYP9K1, and CYP6P5 and the Gluthatione S-tranferase GSTe2 in An. funestus from Mayuge (C) and Busia (D), error bars represent standard error of the mean.
The A296S-RDL mutation conferring dieldrin resistance [11] and the CYP6P9a_R allele conferring pyrethroid resistance were completely absent in oviposited and non oviposited females from both locations (Figure 4A). The PCR-RFLP recently designed to detect the resistant allele at the CYP6P9b locus and a multiplex PCR designed for the 6.5 kb-sv failed to amplify (Figure 4A).
2.7. Distribution of Insecticide Resistance Markers in An. gambiae
The L1014F-KdrW mutation was completely absent in both An. gambiae and An. arabiensis from Mayuge and Busia. However, the L1014S-KdrE mutation was fixed in An. gambiae (100%RR) and completely absent in An. arabiensis. The N1575Y-kdr mutation conferring pyrethroid resistance and the G119S-Ace1 mutation conferring carbamate resistance were completely absent in both species in both localities.
2.8. Transcriptional Profiling Of Metabolic Resistance Genes in An. funestus s.s.
Transcription analysis of CYP9K1 and the duplicated P450 genes CYP6P9a and CYP6P9b known to confer pyrethroid resistance in An. funestus [12,13] reveals a high up-regulation of these genes in deltamethrin resistant mosquitoes from both site particularly the CYP9K1 (fold change, FC = 36.7 ± 10.3; FC = 34.15 ± 6.1; FC = 32.9 ± 6.3; FC = 41.1 ± 24.1 for deltamethrin alive 1×, 5×, 10× and unexposed respectively) and CYP6P9b (fold change, FC = 22.7 ± 9.3; FC = 30.4 ± 10.6; FC = 26.8 ± 6.2; FC = 23.7 ± 18.7 deltamethrin alive 1×, 5×, 10× and unexposed respectively) compared to the susceptible FANG strain (p < 0.001) (Figure 4C). However, the expression of these genes was not statistically different between mosquitoes alive to DC, 5× DC, and 10× DC of deltamethrin showing that these genes might not play a major role in the increased resistance. The CYP6P5 and the GSTe2 (conferring DDT/permethrin resistance in West/Central Africa [14]) were not significantly upregulated in Uganda (Figure 4D).
3. Discussion
Worrying cases of high resistance levels to insecticides are emerging in major malaria vectors leading to extensive loss of efficacy of current and probably future vector control tools. Quantifying the resistance intensity and establishing the molecular drivers of such resistance escalation is crucial for designing resistance management strategies and prevent malaria resurgence.
This study revealed a high intensity of resistance to pyrethroids with significantly reduced efficacy of insecticidal treated LLINs including standard nets (Olyset® Net and PermaNet® 2.0) and PBO-based net (Olyset plus) in An. funestus s.s. from Eastern Uganda. Similar results were obtained recently in An. funestus s.s. from Southern Mozambique revealing a very low efficacy of the two most common commercial LLINs used across Africa, Olyset® Net, and PermaNet® 2.0 [4,8]. The same loss was also reported Malawi (<5% mortality) [15] and in Democratic Republic of Congo (<35% mortality) [3]. This loss in efficacy of bednets in Mayuge and Busia was in line with the results of WHO tube assays, pointing a high intensity of resistance to permethrin and deltamethrin, the insecticides used in these nets. These An. funestus populations were resistant to permethrin and deltamethrin at all diagnostic concentrations of 1×, 5×, and 10×. However, the An. gambiae s.l populations from both sites are resistant to permethrin at 1× and 5× but not 10×. These results for An. gambiae is similar to the observations of Okia et al. (2018) in Tororo [2]. However, this study is the first evidence of resistance escalation in An. funestus in Uganda using the 5× and 10× DC of pyrethroids. However, the high level of resistance to the diagnostic dose (1×) of pyrethroids in An. funestus is similar to the temporal increase in pyrethroid resistance observed in Eastern Uganda between 2009 and 2013 [16]. Probable resistance was observed to bendiocarb in An. funestus population from both locations showing that the carbamate insecticides could not be an alternative to pyrethroid for IRS. Furthermore, the full susceptibility to the organophosphate pyrimiphos-methyl, as observed in many other African countries across the continent, suggests that this insecticide class is the most suitable for IRS against this species and justifies the use of Arctellic for IRS in many districts in Uganda.
This study reported low mortality after exposure to permethrin+PBO and deltamethrin + PBO together with the loss in the efficacy of the new generation of PBO-based nets particularly the Olyset plus. This reduced efficacy of PBO-based tools could be linked to the fact that PBO-net are already in use in the study site and could be contributing to the selection of other resistance mechanisms beyond cytochrome P450s. The ability of mosquitoes to survive exposure to the high intensity of pyrethroids and PBO-based nets in this population is problematic for malaria control programs. These observations are similar to the results obtained with An. funestus in Cameroon [17] and An. coluzzi in Chad [6] for which no mortality was noticed after exposure to the synergist net Olyset plus. However, the reduced efficacy of PBO nets has not previously been observed in An. funestus as higher mortality rates (>80%) have so far been reported when testing PBO-based nets (Olyset Plus) against other pyrethroid-resistant populations like in Malawi [15] and DR Congo [3]. The PermaNet 3.0 Top (containing PBO) showed a higher efficacy with 100% mortality. Nevertheless, the low mortality with PermaNet 3.0 (side) obtained in this study is similar to the results obtained in Cameroon [17], Mozambique [4], and Chad [6] but contrary to the higher mortality rate (88%) observed in DRC [3] suggesting also an overall loss of efficacy of PermaNet 3.0 in Uganda. The causes of such high resistance could be associated with the scale-up of LLINs distribution across the country as PB0-nets were present in both locations. The massive use of pyrethroids in agriculture in Uganda could be another factor in selecting for resistance in malaria vectors [2].
Genotyping of resistant markers showed the absence of CYP6P9a_R resistant allele, the predominant pyrethroids resistance marker in southern Africa [18] showing that other mechanisms are responsible for pyrethroids resistance in these An. funestus mosquitoes. Furthermore, the moderate resistance to DDT supports the low frequency of L119F-GSTe2 in these populations compared to West and Central Africa. The CYP9K1 was the most over-expressed gene in deltamethrin-resistant mosquitoes from both localities showing that this gene is likely the main driver of pyrethroid resistance as previously shown for the ortholog of this gene in An. gambiae [19,20]. However, the expression of this gene did not vary significantly between resistant mosquitoes at 10× and 5× compared to 1× showing that other mechanisms are playing a major role in the escalation of resistance in these An. funestus populations. The duplicated CYP6P9a and CYP6P9b were also overexpressed but at a reduced level compared to southern Africa (Mozambique and Malawi) where these genes are highly over-expressed [5,13,14]. The GSTe2 gene conferring DDT resistance in West/Central Africa was also significantly over-expressed in both localities but at very low level compared to Benin with more than 80 fold-change observed in resistant mosquitoes [14]. Nevertheless, the expression level of the gene was higher than the four (4)-fold change observed in Uganda in 2014 [16].
The sporozoite infection rates of An. funestus and An. gambiae populations of Busia and Mayuge is similar to the rates generally reported in this country [16,21] and across the continent. However, the infection rate observed in these two locations was higher compared to the areas of the country where IRS have been implemented such as Tororo where a recent study showed a significant reduction in plasmodium infection rate [22]. Therefore, the high infection rates of An. gambiae and An. funestus in Busia and Mayuge highlights the need of giving further attention to both species when it comes to vector control. This observation also may indicate that IRS with organophosphate could be the most appropriate control intervention compared to LLINS (impregnated with pyrethroid mainly).
4. Materials and Methods
4.1. Mosquito Collection
Indoor resting and blood-fed female Anopheles mosquitoes were collected in two districts in Eastern Uganda. Initially, four districts were selected (Tororo, Busia, Jinja, and Mayuge), and due to the low density of mosquitoes, collections were performed mainly in two districts (Busia and Mayuge). In each district, one village was chosen: Bumanji (0°27′08.4″ N, 34°06′38.1″ E) in Busia and Bubbalya (0°23′10.8″ N, 33°37′16.5″ E) in Mayuge. The collection was performed for 7 days in Busia and 4 consecutive days in Mayuge in February 2020. Mosquitoes were collected using electric aspirators after recording the information on the types of houses and the bed-nets use.
Anopheles females mosquitoes collected were morphologically identified as belonging to An. funestus group or An. gambiae s.l complex according to morphological keys [23]. These mosquitoes were kept in carton cups and fed with sugar until they became fully gravid before forced egg-laying in 1.5 mL micro-centrifuge tubes and larvae reared to adults as previously described [24].
4.2. Molecular Identification of Field-Collected Females
Oviposited and non-oviposited females An. funestus s.l. and An. gambiae s.l were cutted into head/thorax and abdomen for genomic DNA (gDNA) extraction using the Livak method [25]. A cocktail polymerase chain reaction (PCR) was used for species identification of An. funestus members as previously described [26]. The SINE PCR assay [27] was used for the identification of the An. gambiae species.
4.3. Plasmodium Infection Rates
Plasmodium sporozoite infection rate was assessed using TaqMan assay in heads plus thoraxes gDNA from F0 An. funestus s.s. and An. gambiae s.l as previously described [28].
4.4. Insecticide Susceptibility Assays
The susceptibility pattern of both An. funestus and An gambiae s.l to various insecticides were assessed using the WHO-tube bioassays [10]. An. funestus s.s. mosquitoes from both locations were tested to the pyrethroids type I permethrin (0.75%) and type II deltamethrin (0.05%), the organochlorine DDT (4%), the carbamate bendiocarb (0.1%), and the organophosphate pyrimiphos-methyl (0.25%). In addition, the new insecticide, clothianidin, was also tested using the SumiShield formulated product at a dosage of 13.2 mg/m2. Because of the low number of An. gambiae s.l collected, tests were performed only for permethrin. All the tests were performed at standard insectary conditions of 25 ± 1 ° C temperature and 70–80% relative humidity. For each test, four replicates of 20–25 F1 female mosquitoes of 2–5 day-old were exposed to insecticide-impregnated papers for 1 h. After the exposure, mosquitoes were transferred to a holding tube provided with cotton soaked in a 10% sugar solution. Mortality was determined 24 h later. Control tubes with non-impregnated papers were performed for each bioassay.
Based on the results of resistance status with 1× (discriminating concentration (DC)) of pyrethroid (permethrin and deltamethrin), intensity bioassays were carried out with 5× DC and 10× DC of these insecticides. The intensity bioassays with 5× and 10× DC were performed following the WHO 2016 test procedure [10]. Synergist assays with piperonyl butoxide (PBO; an inhibitor of cytochrome P450s) were performed for the potential involvement of P450′s genes.
4.5. Insecticide-Treated Bed Nets Efficacy Assays
The effectiveness of the LLINs was estimated following the WHO guidelines for cone bioassays [29]. The nets tested were Olyset® Net (permethrin 2%) and Olyset® Plus net roof (permethrin 2% plus PBO 1% in the roof) for the PBO-nets; PermaNet® 2.0 (deltamethrin 0.18%) and PermaNet® 3.0 side (deltamethrin 0.28%) for the standard nets. An untreated mosquito net was used as a control. Five replicates of ten F1 2–5 days old females were placed in plastic cones enclosed with the mosquito net for 3 min. Mosquitoes were then transferred in small holding paper cups with cotton soaked in a 10% sugar solution. Mortality was determined 24 h later.
4.6. Genotyping of Resistance Markers in An. funestus s.s.
The presence of resistance markers including L119F-GSTe2 (DDT/permethrin), CYP6P9a, CYP6P9b, 6.5kb-SV (pyrethroid), and A296S-RDL (dieldrin) was assessed in An. funestus s.s. The A296S-RDL mutations were genotyped using TaqMan assays as previously described [15], an allele-specific PCR (AS-PCR) was used to genotype the L119F-GSTe2 [30,31] whereas the presence of the CYP6P9a/b_R allele was assessed using PCR-RFLP assays recently designed [18,32]. Finally, the 6.5kb-SV was genotyped using a multiplex PCR [33].
Furthermore, the L119F mutation was genotyped in deltamethrin alive and dead (1×, 5×, and 10×) to assess a potential association between this marker and the aggravation of deltamethrin resistance.
4.7. Genotyping of Resistance Markers in An. gambiae.s.l
TaqMan assays with two labeled fluorochromes probes FAM and HEX were used to screen for the L1014F and L1014S-kdr [28] and the N1575Y [28] mutation associated with DDT and pyrethroid resistance in An. gambiae s.l. Further, the G119S-ace-1 responsible for organophosphate and carbamate resistance in An. gambiae s.l. was also genotyped in Mayuge and Busia mosquitoes using TaqMan assays.
4.8. Transcription Profile of Resistance Genes in An. funestus s.s.
The transcription patterns of CYP9K1, CYP6P5, CYP6P9a, CYP6P9b, and GSTe2) major pyrethroid resistance genes [34] were assessed by a quantitative reverse transcription PCR (qRT-PCR) in deltamethrin 1× alive, 5× alive, 10× alive and unexposed mosquitoes relatively to the susceptible strain FANG. Total RNA was extracted from 3 batches of 10 mosquitoes for each group and similarly from the susceptible laboratory strain FANG, as previously described [12]. The relative expression was calculated individually according to the 2−ΔΔCT method [35] and compared between different groups.
5. Conclusions
The extremely high intensity of resistance coupled with the loss of efficacy of impregnated bed nets against An. funestus s.s. in Uganda represents a serious threat for vector control in Uganda. The common resistant P450-based mechanisms were not found to play a role in the escalation of resistance highlighting the urgent need to investigate the causes such super-resistance and to monitor the spread of such operationally significant resistance in other mosquito populations and assess its impact on malaria transmission.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10040415/s1, Table S1: Type of houses in the collection sites Table S2: Number of mosquitoes collected and the oviposition rate per site.
Author Contributions
C.S.W. designed the study; M.T. (Magellan Tchouakui), and J.N.T.K. collected the samples on the field; M.T. (Magellan Tchouakui), W.T., L.M.J.M. and B.D.M. performed the resistance testing in the insectary, M.T. (Magellan Tchouakui). M.T. (Micareme Tchoupo), L.M.J.M. and M.J.W. performed the molecular experiments; M.T. (Magellan Tchouakui) and C.S.W. analyzed the data; M.T. (Magellan Tchouakui) and C.S.W. wrote the manuscript with contribution from all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Wellcome Trust Senior Research Fellowship in Biomedical Sciences to Charles S. Wondji (217188/Z/19/Z).
Data Availability Statement
All the data is present in the manuscript and supplemental files.
Conflicts of Interest
All the authors declare that there is no conflict of interest concerning the research conducted and the publication of the results obtained.
References
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Okia, M.; Hoel, D.F.; Kirunda, J.; Rwakimari, J.B.; Mpeka, B.; Ambayo, D.; Price, A.; Oguttu, D.W.; Okui, A.P.; Govere, J. Insecticide resistance status of the malaria mosquitoes: Anopheles gambiae and Anopheles funestus in eastern and northern Uganda. Malar. J. 2018, 17, 1–12. [Google Scholar] [CrossRef]
- Riveron, J.M.; Watsenga, F.; Irving, H.; Irish, S.R.; Wondji, C.S. High Plasmodium Infection Rate and Reduced Bed Net Efficacy in Multiple Insecticide-Resistant Malaria Vectors in Kinshasa, Democratic Republic of Congo. J. Infect. Dis. 2018, 217, 320–328. [Google Scholar] [CrossRef]
- Riveron, J.M.; Huijben, S.; Tchapga, W.; Tchouakui, M.; Wondji, M.J.; Tchoupo, M.; Irving, H.; Cuamba, N.; Maquina, M.; Paaijmans, K.; et al. Escalation of Pyrethroid Resistance in the Malaria Vector Anopheles funestus Induces a Loss of Efficacy of Piperonyl Butoxide–Based Insecticide-Treated Nets in Mozambique. J. Infect. Dis. 2019, 220, 467–475. [Google Scholar] [CrossRef]
- Tchouakui, M.; Fossog, B.T.; Ngannang, B.V.; Djonabaye, D.; Tchapga, W.; Njiokou, F.; Wondji, C.S. Investigation of the influence of a glutathione S-transferase metabolic resistance to pyrethroids/DDT on mating competitiveness in males Anopheles funestus, African malaria vector. Wellcome Open Res. 2019, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Fadel, A.N.; Tchouakui, M.; Terence, E.; Wondji, M.J.; Tchoupo, M.; Kérah-Hinzoumbé, C.; Wanji, S.; Wondji, C.S. High insecticide resistance in the major malaria vector Anopheles coluzzii in Chad Republic. Infect. Dis. Poverty 2019, 8, 1–12. [Google Scholar] [CrossRef]
- Hemingway, J. The way forward for vector control. Science 2017, 358, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Glunt, K.D.; Abilio, A.P.; Bassat, Q.; Bulo, H.; Gilbert, A.E.; Huijben, S.; Manaca, M.N.; Macete, E.; Alonso, P.; Paaijmans, K.P. Long-lasting insecticidal nets no longer effectively kill the highly resistant Anopheles funestus of southern Mozambique. Malar. J. 2015, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Toé, K.H.; Jones, C.M.; N’Fale, S.; Ismail, H.M.; Dabiré, R.K.; Ranson, H. Increased Pyrethroid Resistance in Malaria Vectors and Decreased Bed Net Effectiveness, Burkina Faso. Emerg. Infect. Dis. 2014, 20, 1691–1696. [Google Scholar] [CrossRef]
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Wondji, C.S.; Dabire, R.K.; Tukur, Z.; Irving, H.; Djouaka, R.; Morgan, J.C. Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect Biochem. Mol. Biol. 2011, 41, 484–491. [Google Scholar] [CrossRef]
- Riveron, J.M.; Irving, H.; Ndula, M.; Barnes, K.G.; Ibrahim, S.S.; Paine, M.J.I.; Wondji, C.S. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 2012, 110, 252–257. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Riveron, J.M.; Bibby, J.; Irving, H.; Yunta, C.; Paine, M.J.I.; Wondji, C.S. Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector. PLoS Genet. 2015, 11, e1005618. [Google Scholar] [CrossRef]
- Riveron, J.M.; Yunta, C.; Ibrahim, S.S.; Djouaka, R.; Irving, H.; Menze, B.D.; Ismail, H.M.; Hemingway, J.; Ranson, H.; Albert, A.; et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014, 15, R27. [Google Scholar] [CrossRef]
- Riveron, J.M.; Chiumia, M.; Menze, B.D.; Barnes, K.G.; Irving, H.; Ibrahim, S.S.; Weedall, G.D.; Mzilahowa, T.; Wondji, C.S. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: A major concern for malaria vector control. Malar. J. 2015, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mulamba, C.; Riveron, J.M.; Ibrahim, S.S.; Irving, H.; Barnes, K.G.; Mukwaya, L.G.; Birungi, J.; Wondji, C.S. Widespread Pyrethroid and DDT Resistance in the Major Malaria Vector Anopheles funestus in East Africa Is Driven by Metabolic Resistance Mechanisms. PLoS ONE 2014, 9, e110058. [Google Scholar] [CrossRef] [PubMed]
- Menze, B.D.; Wondji, M.J.; Tchapga, W.; Tchoupo, M.; Riveron, J.M.; Wondji, C.S. Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malar. J. 2018, 17, 317. [Google Scholar] [CrossRef]
- Weedall, G.D.; Mugenzi, L.M.J.; Menze, B.D.; Tchouakui, M.; Ibrahim, S.S.; Amvongo-Adjia, N.; Irving, H.; Wondji, M.J.; Tchoupo, M.; Djouaka, R.; et al. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Transl. Med. 2019, 11, eaat7386. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.; Grigoraki, L.; Morgan, J.; Tsakireli, D.; Fuseini, G.; Segura, L.; De Carvalho, J.N.; Nguema, R.; Weetman, D.; Slotman, M.A.; et al. Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc. Natl. Acad. Sci. USA 2018, 115, 4619–4624. [Google Scholar] [CrossRef]
- Oumbouke, W.A.; Pignatelli, P.; Barreaux, A.M.G.; Tia, I.Z.; Koffi, A.A.; Alou, L.P.A.; Sternberg, E.D.; Thomas, M.B.; Weetman, D.; N’Guessan, R. Fine scale spatial investigation of multiple insecticide resistance and underlying target-site and metabolic mechanisms in Anopheles gambiae in central Côte d’Ivoire. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Mulamba, C.; Irving, H.; Riveron, J.M.; Mukwaya, L.G.; Birungi, J.; Wondji, C.S. Contrasting Plasmodium infection rates and insecticide susceptibility profiles between the sympatric sibling species Anopheles parensis and Anopheles funestus s.s: A potential challenge for malaria vector control in Uganda. Parasites Vectors 2014, 7, 71. [Google Scholar] [CrossRef]
- Musiime, A.K.; Smith, D.L.; Kilama, M.; Rek, J.; Arinaitwe, E.; Nankabirwa, J.I.; Kamya, M.R.; Conrad, M.D.; Dorsey, G.; Akol, A.M.; et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar. J. 2019, 18, 445–449. [Google Scholar] [CrossRef]
- Gillies, M.; Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara. Publ. S. Afr. Inst. Med. Res. 1987, 55, 1–143. [Google Scholar]
- Morgan, J.C.; Irving, H.; Okedi, L.M.; Steven, A.; Wondji, C.S. Pyrethroid Resistance in an Anopheles funestus Population from Uganda. PLoS ONE 2010, 5, e11872. [Google Scholar] [CrossRef]
- Livak, K.J. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar] [CrossRef] [PubMed]
- Koekemoer, L.L.; Kamau, L.; Coetzee, M.; Hunt, R.H. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 2002, 66, 804–811. [Google Scholar] [CrossRef]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; Della Torre, A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Blagborough, A.M.; Vontas, J.; E Sinden, R.; Williamson, M.S.; Field, L.M. PCR-based detection of Plasmodium in Anopheles mosquitoes: A comparison of a new high-throughput assay with existing methods. Malar. J. 2008, 7, 177. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field-Testing of long-Lasting Insecticidal Nets; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Tchouakui, M.; Riveron, J.M.; Djonabaye, D.; Tchapga, W.; Irving, H.; Takam, P.S.; Njiokou, F.; Wondji, C.S. Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles Funestus. Genes 2018, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Chiang, M.-C.; Ndo, C.; Kuicheu, C.K.; Amvongo-Adjia, N.; Wondji, M.J.; Tchoupo, M.; Kusimo, M.O.; Riveron, J.M.; Wondji, C.S. A marker of glutathione S-transferase-mediated resistance to insecticides is associated with higher Plasmodium infection in the African malaria vector Anopheles funestus. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Mugenzi, L.M.J.; Menze, B.D.; Tchouakui, M.; Wondji, M.J.; Irving, H.; Tchoupo, M.; Hearn, J.; Weedall, G.D.; Riveron, J.M.; Wondji, C.S. Cis-regulatory CYP6P9b P450 variants associated with loss of insecticide-treated bed net efficacy against Anopheles funestus. Nat. Commun. 2019, 10, 4652. [Google Scholar] [CrossRef] [PubMed]
- Mugenzi, M.M.; Benjamin, M.D.; Magellan, T.; Murielle, W.J.; Helen, I.; Micareme, T.; Jack, H.; Gareth, D.W.; Jacob, M.R.; Fidelis, C.-N.; et al. A 6.5 kb intergenic structural variation enhances P450-mediated resistance to pyrethroids in malaria vectors lowering bed net efficacy. BioRxiv 2020, 29, 4395–4411. [Google Scholar] [CrossRef] [PubMed]
- Sandeu, M.M.; Mulamba, C.; Weedall, G.D.; Wondji, C.S. A differential expression of pyrethroid resistance genes in the malaria vector Anopheles funestus across Uganda is associated with patterns of gene flow. PLoS ONE 2020, 15, e0240743. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).