Association of Bovine Leukemia Virus-Induced Lymphoma with BoLA-DRB3 Polymorphisms at DNA, Amino Acid, and Binding Pocket Property Levels
Abstract
:1. Introduction
2. Results
2.1. BoLA-DRB3 Genotyping in Asymptomatic and Lymphoma Cattle
2.2. Association Study of BoLA-DRB3 with BLV-Induced Lymphoma
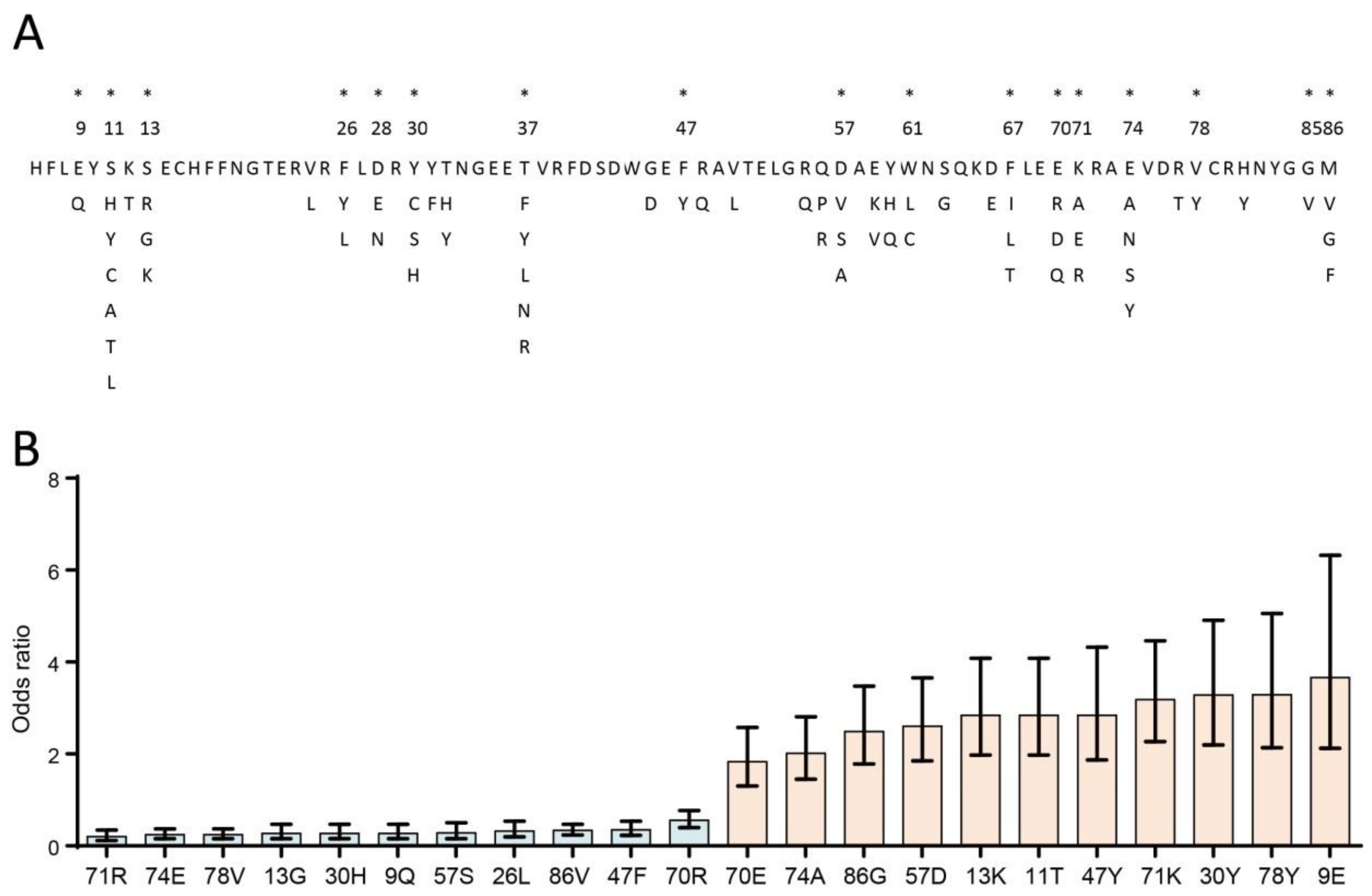
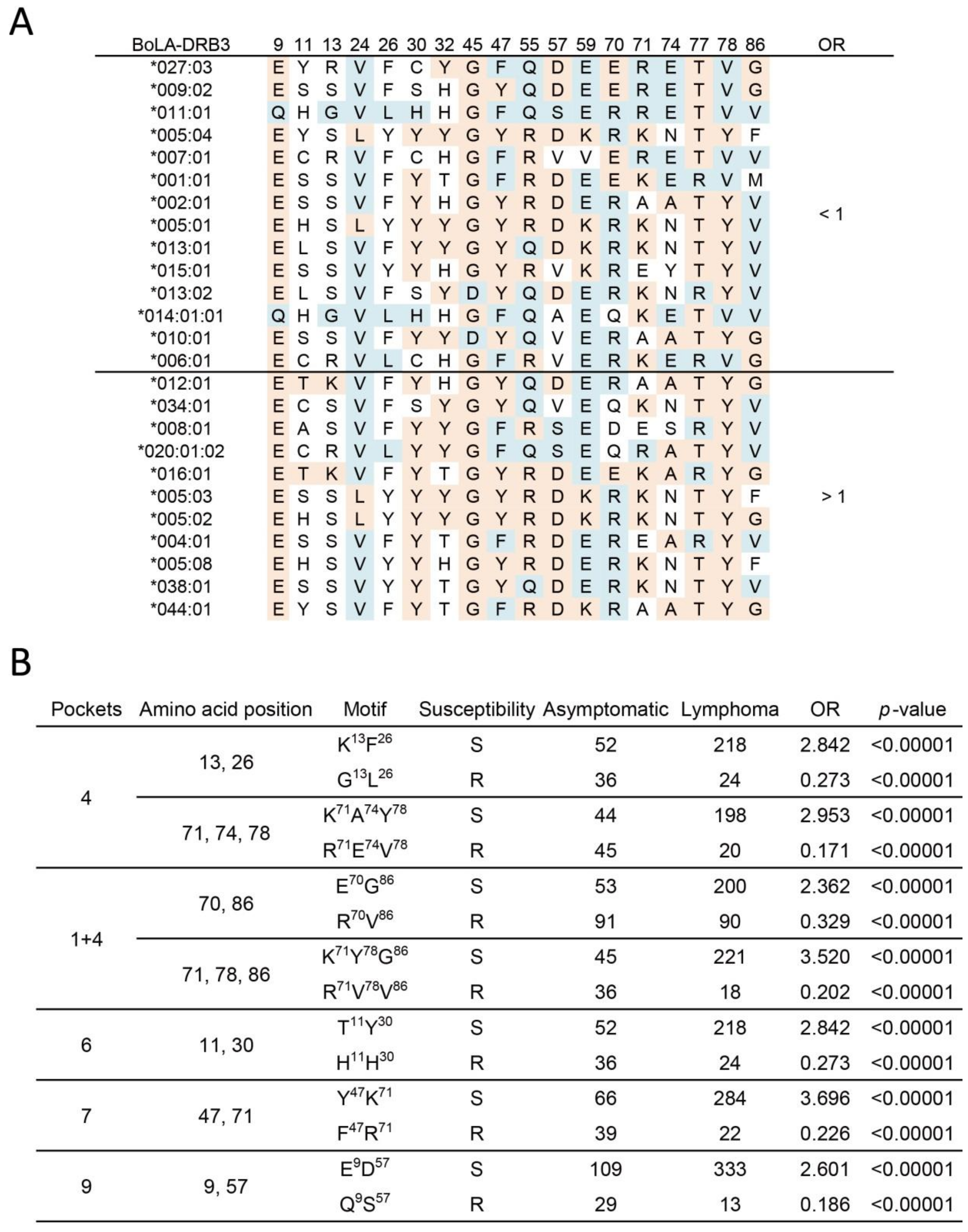
2.3. Association Study of BoLA-DRβ with BLV-Induced Lymphoma at the Amino Acid Level
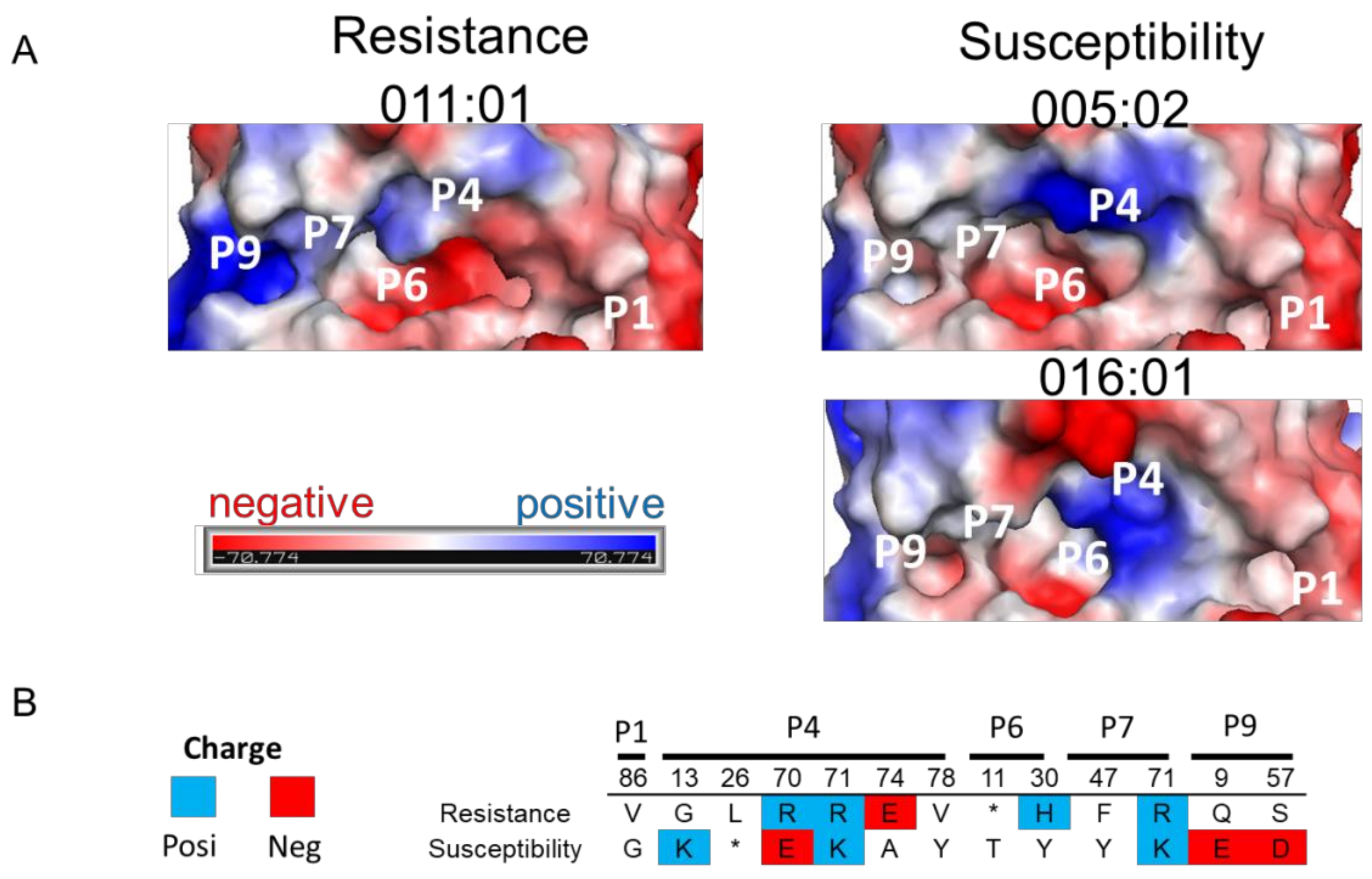
2.4. 3D Structure and Electrostatic Charge Analysis of BoLA-DRβ Binding Pocket
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Diagnosis
4.2. BLV Infection Determination Using Enzyme-Linked Immunosorbent Assays (ELISA)
4.3. BoLA-DRB3 Genotyping
4.4. Characterization of Amino Acid Properties
4.5. 3-Dimensional (3D) Protein Structure Modeling of BoLA-DRB3 Molecules
4.6. Association Study and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Underwood, W.J.; Blauwiekel, R.; Delano, M.L.; Gillesby, R.; Mischler, S.A.; Schoell, A. Chapter 15—Biology and diseases of ruminants (sheep, goats, and cattle). In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, J.M., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 623–694. [Google Scholar]
- Aida, Y.; Murakami, H.; Takahashi, M.; Takeshima, S.-N. Mechanisms of pathogenesis induced by bovine leukemia virus as a model for human T-cell leukemia virus. Front. Microbiol. 2013, 4, 328. [Google Scholar] [CrossRef] [Green Version]
- OIE. Enzootic bovine leukosis. In OIE Terrestrial Manual World Organisation for Animal Health; OIE: Paris, France, 2012; pp. 1–11. [Google Scholar]
- Bartlett, P.C.; Ruggiero, V.J.; Hutchinson, H.C.; Droscha, C.J.; Norby, B.; Sporer, K.R.B.; Taxis, T.M. Current Developments in the Epidemiology and Control of Enzootic Bovine Leukosis as Caused by Bovine Leukemia Virus. Pathogens 2020, 9, 1058. [Google Scholar] [CrossRef]
- Gillet, N.; Florins, A.; Boxus, M.; Burteau, C.; Nigro, A.; Vandermeers, F.; Balon, H.; Bouzar, A.-B.; Defoiche, J.; Burny, A.; et al. Mechanisms of leukemogenesis induced by bovine leukemia virus: Prospects for novel anti-retroviral therapies in human. Retrovirology 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barez, P.-Y.; De Brogniez, A.; Carpentier, A.; Gazon, H.; Gillet, N.; Gutiérrez, G.; Hamaidia, M.; Jacques, J.-R.; Perike, S.; Neelature Sriramareddy, S.; et al. Recent Advances in BLV Research. Viruses 2015, 7, 6080–6088. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, S.-N.; Aida, Y. Structure, function and disease susceptibility of the bovine major histocompatibility complex. Anim. Sci. J. 2006, 77, 138–150. [Google Scholar] [CrossRef]
- Aida, Y.; Takeshima, S.N.; Baldwin, C.L.; Kaushik, A.K.; Ruvinsky, A. Bovine immunogenetics. In The Genetics of Cattle; Garrick, D.J., Ruvinsky, A., Eds.; C.A.B. International: Wallingford, UK, 2015. [Google Scholar]
- Lei, W.; Liang, Q.; Jing, L.; Wang, C.; Wu, X.; He, H. BoLA-DRB3 gene polymorphism and FMD resistance or susceptibility in Wanbei cattle. Mol. Biol. Rep. 2012, 39, 9203–9209. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Mukoyama, H.; Furuta, H.; Kondo, Y.; Takeshima, S.N.; Aida, Y.; Kosugiyama, M.; Tomogane, H. Association of the amino acid motifs of BoLA-DRB3 alleles with mastitis pathogens in Japanese Holstein cows. Anim. Sci. J. 2009, 80, 510–519. [Google Scholar] [CrossRef]
- Morales, J.P.A.; López-Herrera, A.; Zuluaga, J.E. Association of BoLA DRB3 gene polymorphisms with BoHV-1 infection and zootechnical traits. Open Vet. J. 2020, 10, 331–339. [Google Scholar] [CrossRef]
- Takeshima, S.-N.; Ohno, A.; Aida, Y. Bovine leukemia virus proviral load is more strongly associated with bovine major histocompatibility complex class II DRB3 polymorphism than with DQA1 polymorphism in Holstein cow in Japan. Retrovirology 2019, 16, 1–6. [Google Scholar] [CrossRef]
- Miyasaka, T.; Takeshima, S.-N.; Jimba, M.; Matsumoto, Y.; Kobayashi, N.; Matsuhashi, T.; Sentsui, H.; Aida, Y. Identification of bovine leukocyte antigen class II haplotypes associated with variations in bovine leukemia virus proviral load in Japanese Black cattle. Tissue Antigens 2012, 81, 72–82. [Google Scholar] [CrossRef]
- Udina, I.G.; Karamysheva, E.E.; Turkova, S.O.; Orlova, A.R.; Sulimova, G.E. Genetic mechanisms of resistance and susceptibility to leukemia in Ayrshire and black pied cattle breeds determined by allelic distribution of gene Bola-DRB3. Russ. J. Genet. 2003, 39, 306–317. [Google Scholar] [CrossRef]
- Forletti, A.; Lützelschwab, C.M.; Cepeda, R.; Esteban, E.N.; Gutiérrez, S.E. Early events following bovine leukaemia virus infection in calves with different alleles of the major histocompatibility complex DRB3 gene. Vet. Res. 2020, 51, 4. [Google Scholar] [CrossRef] [Green Version]
- Jimba, M.; Takeshima, S.N.; Murakami, H.; Kohara, J.; Kobayashi, N.; Matsuhashi, T.; Ohmori, T.; Nunoya, T.; Aida, Y. BLV-CoCoMo-qPCR: A useful tool for evaluating bovine leukemia virus infection status. BMC Vet. Res. 2012, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Juliarena, M.A.; Barrios, C.N.; Ceriani, M.C.; Esteban, E.N. Hot topic: Bovine leukemia virus (BLV)-infected cows with low proviral load are not a source of infection for BLV-free cattle. J. Dairy Sci. 2016, 99, 4586–4589. [Google Scholar] [CrossRef] [Green Version]
- Lützelschwab, C.M.; Forletti, A.; Cepeda, R.; Esteban, E.N.; Confalonieri, O.; Gutiérrez, S.E. Co-infection with Mycobacterium bovis does not alter the response to bovine leukemia virus in BoLA DRB3*0902, genetically resistant cattle. Res. Vet. Sci. 2016, 109, 10–16. [Google Scholar] [CrossRef]
- Lo, C.-W.; Borjigin, L.; Saito, S.; Fukunaga, K.; Saitou, E.; Okazaki, K.; Mizutani, T.; Wada, S.; Takeshima, S.-n.; Aida, Y. BoLA-DRB3 Polymorphism is Associated with Differential Susceptibility to Bovine Leukemia Virus-Induced Lymphoma and Proviral Load. Viruses 2020, 12, 352. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.-J.; Zhang, S.; Wong, H.-S. An effective and effecient peptide binding prediction approach for a broad set of HLA-DR molecules based on ordered weighted averaging of binding pocket profiles. Proteome Sci. 2013, 11, S15. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.S. How the MHC selects Th1/Th2 immunity. Immunol. Today 1998, 19, 157–162. [Google Scholar] [CrossRef]
- Maloy, K.J.; Burkhart, C.; Junt, T.M.; Odermatt, B.; Oxenius, A.; Piali, L.; Zinkernagel, R.M.; Hengartner, H. CD4(+) T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 2000, 191, 2159–2170. [Google Scholar] [CrossRef]
- Boasso, A. Type I interferon at the interface of antiviral immunity and immune regulation: The curious case of HIV-1. Scientifica 2013, 2013, 580968. [Google Scholar] [CrossRef] [Green Version]
- Sajiki, Y.; Konnai, S.; Okagawa, T.; Maekawa, N.; Nakamura, H.; Kato, Y.; Suzuki, Y.; Murata, S.; Ohashi, K. A TLR7 agonist activates bovine Th1 response and exerts antiviral activity against bovine leukemia virus. Dev. Comp. Immunol. 2021, 114, 103847. [Google Scholar] [CrossRef]
- Ohishi, K.; Suzuki, H.; Yasutomi, Y.; Onuma, M.; Okada, K.; Numakunai, S.; Ohshima, K.-I.; Yoji Ikawa, Y.; Sugimoto, M. Augmentation of bovine leukemia virus (BLV)-specific lymphocyte proliferation responses in ruminants by inoculation with BLV ENV-recombinant vaccinia virus: Their role in the suppression of BLV replication. Microbiol. Immunol. 1992, 36, 1317–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaoka, Y.; Kabeya, H.; Onuma, M.; Kasai, N.; Okada, K.; Aida, Y. Ovine MHC class. II. Alleles associated with resistance or susceptibility to development of bovine leukemia virus-induced ovine lymphoma. Cancer Res. 1999, 59, 975. [Google Scholar] [PubMed]
- Konnai, S.; Takeshima, S.N.; Tajima, S.; Yin, S.A.; Okada, K.; Onuma, M.; Aida, Y. The influence of ovine MHC Class. II DRB1 alleles on immune response in bovine leukemia virus infection. Microbiol. Immunol. 2003, 47, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.Y.; Fugger, L.; Strominger, J.L.; Siebold, C. MHC class II proteins and disease: A structural perspective. Nat. Rev. Immunol. 2006, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Brujeni, N.G.; Ghorbanpour, R.; Esmailnejad, A. Association of BoLA-DRB3.2 alleles with BLV infection profiles (persistent lymphocytosis/lymphosarcoma) and lymphocyte subsets in iranian holstein cattle. Biochem. Genet. 2016, 54, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Bondinas, G.P.; Moustakas, A.K.; Papadopoulos, G.K. The spectrum of HLA-DQ and HLA-DR alleles, 2006: A listing correlating sequence and structure with function. Immunogenetics 2007, 59, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, S.-N.; Sarai, Y.; Saitou, N.; Aida, Y. MHC class II DR classification based on antigen-binding groove natural selection. Biochem. Biophys. Res. Commun. 2009, 385, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Yin, J.; Chen, Y.; Deng, F.; Chen, J.; Gao, X.; Liu, Z.; Yu, X.; Zheng, J. The amino acid variation within the binding pocket 7 and 9 of HLA-DRB1 molecules are associated with primary Sjögren’s syndrome. J. Autoimmun. 2015, 57, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, Y.; Qi, J.; Gao, F.; Liu, Y.; Liu, J.; Zhou, X.; Kaufman, J.; Xia, C.; Gao, G.F. Narrow groove and restricted anchors of MHC class. I molecule BF2*0401 plus peptide transporter restriction can. explain disease susceptibility of B4 chickens. J. Immunol. 2012, 189, 4478. [Google Scholar] [CrossRef] [Green Version]
- Garstka, M.A.; Fish, A.; Celie, P.H.N.; Joosten, R.P.; Janssen, G.M.C.; Berlin, I.; Hoppes, R.; Stadnik, M.; Janssen, L.; Ovaa, H.; et al. The first step of peptide selection in antigen presentation by MHC class I molecules. Proc. Natl. Acad. Sci. USA 2015, 112, 1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-F.; Lin, C.-Y.; Hong, H.-M. In silico design, synthesis and potency of an epitope-based vaccine against foot-and-mouth disease virus. Int. J. Pharmacol. 2017, 13, 122–133. [Google Scholar] [CrossRef]
- Ettinger, R.A.; Papadopoulos, G.K.; Moustakas, A.K.; Nepom, G.T.; Kwok, W.W. Allelic variation in key peptide-binding pockets discriminates between closely related diabetes-protective and diabetes-susceptible alleles. J. Immunol. 2006, 176, 1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.-X.; Pang, X. Electrostatic interactions in protein structure, folding, binding, and condensation. Chem. Rev. 2018, 118, 1691–1741. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, P.T. Electrostatic modifications of the human leukocyte antigen DR P9 peptide-binding pocket in primary sclerosing cholangitis: Back to the future with human leukocyte antigen DRβ. Hepatology 2011, 53, 1798–1800. [Google Scholar] [CrossRef]
- Rosewick, N.; Durkin, K.; Artesi, M.M.; Marçais, A.A.; Hahaut, V.V.; Griebel, P.; Arsic, N.N.; Avettand-Fenoel, V.; Burny, A.; Charlier, C.C.; et al. Cis-perturbation of cancer drivers by the HTLV-1/BLV proviruses is an early determinant of leukemogenesis. Nat. Commun. 2017, 8, 15264. [Google Scholar] [CrossRef]
- Gillet, N.A.; Gutiérrez, G.; Rodriguez, S.M.; De Brogniez, A.; Renotte, N.; Alvarez, I.; Trono, K.; Willems, L. Massive depletion of bovine leukemia virus proviral clones located in genomic transcriptionally active sites during primary infection. PLoS Pathog. 2013, 9, e1003687. [Google Scholar] [CrossRef]
- Zyrianova, I.M.; Kovalchuk, S.N. Bovine leukemia virus tax gene/Tax protein polymorphism and its relation to Enzootic Bovine Leukosis. Virulence 2020, 11, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Willems, L.; Kerkhofs, P.; Dequiedt, F.; Portetelle, D.; Mammerickx, M.; Burny, A.; Kettmann, R. Attenuation of bovine leukemia virus by deletion of R3 and G4 open reading frames. Proc. Natl. Acad. Sci. USA 1994, 91, 11532. [Google Scholar] [CrossRef] [Green Version]
- Tajima, S.; Zhuang, W.Z.; Kato, M.V.; Okada, K.; Ikawa, Y.; Aida, Y. Function and conformation of wild-type p53 protein are influenced by mutations in bovine leukemia virus-induced B-cell lymphosarcoma. Virology 1998, 243, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Dequiedt, F.; Kettmann, R.; Burny, A.; Willems, L. Mutations in the p53 Tumor-Suppressor Gene Are Frequently Associated with Bovine Leukemia Virus-Induced Leukemogenesis in Cattle but Not in Sheep. Virology 1995, 209, 676–683. [Google Scholar] [CrossRef]
- Konnai, S.; Usui, T.; Ikeda, M.; Kohara, J.; Hirata, T.-I.; Okada, K.; Ohashi, K.; Onuma, M. Tumor necrosis factor-alpha genetic polymorphism may contribute to progression of bovine leukemia virus-infection. Microbes Infect. 2006, 8, 2163–2171. [Google Scholar] [CrossRef] [Green Version]
- Debacq, C.; Asquith, B.; Reichert, M.; Burny, A.; Kettmann, R.; Willems, L. Reduced Cell Turnover in Bovine LeukemiaVirus-Infected, Persistently LymphocytoticCattle. J. Virol. 2003, 77, 13073–13083. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Hirose, T.; Assi, W.; Wada, S.; Takeshima, S.-N.; Aida, Y. Bovine Leukemia Virus Infection Affects Host Gene Expression Associated with DNA Mismatch Repair. Pathogens 2020, 9, 909. [Google Scholar] [CrossRef]
- Assi, W.; Hirose, T.; Wada, S.; Matsuura, R.; Takeshima, S.-N.; Aida, Y. PRMT5 Is Required for Bovine Leukemia Virus Infection In Vivo and Regulates BLV Gene Expression, Syncytium Formation, and Glycosylation In Vitro. Viruses 2020, 12, 650. [Google Scholar] [CrossRef]
- Tsutsui, T.; Kobayashi, S.; Hayama, Y.; Yamamoto, T. Fraction of bovine leukemia virus-infected dairy cattle developing enzootic bovine leukosis. Prev. Veter. Med. 2016, 124, 96–101. [Google Scholar] [CrossRef]
- Tajima, S.; Ikawa, Y.; Aida, Y. Complete Bovine Leukemia Virus (BLV) Provirus Is Conserved in BLV-Infected Cattle throughout the Course of B-Cell Lymphosarcoma Development. J. Virol. 1998, 72, 7569–7576. [Google Scholar] [CrossRef] [Green Version]
- Takeshima, S.-N.; Matsumoto, Y.; Miyasaka, T.; Arainga-Ramirez, M.; Saito, H.; Onuma, M.; Aida, Y. A new method for typing bovine major histocompatibility complex class II DRB3 alleles by combining two established PCR sequence-based techniques. Tissue Antigens 2011, 78, 208–213. [Google Scholar] [CrossRef]
- Pommié, C.; Levadoux, S.; Sabatier, R.; Lefranc, G.; Lefranc, M.-P. IMGT standardized criteria for statistical analysis of immunoglobulin V-REGION amino acid properties. J. Mol. Recognit. 2004, 17, 17–32. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Stern, L.J.; Brown, J.H.; Jardetzky, T.S.; Gorga, J.C.; Urban, R.G.; Strominger, J.L.; Wiley, D.C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nat. Cell Biol. 1994, 368, 215–221. [Google Scholar] [CrossRef] [PubMed]
| Allele | Asymptomatic (n. = 212) 1 | Lymphoma (n. = 454) | OR 2 | 95% CI 3 | p-Value | Susceptibility 4 |
|---|---|---|---|---|---|---|
| *001:01 | 9 | 8 | 0.405 | 0.154–1.064 | 0.0677 | - |
| *002:01 | 18 | 17 | 0.419 | 0.212–0.831 | 0.0147 | (R) 5 |
| *004:01 | 0 | 1 | 1.406 | 0.057–34.653 | 1 | - |
| *005:01 | 1 | 1 | 0.466 | 0.029–7.483 | 0.5356 | - |
| *005:02 | 1 | 23 | 11.260 | 1.510–83.945 | 0.0014 | S |
| *005:03 | 3 | 31 | 5.106 | 1.543–16.894 | 0.0021 | (S) |
| *005:04 | 2 | 1 | 0.232 | 0.021–2.571 | 0.2391 | - |
| *005:08 | 0 | 3 | 3.295 | 0.169–64.072 | 0.5552 | - |
| *006:01 | 1 | 2 | 0.934 | 0.084–10.354 | 1 | - |
| *007:01 | 7 | 5 | 0.326 | 0.102–1.040 | 0.0606 | - |
| *008:01 | 1 | 4 | 1.876 | 0.208–16.884 | 1 | - |
| *009:02 | 7 | 2 | 0.130 | 0.027–0.629 | 0.0059 | (R) |
| *010:01 | 27 | 45 | 0.754 | 0.454–1.253 | 0.2855 | - |
| *011:01 | 29 | 13 | 0.186 | 0.095–0.366 | <0.00001 | R |
| *012:01 | 8 | 20 | 1.175 | 0.509–2.713 | 0.8369 | - |
| *013:01 | 1 | 1 | 0.466 | 0.029–7.483 | 0.5356 | - |
| *013:02 | 13 | 20 | 0.705 | 0.344–1.447 | 0.3426 | - |
| *014:01:01 | 7 | 11 | 0.727 | 0.278–1.903 | 0.6086 | - |
| *015:01 | 29 | 34 | 0.511 | 0.302–0.864 | 0.0024 | (R) |
| *016:01 | 44 | 198 | 2.953 | 2.019–4.319 | <0.00001 | S |
| *027:03 | 2 | 0 | 0.093 | 0.004–1.938 | 0.1010 | - |
| *020:01:02 | 1 | 4 | 1.876 | 0.208–16.884 | 1 | - |
| *034:01 | 1 | 3 | 1.404 | 0.145–13.573 | 1 | - |
| *038:01 | 0 | 3 | 3.295 | 0.169–64.072 | 0.5552 | - |
| *044:01 | 0 | 4 | 4.245 | 0.228–79.214 | 0.3126 | - |
| Genotype 1 | Asymptomatic (n. = 106) 2 | Lymphoma (n. = 227) | OR 3 | 95% CI 4 | p-Value | Susceptibility 5 |
|---|---|---|---|---|---|---|
| *001:01/*016:01 | 2 | 3 | 0.696 | 0.115–4.231 | 0.6551 | - |
| *002:01/*005:03 | 1 | 3 | 1.406 | 0.145–13.681 | 1 | - |
| *002:01/*010:01 | 5 | 2 | 0.180 | 0.034–0.941 | 0.0356 | (R) 6 |
| *002:01/*011:01 | 5 | 0 | 0.041 | 0.002–0.741 | 0.0031 | (R) |
| *002:01/*016:01 | 1 | 7 | 3.341 | 0.406–27.507 | 0.4438 | - |
| *005:02/*016:01 | 0 | 13 | 13.405 | 0.789–227.680 | 0.0115 | (S) |
| *005:03/*012:01 | 0 | 4 | 4.289 | 0.229–80.387 | 0.311 | - |
| *005:03/*016:01 | 0 | 10 | 10.283 | 0.597–177.152 | 0.0341 | (S) |
| *010:01/*010:01 | 2 | 3 | 0.696 | 0.117–4.312 | 0.6551 | - |
| *010:01/*011:01 | 3 | 3 | 0.460 | 0.091–2.317 | 0.3873 | - |
| *010:01/*015:01 | 3 | 4 | 0.616 | 0.135–2.802 | 0.6837 | - |
| *010:01/*016:01 | 7 | 19 | 1.292 | 0.526–3.175 | 0.6654 | - |
| *011:01/*015:01 | 4 | 0 | 0.050 | 0.003–0.939 | 0.0099 | (R) |
| *011:01/*016:01 | 7 | 4 | 0.254 | 0.073–0.886 | 0.0415 | (R) |
| *012:01/*015:01 | 2 | 3 | 0.696 | 0.115–4.231 | 0.6551 | - |
| *012:01/*016:01 | 0 | 5 | 5.265 | 0.289–96.103 | 0.1822 | - |
| *013:02/*016:01 | 3 | 10 | 1.582 | 0.426–5.872 | 0.7621 | - |
| *015:01/*016:01 | 10 | 15 | 0.679 | 0.295–1.567 | 0.3769 | - |
| *016:01/*016:01 | 4 | 49 | 7.020 | 2.462–20.017 | <0.00001 | S |
| *016:01/*020:01:02 | 1 | 3 | 1.406 | 0.145–13.681 | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, C.-W.; Takeshima, S.-n.; Okada, K.; Saitou, E.; Fujita, T.; Matsumoto, Y.; Wada, S.; Inoko, H.; Aida, Y. Association of Bovine Leukemia Virus-Induced Lymphoma with BoLA-DRB3 Polymorphisms at DNA, Amino Acid, and Binding Pocket Property Levels. Pathogens 2021, 10, 437. https://doi.org/10.3390/pathogens10040437
Lo C-W, Takeshima S-n, Okada K, Saitou E, Fujita T, Matsumoto Y, Wada S, Inoko H, Aida Y. Association of Bovine Leukemia Virus-Induced Lymphoma with BoLA-DRB3 Polymorphisms at DNA, Amino Acid, and Binding Pocket Property Levels. Pathogens. 2021; 10(4):437. https://doi.org/10.3390/pathogens10040437
Chicago/Turabian StyleLo, Chieh-Wen, Shin-nosuke Takeshima, Kosuke Okada, Etsuko Saitou, Tatsuo Fujita, Yasunobu Matsumoto, Satoshi Wada, Hidetoshi Inoko, and Yoko Aida. 2021. "Association of Bovine Leukemia Virus-Induced Lymphoma with BoLA-DRB3 Polymorphisms at DNA, Amino Acid, and Binding Pocket Property Levels" Pathogens 10, no. 4: 437. https://doi.org/10.3390/pathogens10040437







