Virulence of Clinical Candida Isolates
Abstract
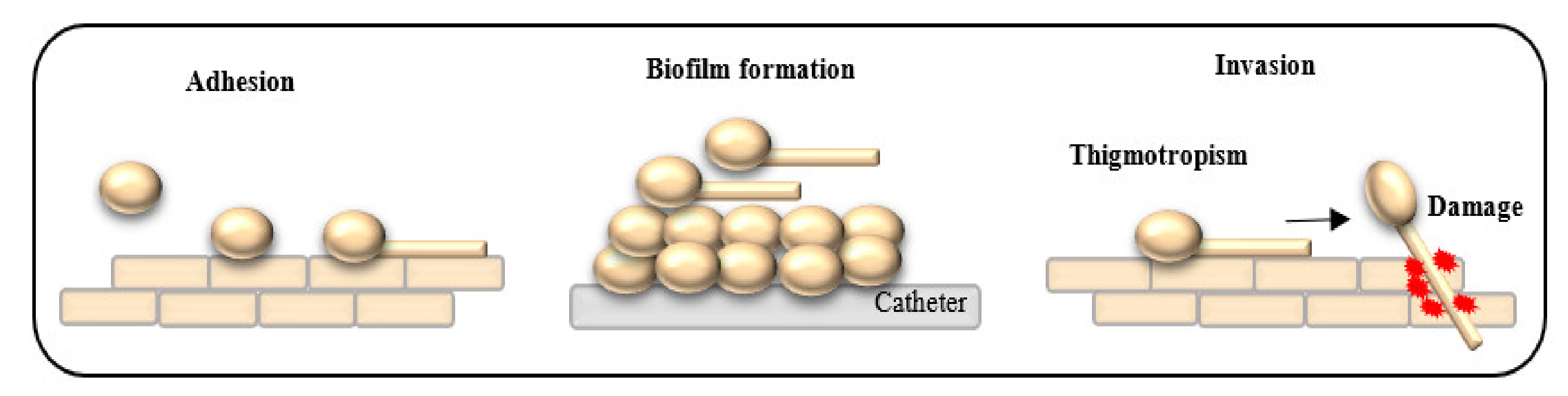
:1. Introduction
2. Results
2.1. Candida Virulence Investigation Using G. mellonella as Host Model
2.2. Examination of Virulence Factor Production
3. Discussion
4. Materials and Methods
4.1. Candida spp.
4.2. Candida spp. Virulence Investigation Using G. mellonella as Host Model
4.3. Examination of Virulence Factors Production
5. Conclusions
- C. albicans isolates were the most virulent and produce the highest number of extracellular virulence factors;
- A positive correlation between the biofilm formation and the MIC values of echinocandins was confirmed among the tested Candida isolates;
- The virulence of Candida isolates is related to the expression of proteases, hemolysins, and esterases;
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brillowska-Dabrowska, A.; Bergmann, O.; Jensen, I.M.; Jarløv, J.O.; Arendrup, M.C. Typing of Candida Isolates from Patients with Invasive Infection and Concomitant Colonization. Scand. J. Infect. Dis. 2010, 42, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida Species: Current Epidemiology, Pathogenicity, Biofilm Formation, Natural Antifungal Products and New Therapeutic Options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, S.C.; Saini, S.; Mathew, S. Virulence Factors Contributing to Pathogenicity of Candida Tropicalis and Its Antifungal Susceptibility Profile. Int. J. Microbiol. 2014, 2014, e456878. [Google Scholar] [CrossRef] [Green Version]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida Albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.N.; Solis, N.V.; Phan, Q.T.; Bajwa, J.S.; Kashleva, H.; Thompson, A.; Liu, Y.; Dongari-Bagtzoglou, A.; Edgerton, M.; Filler, S.G. Host Cell Invasion and Virulence Mediated by Candida Albicans Ssa1. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Yan, L.; Wu, C.; Zhao, X.; Tang, J. Fungal Invasion of Epithelial Cells. Microbiol. Res. 2014, 169, 803–810. [Google Scholar] [CrossRef]
- Wächtler, B.; Wilson, D.; Haedicke, K.; Dalle, F.; Hube, B. From Attachment to Damage: Defined Genes of Candida Albicans Mediate Adhesion, Invasion and Damage during Interaction with Oral Epithelial Cells. PLoS ONE 2011, 6, e17046. [Google Scholar] [CrossRef] [Green Version]
- Swidergall, M.; Filler, S.G. Oropharyngeal Candidiasis: Fungal Invasion and Epithelial Cell Responses. PLoS Pathog. 2017, 13, e1006056. [Google Scholar] [CrossRef]
- Cafarchia, C.; Romito, D.; Coccioli, C.; Camarda, A.; Otranto, D. Phospholipase Activity of Yeasts from Wild Birds and Possible Implications for Human Disease. Med. Mycol. 2008, 46, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Furlaneto-Maia, L.; Specian, A.F.; Bizerra, F.C.; de Oliveira, M.T.; Furlaneto, M.C. In Vitro Evaluation of Putative Virulence Attributes of Oral Isolates of Candida Spp. Obtained from Elderly Healthy Individuals. Mycopathologia 2008, 166, 209. [Google Scholar] [CrossRef]
- Galán-Ladero, M.A.; Blanco, M.T.; Sacristán, B.; Fernández-Calderón, M.C.; Pérez-Giraldo, C.; Gómez-García, A.C. Enzymatic Activities of Candida Tropicalis Isolated from Hospitalized Patients. Med. Mycol. 2010, 48, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida Glabrata, Candida Parapsilosis and Candida Tropicalis: Biology, Epidemiology, Pathogenicity and Antifungal Resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.; Menon, T.; Nalini, S.; Thirunarayan, M.A.; Rajasekaran, S.; Venkatadesikalu, M. Esterase Activity of Candida Species Isolated from Immunocompromised Hosts. Eur. PMC 2006, 23, 101–103. [Google Scholar]
- Gácser, A.; Trofa, D.; Schäfer, W.; Nosanchuk, J.D. Targeted Gene Deletion in Candida Parapsilosis Demonstrates the Role of Secreted Lipase in Virulence. J. Clin. Investig. 2007, 117, 3049–3058. [Google Scholar] [CrossRef] [Green Version]
- Al-Fattani, M.A. Biofilm Matrix of Candida Albicans and Candida Tropicalis: Chemical Composition and Role in Drug Resistance. J. Med. Microbiol. 2006, 55, 999–1008. [Google Scholar] [CrossRef]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of Non-Candida Albicans Candida Species: Quantification, Structure and Matrix Composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida Auris and Other Key Pathogenic Candida Species. mSphere 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Fanning, S.; Mitchell, A.P. Fungal Biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef] [Green Version]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on Current Status of Echinocandins Use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroschein-Stevenson, S.L.; Foley, E.; O’Farrell, P.H.; Johnson, A.D. Identification of Drosophila Gene Products Required for Phagocytosis of Candida Albicans. PLoS Biol. 2005, 4, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Riveros, M.; De-la-Pinta, I.; Marcos-Arias, C.; Ezpeleta, G.; Quindós, G.; Eraso, E. Usefulness of the Non-Conventional Caenorhabditis Elegans Model to Assess Candida Virulence. Mycopathologia 2017, 182, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, H. Contamination of Cockroaches (Insecta: Blattaria) to Medically Fungi: A Systematic Review and Meta-Analysis. J. Mycol. Médicale 2017, 27, 427–448. [Google Scholar] [CrossRef]
- Nwibo, D.D.; Hamamoto, H.; Matsumoto, Y.; Kaito, C.; Sekimizu, K. Current Use of Silkworm Larvae (Bombyx Mori) as an Animal Model in Pharmaco-Medical Research. Drug Discov. Ther. 2015, 9, 133–135. [Google Scholar] [CrossRef] [Green Version]
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Cristina, A.; Prata, M.; Jorge, A.O.C.; Junqueira, J.C. From Moths to Caterpillars: Ideal Conditions for Galleria Mellonella Rearing for in Vivo Microbiological Studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, B.B.; O’Brien, E.; Khoury, J.B.E.; Mylonakis, E. Methods for Using Galleria Mellonella as a Model Host to Study Fungal Pathogenesis. Virulence 2010, 1, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Mowlds, P.; Kavanagh, K. Effect of Pre-Incubation Temperature on Susceptibility of Galleria Mellonella Larvae to Infection by Candida Albicans. Mycopathologia 2008, 165, 5–12. [Google Scholar] [CrossRef]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Galleria Mellonella: An Invertebrate Model to Study Pathogenicity in Correctly Defined Fungal Species. Fungal Biol. 2016, 120, 288–295. [Google Scholar] [CrossRef]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between Virulence of Candida Albicans Mutants in Mice and Galleria Mellonella Larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Slater, J.L.; Gregson, L.; Denning, D.W.; Warn, P.A. Pathogenicity of Aspergillus Fumigatus Mutants Assessed in Galleria Mellonella Matches That in Mice. Med. Mycol. 2011, 49, S107–S113. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.J.; Hamblin, K.A.; Armstrong, S.J.; Müller, C.M.; Bokori-Brown, M.; Goldman, S.; Atkins, H.S.; Titball, R.W. Galleria Mellonella as a Model System to Test the Pharmacokinetics and Efficacy of Antibiotics against Burkholderia Pseudomallei. Int. J. Antimicrob. Agents 2013, 41, 330–336. [Google Scholar] [CrossRef]
- Thomaz, L.; García-Rodas, R.; Guimarães, A.J.; Taborda, C.P.; Zaragoza, O.; Nosanchuk, J.D. Galleria Mellonella as a Model Host to Study Paracoccidioides Lutzii and Histoplasma Capsulatum. Virulence 2013, 4, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Fallon, J.P.; Troy, N.; Kavanagh, K. Pre-Exposure of Galleria Mellonella Larvae to Different Doses of Aspergillus Fumigatus Conidia Causes Differential Activation of Cellular and Humoral Immune Responses. Virulence 2011, 2, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Firacative, C.; Duan, S.; Meyer, W. Galleria Mellonella Model Identifies Highly Virulent Strains among All Major Molecular Types of Cryptococcus Gattii. PLoS ONE 2014, 9, e105076. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Lucas, M.P.; Mesa-Arango, A.C.; Fusco-Almeida, A.M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M.J.; Zaragoza, O. Antifungal Efficacy during Candida Krusei Infection in Non-Conventional Models Correlates with the Yeast in Vitro Susceptibility Profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef]
- Borghi, E.; Romagnoli, S.; Fuchs, B.B.; Cirasola, D.; Perdoni, F.; Tosi, D.; Braidotti, P.; Bulfamante, G.; Morace, G.; Mylonakis, E. Correlation between Candida Albicans Biofilm Formation and Invasion of the Invertebrate Host Galleria Mellonella. Future Microbiol. 2014, 9, 163–173. [Google Scholar] [CrossRef]
- Fuchs, B.B.; Eby, J.; Nobile, C.J.; El Khoury, J.B.; Mitchell, A.P.; Mylonakis, E. Role of Filamentation in Galleria Mellonella Killing by Candida Albicans. Microbes Infect. 2010, 12, 488–496. [Google Scholar] [CrossRef] [Green Version]
- De Paula Menezes, R.; de Melo Riceto, É.B.; Borges, A.S.; de Brito Röder, D.V.D.; dos Santos Pedroso, R. Evaluation of Virulence Factors of Candida Albicans Isolated from HIV-Positive Individuals Using HAART. Arch. Oral Biol. 2016, 66, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The Emerging Pathogen Candida Auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef] [Green Version]
- Neji, S.; Hadrich, I.; Trabelsi, H.; Abbes, S.; Cheikhrouhou, F.; Sellami, H.; Makni, F.; Ayadi, A. Virulence Factors, Antifungal Susceptibility and Molecular Mechanisms of Azole Resistance among Candida Parapsilosis Complex Isolates Recovered from Clinical Specimens. J. Biomed. Sci. 2017, 24, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumdar, T.; Mullick, J.; Bir, R.; Roy, J.; Sil, S. Determination of Virulence Factors and Biofilm Formation among Isolates of Vulvovaginal Candidiasis. J. Med. Sci. 2016, 36, 53. [Google Scholar] [CrossRef]
- De Souza Ramos, L.; Barbedo, L.S.; Silva, L.A.B.; dos Santos, A.L.S.; Pinto, M.R.; da Graça Sgarbi, D.B. Protease and Phospholipase Activities of “Candida” Spp. Isolated from Cutaneous Candidiasis. Rev. Iberoam. Micol. 2015, 32, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.V.; Silva, L.B.; de Oliveira, D.B.C.; da Silva, P.R.; Ferreira-Paim, K.; Andrade-Silva, L.E.; Silva-Vergara, M.L.; Andrade, A.A. Species Distribution, Virulence Factors, and Antifungal Susceptibility Among Candida Parapsilosis Complex Isolates Recovered from Clinical Specimens. Mycopathologia 2015, 180, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Abi-Chacra, É.A.; Souza, L.O.P.; Cruz, L.P.; Braga-Silva, L.A.; Gonçalves, D.S.; Sodré, C.L.; Ribeiro, M.D.; Seabra, S.H.; Figueiredo-Carvalho, M.H.G.; Barbedo, L.S.; et al. Phenotypical Properties Associated with Virulence from Clinical Isolates Belonging to the Candida Parapsilosis Complex. FEMS Yeast Res. 2013, 13, 831–848. [Google Scholar] [CrossRef] [Green Version]
- Kalaiarasan, K.; Singh, R.; Chaturvedula, L. Changing Virulence Factors among Vaginal Non-Albicans Candida Species. Indian J. Med. Microbiol. 2018, 36, 364. [Google Scholar] [CrossRef]
- Udayalaxmi, J.; Shenoy, N. Comparison Between Biofilm Production, Phospholipase and Haemolytic Activity of Different Species of Candida Isolated from Dental Caries Lesions in Children. J. Clin. Diagn. Res. 2016, 10, DC21–DC23. [Google Scholar] [CrossRef]
- Fule, S.R.; Das, D.; Fule, R.P. Detection of Phospholipase Activity of Candida Albicans and Non Albicans Isolated from Women of Reproductive Age with Vulvovaginal Candidiasis in Rural Area. Indian J. Med. Microbiol. 2015, 33, 92. [Google Scholar] [CrossRef]
- Pakshir, K.; Zomorodian, K.; Karamitalab, M.; Jafari, M.; Taraz, H.; Ebrahimi, H. Phospholipase, Esterase and Hemolytic Activities of Candida Spp. Isolated from Onychomycosis and Oral Lichen Planus Lesions. J. Mycol. Médicale 2013, 23, 113–118. [Google Scholar] [CrossRef]
- Slifkin, M. Tween 80 Opacity Test Responses of Various Candida Species. J. Clin. Microbiol. 2000, 38, 4626–4628. [Google Scholar] [CrossRef] [Green Version]
- El-Houssaini, H.H.; Elnabawy, O.M.; Nasser, H.A.; Elkhatib, W.F. Correlation between Antifungal Resistance and Virulence Factors in Candida Albicans Recovered from Vaginal Specimens. Microbial. Pathog. 2019, 128, 13–19. [Google Scholar] [CrossRef]
- Cotter, G.; Doyle, S.; Kavanagh, K. Development of an Insect Model for the in Vivo Pathogenicity Testing of Yeasts. FEMS Immunol. Med. Microbiol. 2000, 27, 163–169. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Fuchs, B.B.; Muhammed, M.; Coleman, J.J.; Suleiman, J.M.; Vilela, S.F.; Costa, A.C.; Rasteiro, V.M.; Jorge, A.O.; Mylonakis, E. Oral Candida Albicans Isolates from HIV-Positive Individuals Have Similar in Vitro Biofilm-Forming Ability and Pathogenicity as Invasive Candida Isolates. BMC Microbiol. 2011, 11, 247. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Zambrano, L.J.; Bordallo-Cardona, M.Á.; Borghi, E.; Falleni, M.; Tosi, D.; Muñoz, P.; Escribano, P.; Guinea, J. Candida Isolates Causing Candidemia Show Different Degrees of Virulence in Galleria Mellonella. Med. Mycol. 2020, 58, 83–92. [Google Scholar] [CrossRef]
- Perini, H.F.; Moralez, A.T.P.; Almeida, R.S.C.; Panagio, L.A.; Junior, A.O.G.; Barcellos, F.G.; Furlaneto-Maia, L.; Furlaneto, M.C. Phenotypic Switching in Candida Tropicalis Alters Host-Pathogen Interactions in a Galleria Mellonella Infection Model. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Németh, T.; Tóth, A.; Szenzenstein, J.; Horváth, P.; Nosanchuk, J.D.; Grózer, Z.; Tóth, R.; Papp, C.; Hamari, Z.; Vágvölgyi, C.; et al. Characterization of Virulence Properties in the C. Parapsilosis Sensu Lato Species. PLoS ONE 2013, 8, e68704. [Google Scholar] [CrossRef] [Green Version]
- Cirasola, D.; Sciota, R.; Vizzini, L.; Ricucci, V.; Morace, G.; Borghi, E. Experimental Biofilm-Related Candida Infections. Future Microbiol. 2013, 8, 799–805. [Google Scholar] [CrossRef]
- Rossoni, R.D.; Barbosa, J.O.; Vilela, S.F.G.; dos Santos, J.D.; Jorge, A.O.C.; Junqueira, J.C. Correlation of Phospholipase and Proteinase Production of Candida with in Vivo Pathogenicity in Galleria Mellonella. Braz. J. Oral Sci. 2013, 12, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Mroczyńska, M.; Brillowska-Dabrowska, A. First Report on Echinocandin Resistant Polish Candida Isolates. Acta Biochim. Pol. 2019, 66, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate Method for Detection of Phospholipase Activity in Candida Albicans. Sabouraudia J. Med Vet. Mycol. 1982, 20, 7–14. [Google Scholar] [CrossRef]
- Pham, L.T.T.; Pharkjaksu, S.; Chongtrakool, P.; Suwannakarn, K.; Ngamskulrungroj, P. A Predominance of Clade 17 Candida Albicans Isolated From Hemocultures in a Tertiary Care Hospital in Thailand. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- De Melo Riceto, É.B.; de Paula Menezes, R.; Penatti, M.P.A.; dos Santos Pedroso, R. Enzymatic and Hemolytic Activity in Different Candida Species. Rev. Iberoam. Micol. 2015, 32, 79–82. [Google Scholar] [CrossRef]
- Ranjith, K.; Chakravarthy, S.K.; Adicherla, H.; Sharma, S.; Shivaji, S. Temporal Expression of Genes in Biofilm-Forming Ocular Candida Albicans Isolated from Patients with Keratitis and Orbital Cellulitis. Invest. Ophthalmol. Vis. Sci. 2018, 59, 528–538. [Google Scholar] [CrossRef] [Green Version]



| MIC Range | Candida spp. | Isolate No. | Hemolytic Activity | Phospholipase Activity | Protease Activity | Esterase Activity | Biofilm | Survival |
|---|---|---|---|---|---|---|---|---|
| Hz Value | Pz Value | PRz Value | Ez Value | Abs600 | 6th Day | |||
| MIC value ≤ 0.016 mg/L | C. albicans | 71 | 0.59 | 1.0 | 0.35 | 0.40 | 0.51 | 0.6 |
| C. albicans | 380 | 0.52 | 1.0 | 0.39 | 0.45 | 0.89 | 0.1 | |
| C. albicans | 389 | 0.48 | 1.0 | 0.36 | 1.0 | 1.89 | 0.54 | |
| C. albicans | 1010 | 0.48 | 0.48 | 0.39 | 0.44 | 1.27 | 0.50 | |
| C. albicans | 1296 | 0.44 | 0.80 | 0.48 | 0.55 | 0.80 | 0.26 | |
| C. albicans | 1768 | - * | - | - | - | 0.11 | 0.40 | |
| C. albicans | 2023 | 1.0 | 0.48 | 0.36 | 0.44 | 0.49 | 0.15 | |
| C. albicans | 2029 | 0.57 | 0.88 | 0.35 | 0.37 | 1.34 | 0.32 | |
| C. albicans | 2608 | 0.48 | 1.0 | 0.38 | 0.46 | 1.04 | 0.15 | |
| C. palmioleophila | 370 | 0.67 | 0.51 | 0.37 | 1.0 | 1.15 | 0.40 | |
| C. parapsilosis | 395 | 1.0 | 1.0 | 0.50 | 1.0 | 0.42 | 1.0 | |
| MIC value 0.31–0.25 mg/L | C. albicans | 40 | 0.44 | 1.0 | 0.37 | 0.53 | 0.97 | 0.16 |
| C. albicans | 49 | 0.56 | 1.0 | 0.34 | 0.48 | 0.24 | 0.40 | |
| C. albicans | 114 | 0.67 | - | 0.45 | 0.53 | 0.12 | 0.0 | |
| C. albicans | 125 | 0.61 | 0.81 | 0.36 | 0.39 | 1.05 | 0.056 | |
| C. albicans | 286 | 0.48 | 1.0 | 0.35 | 0.44 | 0.97 | 0.33 | |
| C. glabrata | 1150 | 0.50 | - | 0.35 | 1.0 | 0.81 | 0.21 | |
| MIC value ≥ 0.5 mg/L | C. albicans | 54 | 0.48 | 1.0 | 0.32 | - | 1.56 | 0.0 |
| C. krusei | 102 | 0.52 | 1.0 | 0.40 | 1.0 | 0.98 | 1.0 | |
| C. palmioleophila | 4 | 1.0 | 0.63 | 0.40 | 0.52 | 1.05 | 0.44 | |
| C. palmioleophila | 368 | 1.0 | 1.0 | 0.48 | 1.0 | 0.97 | 0.90 | |
| C. parapsilosis | 105 | 1.0 | 1.0 | 0.59 | 1.0 | 2.33 | 1.0 | |
| C. parapsilosis | 441 | 0.59 | 1.0 | 0.39 | 0.63 | 1.01 | 0.7 | |
| C. parapsilosis | 443 | 1.0 | 1.0 | 0.35 | 1.0 | 0.69 | 0.65 | |
| C. inconspicua | 1444 | 0.56 | 1.0 | 0.48 | 1.0 | 1.58 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mroczyńska, M.; Brillowska-Dąbrowska, A. Virulence of Clinical Candida Isolates. Pathogens 2021, 10, 466. https://doi.org/10.3390/pathogens10040466
Mroczyńska M, Brillowska-Dąbrowska A. Virulence of Clinical Candida Isolates. Pathogens. 2021; 10(4):466. https://doi.org/10.3390/pathogens10040466
Chicago/Turabian StyleMroczyńska, Martyna, and Anna Brillowska-Dąbrowska. 2021. "Virulence of Clinical Candida Isolates" Pathogens 10, no. 4: 466. https://doi.org/10.3390/pathogens10040466
APA StyleMroczyńska, M., & Brillowska-Dąbrowska, A. (2021). Virulence of Clinical Candida Isolates. Pathogens, 10(4), 466. https://doi.org/10.3390/pathogens10040466






