Cytomegalovirus Disease in Renal Transplanted Patients: Prevalence, Determining Factors, and Influence on Graft and Patients Outcomes
Abstract
1. Introduction
2. Results
2.1. Cohort Characteristics
CMV Evaluation
2.2. Principal Outcomes
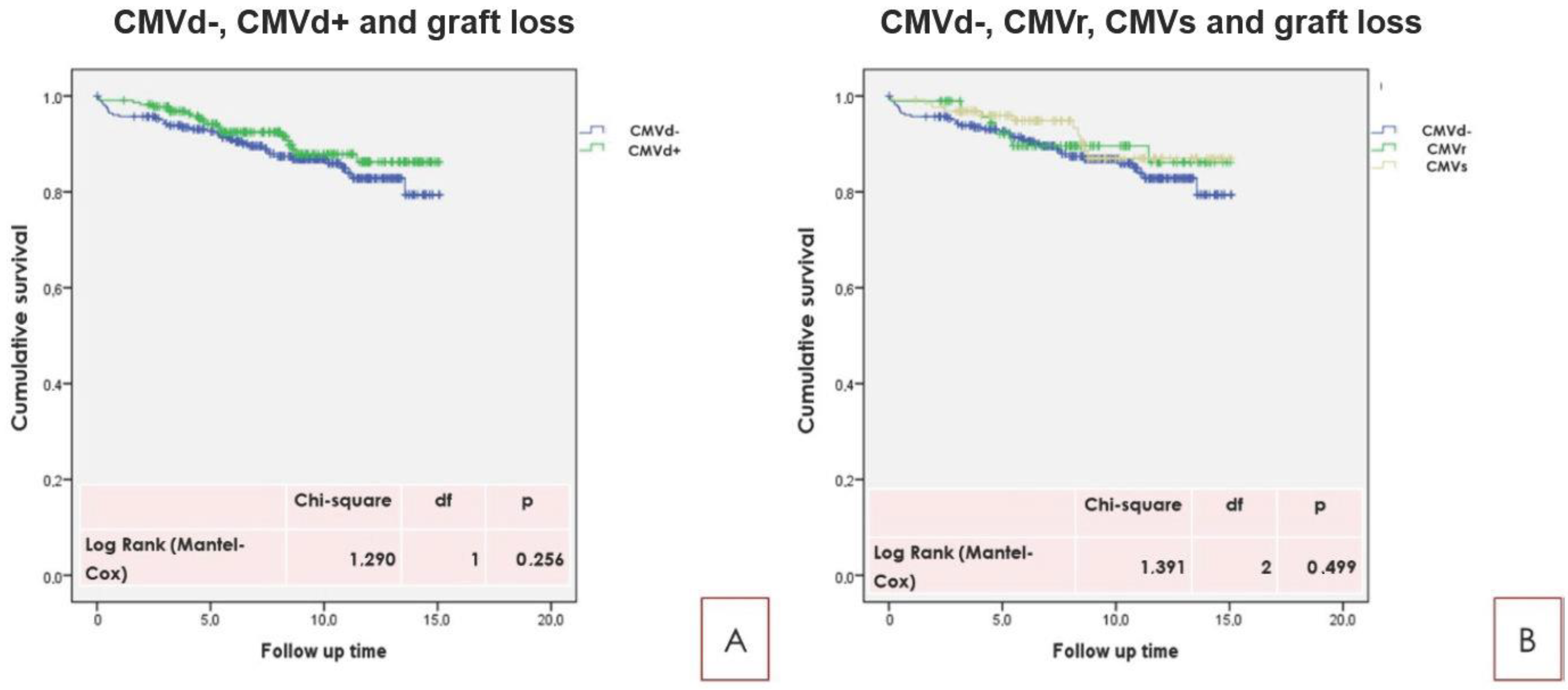
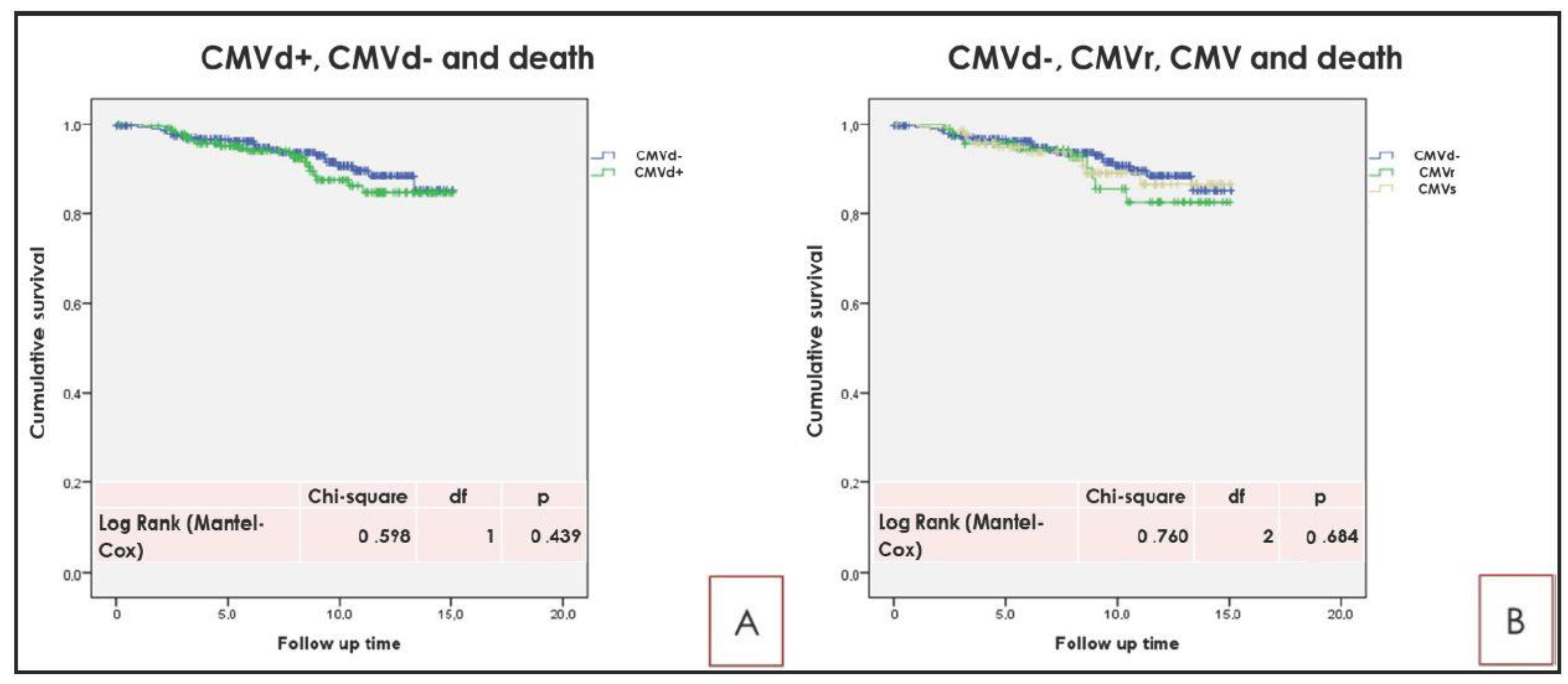
2.3. Principal Long Term Outcome Related to CMVd
3. Discussion
4. Materials and Methods
4.1. Study Design and Characteristics
4.2. Cytomegalovirus Analysis
4.3. Outcomes and Follow up
4.4. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of Chronic Kidney Disease in the United States. JAMA 2007, 298, 2038. [Google Scholar] [CrossRef]
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Jager, K.J. Clinical epidemiology ckd Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef]
- Silkensen, J.R. Long-Term Complications in Renal Transplantation. J. Am. Soc. Nephrol. 2000, 11, 582–588. [Google Scholar]
- Ramanan, P.; Razonable, R.R. Cytomegalovirus infections in solid organ transplantation: A review. Infect. Chemother. 2013, 45, 260–271. [Google Scholar] [CrossRef]
- Karuthu, S.; Blumberg, E.A. Common Infections in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials: Table 1. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [PubMed]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [CrossRef]
- Linares, L.; Sanclemente, G.; Cervera, C.; Hoyo, I.; Cofán, F.; Ricart, M.; Pérez-Villa, F.; Navasa, M.; Marcos, M.; Antón, A.; et al. Influence of Cytomegalovirus Disease in Outcome of Solid Organ Transplant Patients. Transplant. Proc. 2011, 43, 2145–2148. [Google Scholar] [CrossRef]
- Meesing, A.; Razonable, R.R. New Developments in the Management of Cytomegalovirus Infection after Transplantation. Drugs 2018, 78, 1085–1103. [Google Scholar] [CrossRef]
- Eid, A.J.; Razonable, R.R. New Developments in the Management of Cytomegalovirus Infection after Solid Organ Transplantation. Drugs 2010, 70, 965–981. [Google Scholar] [CrossRef]
- Witzke, O.; Hauser, I.A.; Bartels, M.; Wolf, G.; Wolters, H.; Nitschke, M. Valganciclovir Prophylaxis Versus Preemptive Therapy in Cytomegalovirus-Positive Renal Allograft Recipients: 1-Year Results of a Randomized Clinical Trial. Transplantation 2012, 93, 61–68. [Google Scholar] [CrossRef]
- Volpi, A.; Pica, F.; Cauletti, M.; Panà, A.; Rocchi, G. Cytomegalovirus infection in day care centers in Rome, Italy: Viral excretion in children and occupational risk among workers. J. Med. Virol. 1988, 26, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Barbi, M.; Binda, S.; Caroppo, S.; Calvario, A.; Germinario, C.; Bozzi, A.; Tanzi, M.L.; Veronesi, L.; Mura, I.; Piana, A.; et al. Multicity Italian study of congenital cytomegalovirus infection. Pediatr. Infect. Dis. J. 2006, 25, 156–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Kute, V.B.; Vanikar, A.V.; Shah, P.R.; Gumber, M.; Patel, H.; Godara, S.; Munjappa, B.; Sainaresh, V.; Engineer, D.; Jain, S.; et al. Post–Renal Transplant Cytomegalovirus Infection: Study of Risk Factors. Transplant. Proc. 2012, 44, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Selvey, L.A.; Lim, W.H.; Boan, P.; Swaminathan, R.; Slimings, C.; Harrison, A.E.; Chakera, A. Cytomegalovirus viraemia and mortality in renal transplant recipients in the era of antiviral prophylaxis. Lessons from the western Australian experience. BMC Infect. Dis. 2017, 17, 501. [Google Scholar] [CrossRef]
- Arthurs, S.K.; Eid, A.J.; Pedersen, R.A.; Kremers, W.K.; Cosio, F.G.; Patel, R.; Razonable, R.R. Delayed-Onset Primary Cytomegalovirus Disease and the Risk of Allograft Failure and Mortality after Kidney Transplantation. Clin. Infect. Dis. 2008, 46, 840–846. [Google Scholar] [CrossRef]
- Cohen, G.; Hörl, W.H. Immune Dysfunction in Uremia-An Update. Toxins 2012, 4, 962–990. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kilpatrick, R.D.; Kuwae, N.; McAllister, C.J.; Alcorn, H.; Kopple, J.D.; Greenland, S. Revisiting mortality predictability of serum albumin in the dialysis population: Time dependency, longitudinal changes and population-attributable fraction. Nephrol. Dial. Transplant. 2005, 20, 1880–1888. [Google Scholar] [CrossRef]
- Abbott, K. Hospitalizations for Cytomegalovirus Disease after Renal Transplantation in the United States. Ann. Epidemiol. 2002, 12, 402–409. [Google Scholar] [CrossRef]
- Courivaud, C.; Ladriere, M.; Toupance, O.; Caillard, S.; De Ligny, B.H.; Ryckelynck, J.-P.; Moulin, B.; Rieu, P.; Frimat, L.; Chalopin, J.-M.; et al. Impact of pre-transplant dialysis modality on post-transplant diabetes mellitus after kidney transplantation. Clin. Transplant. 2011, 25, 794–799. [Google Scholar] [CrossRef]
- Giakoustidis, D.; Antoniadis, A.; Fouzas, I.; Sklavos, A.; Ouzounidis, N.; Gakis, D.; Koubanagiti, K.; Myserlis, G.; Tsitlakidis, A.; Gerogiannis, I.; et al. Prevalence and clinical impact of cytomegalovirus infection and disease in renal transplantation: Ten years of experience in a single center. Transplant. Proc. 2012, 44, 2715–2717. [Google Scholar] [CrossRef]
- Davis, C.L.; Delmonico, F.L. Living-Donor Kidney Transplantation: A Review of the Current Practices for the Live Donor. J. Am. Soc. Nephrol. 2005, 16, 2098–2110. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I.M. Immune Dysfunction in Patients with Diabetes Mellitus. FEMS Immunol. Med Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Mocarski, E.S. Viral and host control of cytomegalovirus maturation. Trends Microbiol. 2012, 20, 392–401. [Google Scholar] [CrossRef] [PubMed]
- De Keyzer, K.; Van Laecke, S.; Peeters, P.; Vanholder, R. Human Cytomegalovirus and Kidney Transplantation: A Clinician’s Update. Am. J. Kidney Dis. 2011, 58, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Halloran, P.F. Immunosuppressive Drugs for Kidney Transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef]
- Couzi, L.; Helou, S.; Bachelet, T.; Martin, S.; Moreau, K.; Morel, D.; Lafon, M.E.; Garrigue, I.; Merville, P. Preemptive therapy versus valgancyclovir prophylaxis in cytomegalovirus-positive kidney transplant recipients receiving antithymocyte globulin induction. Transplant. Proc. 2012, 44, 2809–2813. [Google Scholar] [CrossRef]
- Issa, N.C.; Fishman, J.A. Infectious Complications of Antilymphocyte Therapies in Solid Organ Transplantation. Clin. Infect. Dis. 2009, 48, 772–786. [Google Scholar] [CrossRef]
- Brennan, D.C.; Legendre, C.; Patel, D.; Mange, K.; Wiland, A.; McCague, K.; Shihab, F.S. Cytomegalovirus Incidence Between Everolimus Versus Mycophenolate in De Novo Renal Transplants: Pooled Analysis of Three Clinical Trials. Am. J. Transplant. 2011, 11, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef]
- Soriano, S.; González, L.; Martín-Malo, A.; Rodríguez, M.; Aljama, P. C-reactive protein and low albumin are predictors of morbidity and cardiovascular events in chronic kidney disease (CKD) 3–5 patients. Clin. Nephrol. 2007, 67, 352–357. [Google Scholar] [CrossRef] [PubMed]
- van Ree, R.M.; Gross, S.; Zelle, D.M.; van der Heide, J.J.H.; Schouten, J.; van Son, W.J.; Gans, R.O.B.; Bakker, S.J.L. Influence of C-Reactive Protein and Urinary Protein Excretion on Prediction of Graft Failure and Mortality by Serum Albumin in Renal Transplant Recipients. Transplantation 2010, 89, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.W. Review article: Albumin as a drug-biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol. Ther. 2002, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Mocarski, E.S. Immune escape and exploitation strategies of cytomegaloviruses: Impact on and imitation of the major histocompatibility system. Cell Microbiol. 2004, 6, 707–717. [Google Scholar] [CrossRef]
- Liu, X.; Palaniyandi, S.; Zhu, I.; Tang, J.; Li, W.; Wu, X.; Ochsner, S.P.; Pauza, C.D.; Cohen, J.I.; Zhu, X. Human cytomegalovirus evades antibody-mediated immunity through endoplasmic reticulum-associated degradation of the FcRn receptor. Nat. Commun. 2019, 10, 3020. [Google Scholar] [CrossRef]
- Nowak, I.; Shaw, L.M. Mycophenolic acid binding to human serum albumin: Characterization and relation to pharmacodynamics. Clin. Chem. 1995, 41, 1011–1017. [Google Scholar] [CrossRef]
- Ma, X.; Yan, J.; Wang, Q.; Wu, D.; Li, H. Spectroscopy study and co-administration effect on the interaction of mycophenolic acid and human serum albumin. Int. J. Biol. Macromol. 2015, 77, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Bodnar, J.; Osman, F.; Jorgenson, M.R.; Astor, B.C.; Mandelbrot, D.A.; Parajuli, S. Serum Albumin Level Before Kidney Transplant Predicts Post-transplant BK and Possibly Cytomegalovirus Infection. Kidney Int. Rep. 2020, 5, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, S.J.; Joh, J.-W.; Shin, M.; Moon, J.; Jung, G.; Choi, G.-S.; Kwon, C.; Lee, S.-K. The Risk Factors for Cytomegalovirus Syndrome and Tissue-invasive Cytomegalovirus Disease in Liver Transplant Recipients Who Have Cytomegalovirus Antigenemia. Transplant. Proc. 2010, 42, 890–894. [Google Scholar] [CrossRef] [PubMed]
- USRDS 2018-Chapter 6: Transplantation. Available online: https://www.usrds.org/annual-data-report/ (accessed on 14 March 2021).
- Zoccali, C.; Massy, Z.; Caskey, F.J.; Couchoud, C.; Evans, M.; Finne, P.; Groothoff, J.W.; Harambat, J.; Heaf, J.G.; Jarraya, F.; et al. ERA-EDTA Registry Annual Report 2016; ERA-EDTA Registry: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Sagedal, S.; Hartmann, A.; Nordal, K.P.; Osnes, K.; Leivestad, T.; Foss, A.; Degré, M.; Fauchald, P.; Rollag, H. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004, 66, 329–337. [Google Scholar] [CrossRef]
- Erdbruegger, U.; Scheffner, I.; Mengel, M.; Schwarz, A.; Verhagen, W.; Haller, H.; Gwinner, W. Impact of CMV infection on acute rejection and long-term renal allograft function: A systematic analysis in patients with protocol biopsies and indicated biopsies. Nephrol. Dial. Transplant. 2012, 27, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; Scheffner, I.; Mengel, M.; Schwarz, A.; Haller, H.; Gwinner, W. Long-term impact of CMV infection on allografts and on patient survival in renal transplant patients with protocol biopsies. Am. J. Physiol. Ren. Physiol. 2015, 309, F925–F932. [Google Scholar] [CrossRef] [PubMed]
- The Modification of Diet in Renal Disease Study: Design, methods, and results from the feasibility study. Am. J. Kidney Dis. 1992, 20, 18–33. [CrossRef]
| Parameters | |
|---|---|
| Number of patients N | 505 |
| Age at RTx (years) | 50 (41–58) |
| Gender (M-F) N | 292–213 |
| Native kidney disease | |
| N (%) | |
| GNF | 106 (21) |
| GNC | 94 (18) |
| ADPKD | 92 (18) |
| OTHER | 89 (18) |
| CNP | 49 (10) |
| NDD | 46 (9) |
| VASCULITES | 26 (5) |
| UES | 3 (1) |
| Dialysis type (HD-PD-No) | |
| N (%) | 372-105-28 (74-21-5) |
| Dialysis vintage (months) | 52 (37–76) |
| RTx type | |
| (deceased-living donor) | |
| N (%) | 458-47 (91-9) |
| Number of RTx | |
| (1-2-3) | |
| N (%) | 412-89-4 (81-18-1) |
| Cold ischemia time (hours) | 13 (11–16) |
| Diabetes at RTx | |
| N (%) | 23 (4) |
| Steroid therapy before RTx | |
| N (%) | 189 (38) |
| CMV Receiving-Donor Serology | |
| N (%) | |
| CMV R−/D− | 17 (3) |
| CMV R−/D+ | 64 (13) |
| CMV R+/D− | 52 (10) |
| CMV R+/D+ | 372 (74) |
| HCV+ before RTx | |
| N (%) | 31 (6) |
| Drugs | T1 | T12 |
|---|---|---|
| Immunosuppressive induction therapy | ||
| Basiliximab N (%) | 442 (87) | NA |
| ATG N (%) | 58 (11) | |
| No Basiliximab—No ATG N (%) | 5 (2) | |
| Maintenance immunosuppressive therapy | ||
| MMF-MPA-Tac-Steroids-CyA-m-Tor-I | 443-479-463-60-15 | 440-407-458-56-34 |
| N (%) | (95-88-92-12-3) | (80-87-91-12-7) |
| Cumulative dose of steroids (mg) | 880 (840–1050) | 2722 (2598–3223) |
| Parameters | T1 | T12 | p |
|---|---|---|---|
| Body Weight (Kg) | 65 (56–73) | 68 (57–75) | <0.001 |
| SBP (mmHg) | 130 (120–140) | 130 (120–140) | 0.7 |
| DBP (mmHg) | 80 (75–90) | 80 (75–85) | 0.59 |
| s-Creatinine (mg/dL) | 1.38 (1.09–1.7) | 1.33 (1.1–1.61) | 0.21 |
| Prot-U (g/24h) | 0.203 (0.143–0.300) | 0.175 (0.113–0.251) | 0.43 |
| eGFR (mL/min) | 53 (40–67) | 51 (41–63) | <0.0001 |
| Uric acid (mg/dL) | 5.8 (4.8–6.9) | 6.4 (5.5–7.5) | <0.0001 |
| Hb (g/dL) | 11 (10.05–12) | 12.7 (11.8–13.8) | <0.0001 |
| s-Albumin (g/dL) | 4.2 (4.4–4.5) | 4.5 (4.2–4.7) | <0.0001 |
| Blood glucose (mg/dL) | 82 (72–93) | 82 (74–94) | 0.54 |
| HbA1C (%) | 5.5 (5.1–5.9) | 5.7 (5.4–6.1) | <0.0001 |
| CRP (mg/dL) | 0.25 (0.1–0.64) | 0.13 (0.1–0.390) | 0.001 |
| Parameters | CMVd+ (N = 225) | CMVd- (N = 280) | p |
|---|---|---|---|
| Age (years) | 53 (45–59) | 47 (39–57) | <0.0001 |
| Dialysis vintage (months) | 48 (26–75) | 51 (36–71) | 0.103 |
| Cold ischemia (hours) | 13 (11–16) | 14 (11–16) | 0.266 |
| Gender M-F | 115–110 | 177–103 | 0.0062 |
| Native kidney disease, N | 0.882 | ||
| GNF | 46 | 59 | |
| ADPKD | 42 | 49 | |
| OTHER | 43 | 47 | |
| GNC | 40 | 53 | |
| CNP | 25 | 24 | |
| NDD | 19 | 29 | |
| VASCULITIS | 9 | 17 | |
| UES | 1 | 2 | |
| Dialysis type (HD-PD-No) | 171-49-5 | 201-56-23 | 0.01 |
| RTx type (deceased-living) | 225-0 | 233-47 | <0.0001 |
| Number of RTx (1-2-3) | 193-32-0 | 218-58-4 | 0.023 |
| BAS-ATG Induction Therapy | 194-26 | 248-32 | 0.889 |
| Calcineurin Inhibitors T1 (Tac-Cya-No) | 199-25-1 | 244-36-0 | 0.412 |
| T1 MMF (Yes-No) | 218-7 | 261-19 | 0.070 |
| T1 m-Tor-I (Yes-No) | 3-222 | 14-266 | 0.015 |
| Calcineurin Inhibitors T12 (Tac-Cya-No) | 189-29-7 | 233-34-13 | 0.746 |
| T12 MMF (Yes-No) | 209-16 | 250-30 | 0.132 |
| T12 m-Tor-I (Yes-No) | 13-212 | 25-255 | 0.284 |
| Diabetes at RTx (Yes-No) | 0.0046 | ||
| N (%) | 18-207 (8–92) | 6-274 (2–98) | |
| Steroid therapy before RTx (Yes-No) | 0.343 | ||
| N (%) | 88-137 (39–61) | 118-162 (42–58) | |
| HCV+ before RTx (Yes-No) | 0.576 | ||
| N (%) | 14-211 (6–94) | 22-258 (8–92) | |
| CMV Recipient/Donor Serology | 0.01 | ||
| N | |||
| CMV R−/D− | 4 | 13 | |
| CMV R−/D+ | 38 | 26 | |
| CMV R+/D− | 18 | 34 | |
| CMV R+/D+ | 165 | 207 |
| Parameters T1 | CMVd+ (N 225) | CMVd- (N 280) | p |
|---|---|---|---|
| Body weight (Kg) | 65 (55–73) | 64.5 (56.7–73.05) | 0.846 |
| SBP (mmHg) | 130 (120–145) | 130 (120–140) | 0.819 |
| DBP (mmHg) | 80 (70–90) | 80 (75–90) | 0.471 |
| s-Creatinine (mg/dL) | 1.39 (1.1–1.77) | 1.37 (1.07–1.67) | 0.225 |
| Prot-U (g/24h) | 0.198 (0.140–0.304) | 0.210 (0.151–0.299) | 0.624 |
| eGFR (mL/min) | 49 (38–64) | 55 (44–69) | 0.006 |
| Uric acid (mg/dL) | 6.1 (5.0–7.2) | 5.7 (4.7–6.7) | 0.015 |
| Hb (g/dL) | 10.80 (9.9–11.8) | 11.1 (10.3–12.1) | 0.026 |
| s-Albumin (g/dL) | 4.2 (3.9–4.3) | 4.3 (4.03–4.50) | <0.0001 |
| Glycemia (mg/dL) | 84 (72–94) | 81 (71.25–93) | 0.123 |
| HbA1C (%) | 5.5 (5.1–5.97) | 5.45 (5–5.8) | 0.125 |
| CRP (mg/dL) | 0.30 (0.10–0.85) | 0.2 (0.09–0.55) | 0.008 |
| Cumulative steroids during the first year of transplantation (mg) | 890.00 (840.00–1125.00) | 870 (835–995) | 0.006 |
| Parameter | Coefficient | Standard Error | Odds Ratio | CI | p |
|---|---|---|---|---|---|
| Age at transplantation | 0.01 | 0.008 | 1.01 | 0.99–1.03 | 0.11 |
| s-Albumin T1 | −0.69 | 0.26 | 0.50 | 0.29–0.84 | 0.009 |
| Hb T1 | 0.01 | 0.07 | 1.01 | 0.87–1.17 | 0.85 |
| Steroids T1 | 0.0004 | 0.0002 | 1.0 | 1.0–1.0 | 0.06 |
| Parameter | Coefficient | Standard Error | Odds Ratio | CI | p |
|---|---|---|---|---|---|
| Age at transplantation | 0.014 | 0.009 | 1.014 | 0.99–1.03 | 0.122 |
| s-Albumin T1 | −0.718 | 0.271 | 0.487 | 0.28–0.82 | 0.008 |
| Hb T1 | −0.0005 | 0.075 | 0.999 | 0.86–1.15 | 0.994 |
| Steroids T1 | 0.0003 | 0.0002 | 1.0004 | 0.999–1.00 | 0.084 |
| CMV serology D+/R- | 0.77 | 0.30 | 2.16 | 1.18–3.95 | 0.01 |
| Parameters | CMVd+ (N = 225) | CMVd− (N = 280) | p |
|---|---|---|---|
| D (%) | 0.20 | ||
| No | 204 | 243 | |
| Yes | 21 | 37 | |
| sCr > 50% + D+ | 0.4672 | ||
| No | 183 | 220 | |
| Yes | 42 | 60 | |
| eGFRr > 50% + D+ | 0.0751 | ||
| No | 197 | 228 | |
| Yes | 28 | 52 | |
| eGFR: Variation/year of FU (mL/min/year) | −0.7 (−1.2/+1.1) | −0.9 (−1.6/+1.1) | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, C.M.; Molinari, P.; Gandolfo, M.; Campise, M.; Cresseri, D.; Regalia, A.; Favi, E.; Li, M.; Ikehata, M.; Delbue, S.; et al. Cytomegalovirus Disease in Renal Transplanted Patients: Prevalence, Determining Factors, and Influence on Graft and Patients Outcomes. Pathogens 2021, 10, 473. https://doi.org/10.3390/pathogens10040473
Alfieri CM, Molinari P, Gandolfo M, Campise M, Cresseri D, Regalia A, Favi E, Li M, Ikehata M, Delbue S, et al. Cytomegalovirus Disease in Renal Transplanted Patients: Prevalence, Determining Factors, and Influence on Graft and Patients Outcomes. Pathogens. 2021; 10(4):473. https://doi.org/10.3390/pathogens10040473
Chicago/Turabian StyleAlfieri, Carlo Maria, Paolo Molinari, Mariateresa Gandolfo, Mariarosaria Campise, Donata Cresseri, Anna Regalia, Evaldo Favi, Min Li, Masami Ikehata, Serena Delbue, and et al. 2021. "Cytomegalovirus Disease in Renal Transplanted Patients: Prevalence, Determining Factors, and Influence on Graft and Patients Outcomes" Pathogens 10, no. 4: 473. https://doi.org/10.3390/pathogens10040473
APA StyleAlfieri, C. M., Molinari, P., Gandolfo, M., Campise, M., Cresseri, D., Regalia, A., Favi, E., Li, M., Ikehata, M., Delbue, S., & Messa, P. (2021). Cytomegalovirus Disease in Renal Transplanted Patients: Prevalence, Determining Factors, and Influence on Graft and Patients Outcomes. Pathogens, 10(4), 473. https://doi.org/10.3390/pathogens10040473








