Modulation of Splenic B Cell Subsets during Experimental Leishmania donovani Infection in BALB/c Mice
Abstract
:1. Introduction
2. Results
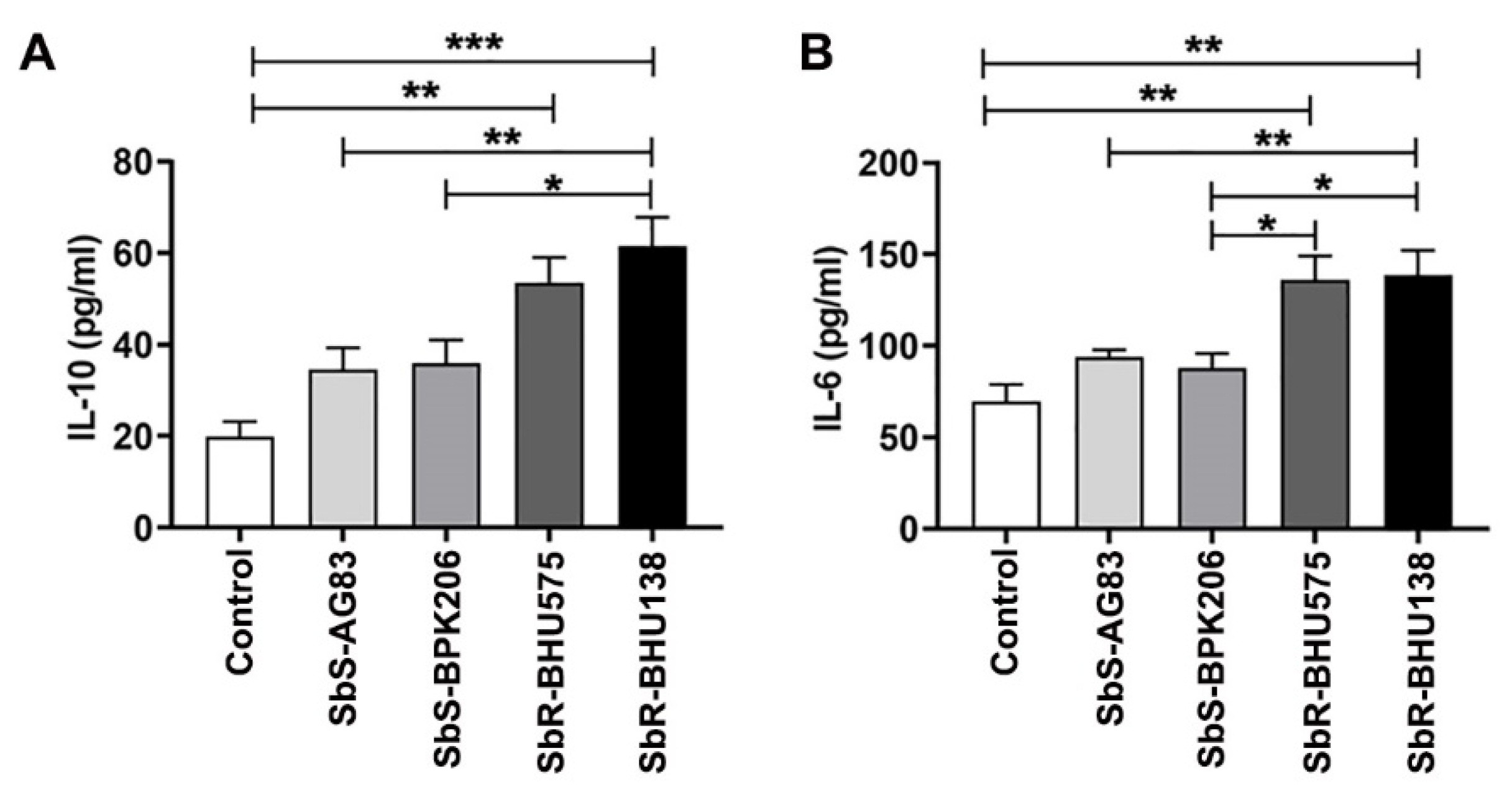
2.1. Interaction between Purified Splenic B Cells with Leishmania Promastigotes of Antimony Resistant Strains Promote Higher Level of IL-10 and IL-6 Secretion as Compared to Its Sensitive Counterpart
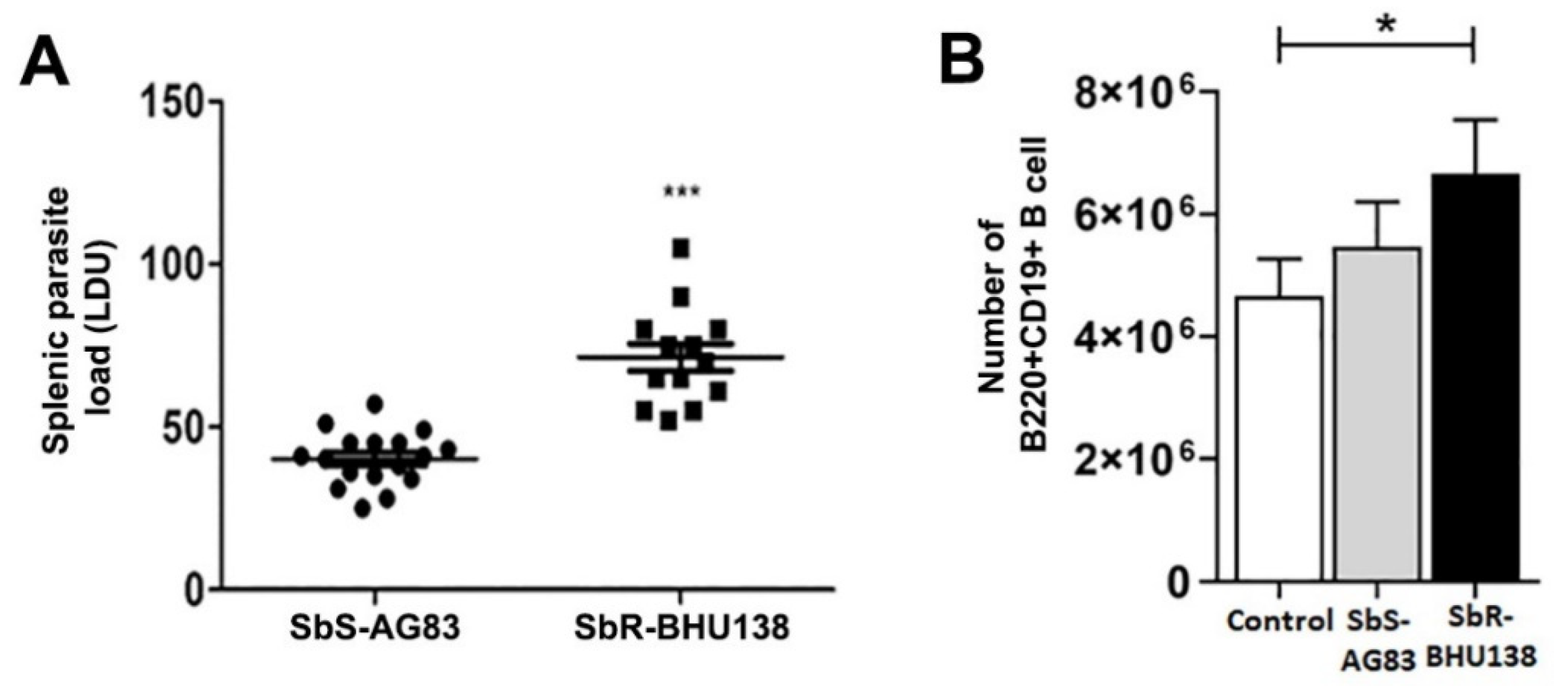
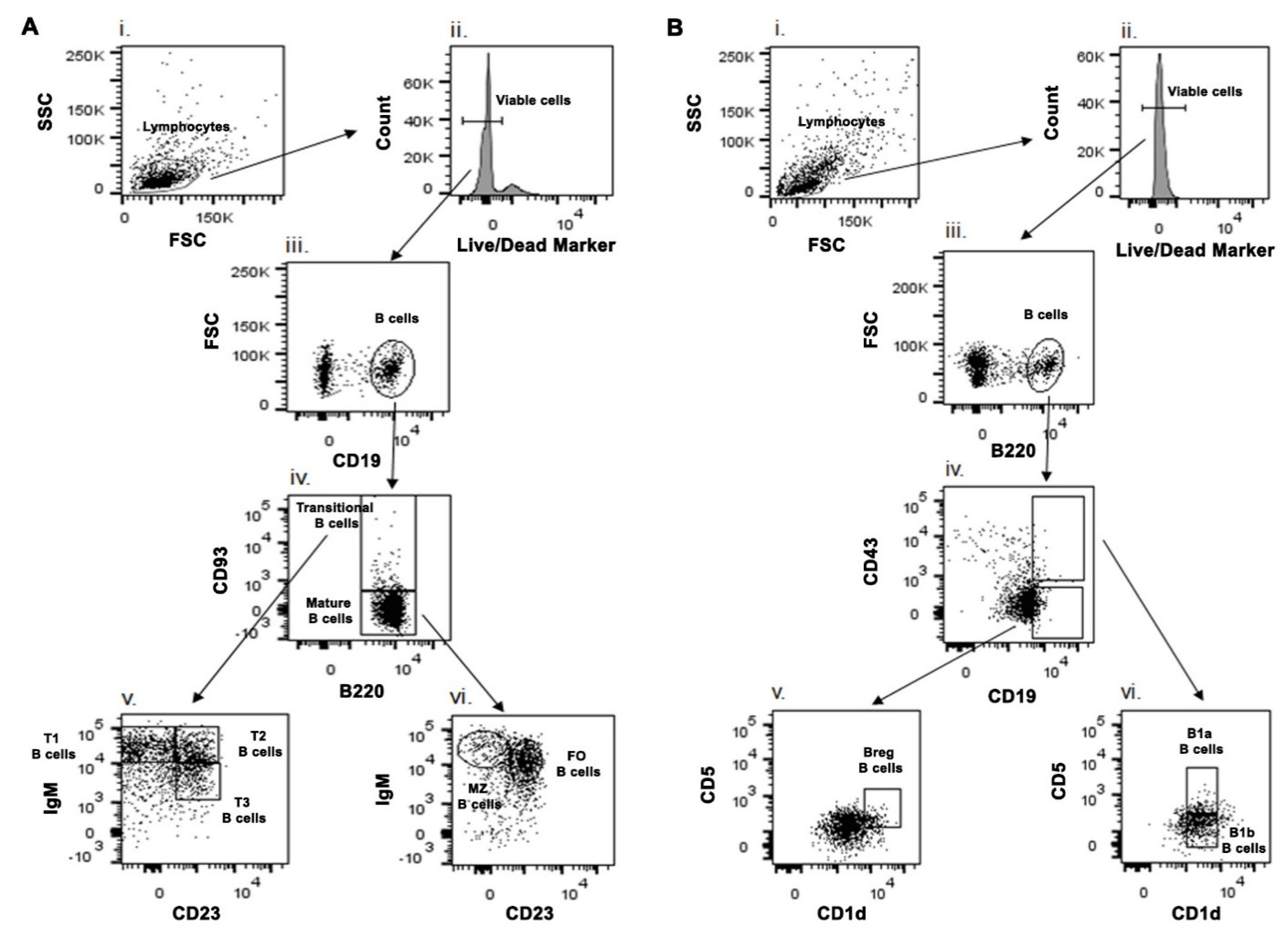
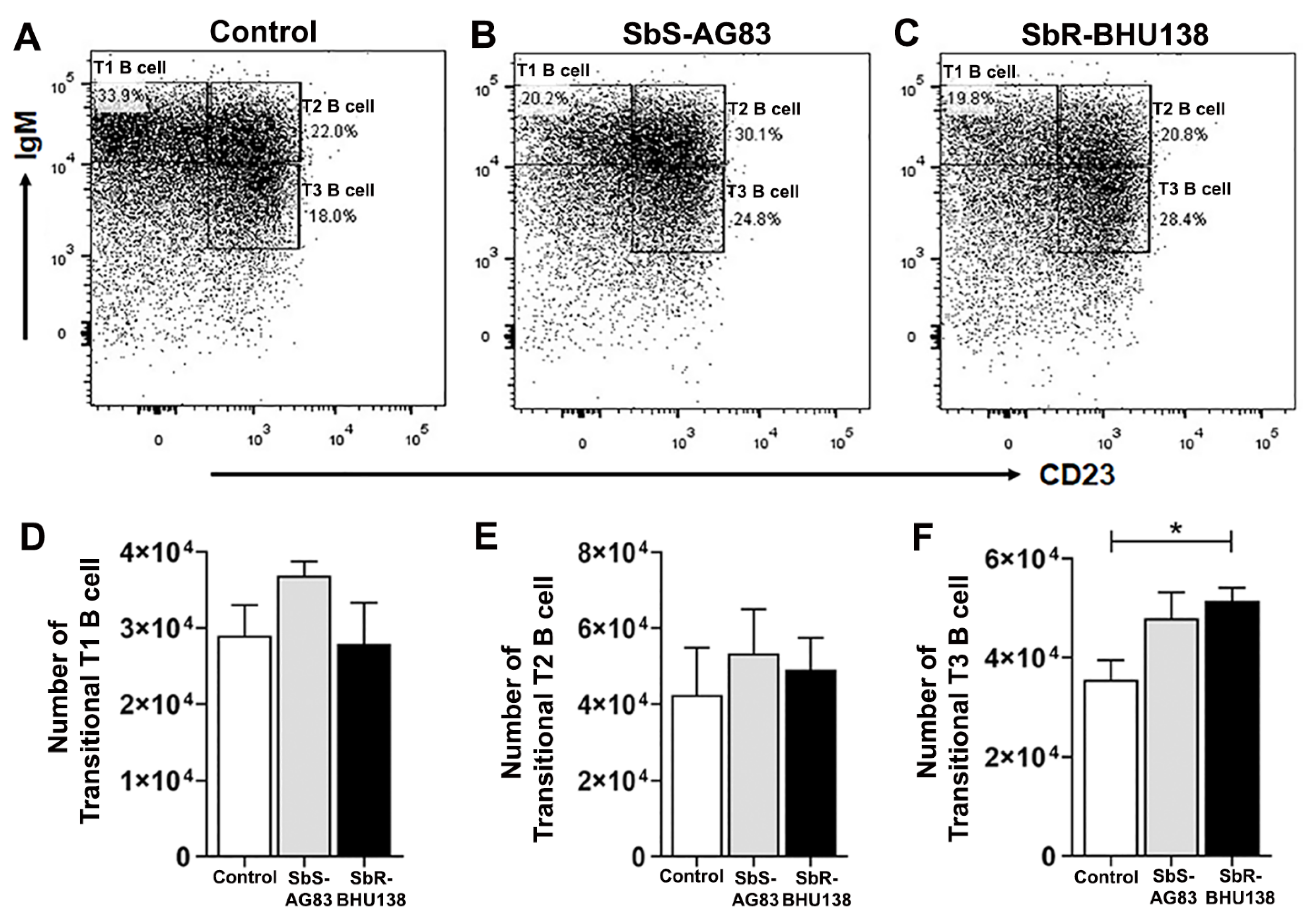
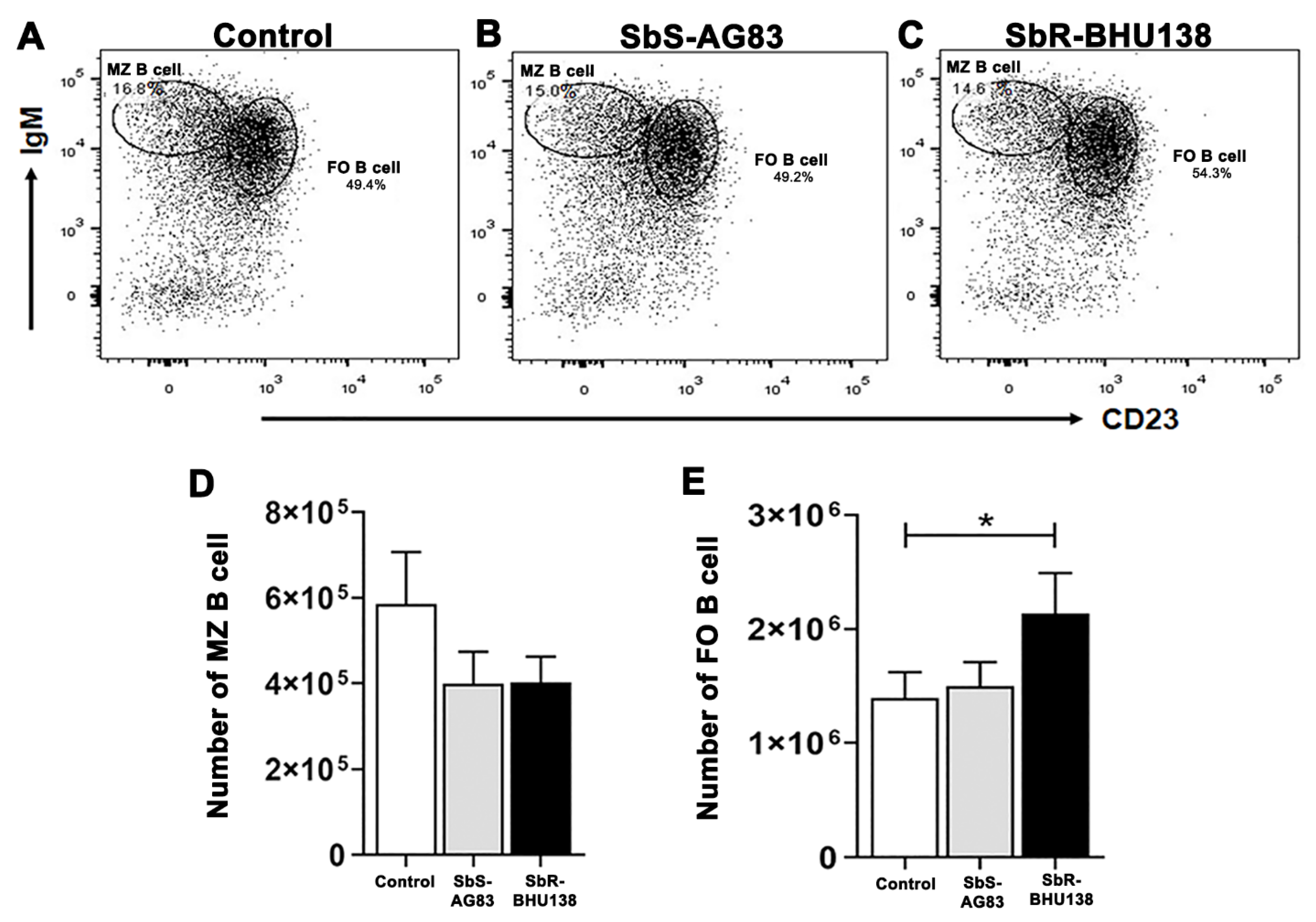
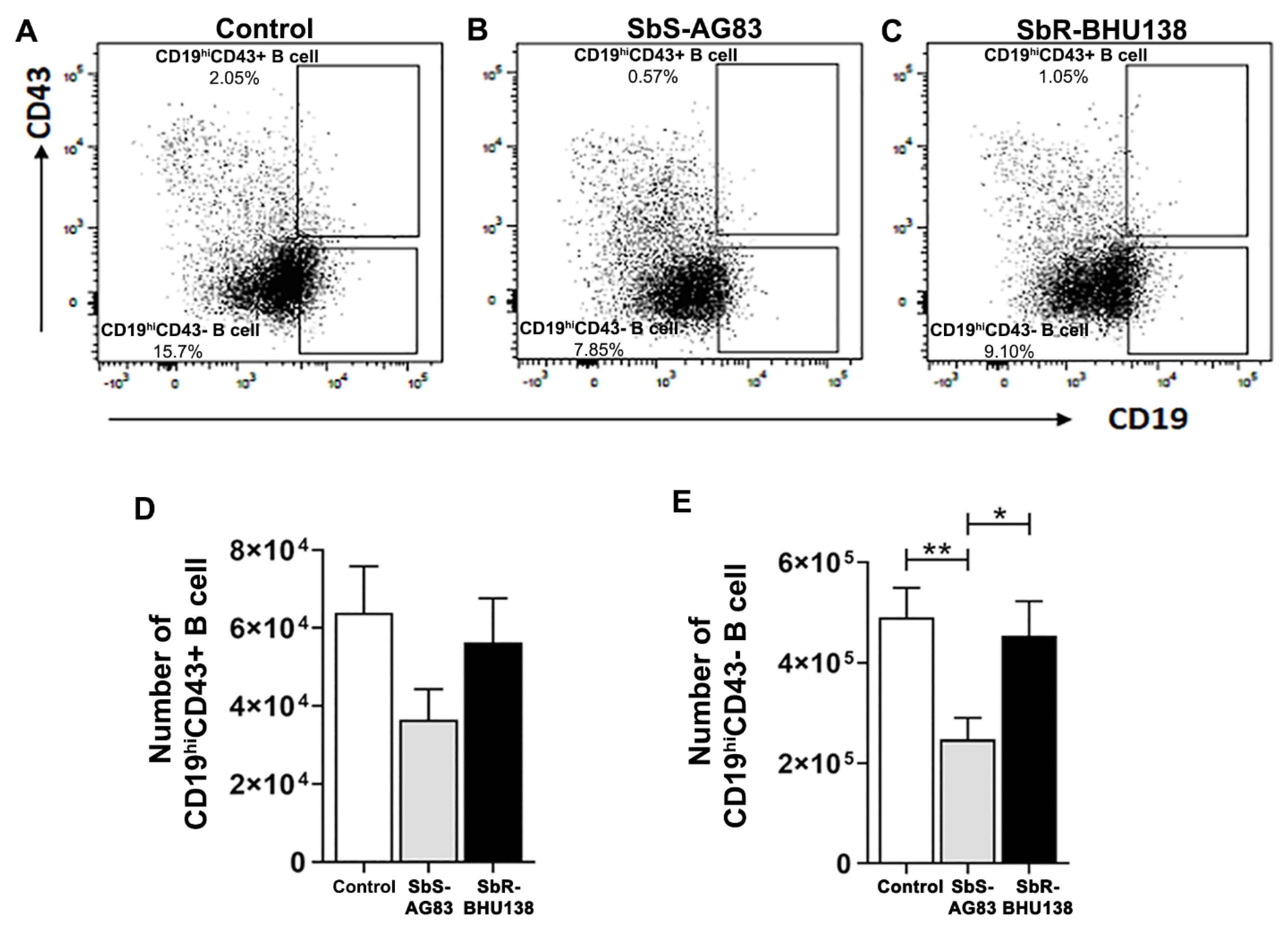
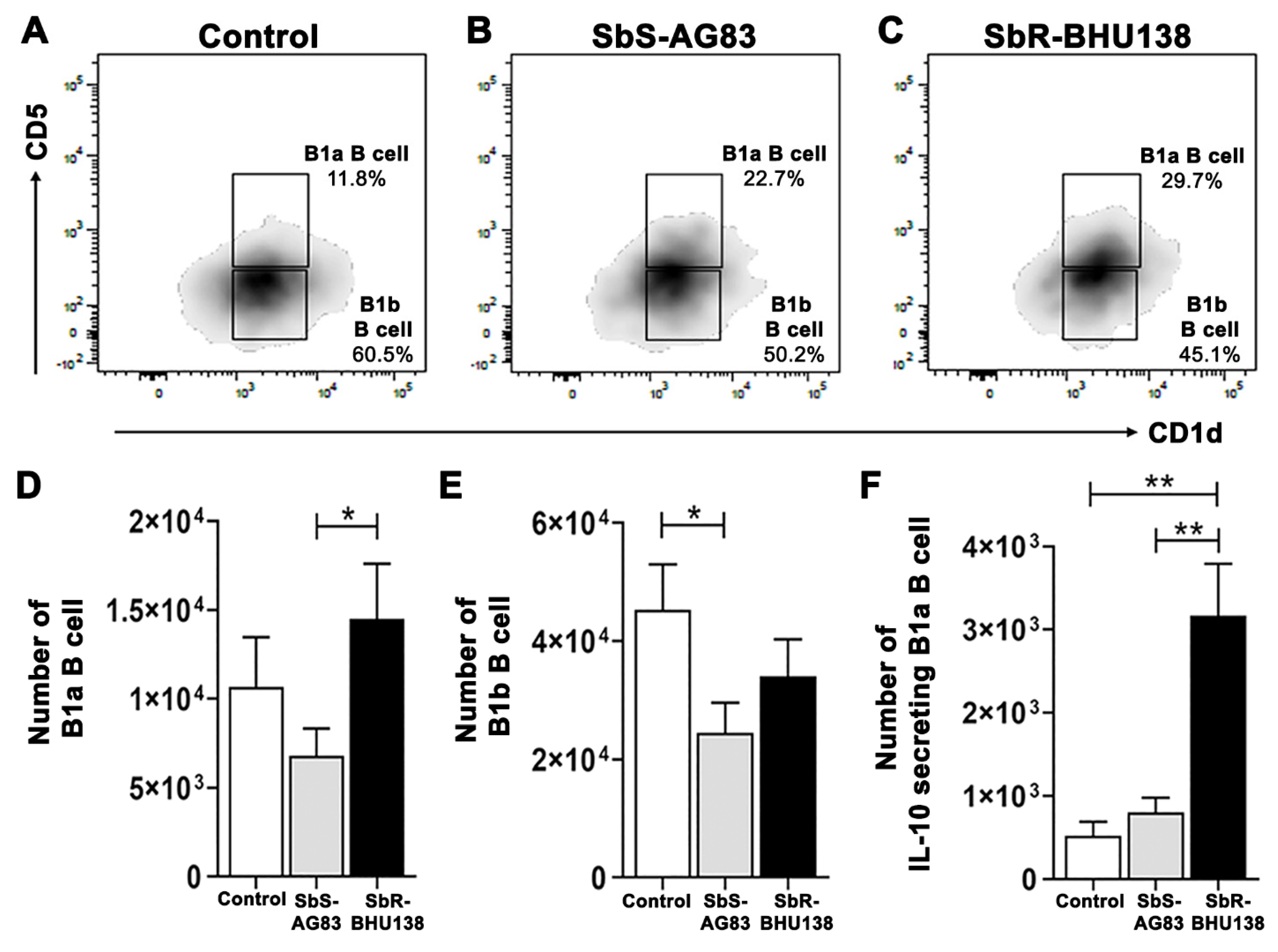
2.2. Identification of Splenic B Cell Subsets as a Source of IL-10 and IL-6 Cytokine Secretion during Leishmania Infection
2.3. Effect on Lyn Kinase While Infection with Leishmania Parasites
3. Discussion
4. Materials and Methods
4.1. Parasites
4.2. Determination of Splenic Parasitic Load
4.3. Reagents and Chemicals
4.4. Phenotypic Characterization of B Cell Subsets Using Different Cell Surface Markers
4.5. Intracellular Staining of Cytokines
4.6. Purification of B Cells and Analysis of Criteria of Purity
4.7. RT-PCR Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Lira, R.; Sundar, S.; Makharia, A.; Kenney, R.; Gam, A.; Saraiva, E.; Sacks, D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 1999, 180, 564–567. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, R.; Mukherjee, S.; Mukherjee, B.; Naskar, K.; Mondal, D.; Decuypere, S.; Ostyn, B.; Prajapati, V.K.; Sundar, S.; Dujardin, J.C.; et al. Characterisation of antimony-resistant Leishmania donovani isolates: Biochemical and biophysical studies and interaction with host cells. Int. J. Parasitol. 2011, 41, 1311–1321. [Google Scholar] [CrossRef]
- Bosque, F.; Saravia, N.G.; Valderrama, L.; Milon, G. Distinct innate and acquired immune responses to Leishmania in putative susceptible and resistant human populations endemically exposed to L. (Viannia) panamensis infection. Scand. J. Immunol. 2000, 51, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Mukherjee, B.; Mukhopadhyay, R.; Naskar, K.; Sundar, S.; Dujardin, J.C.; Roy, S. Imipramine exploits histone deacetylase 11 to increase the IL-12/IL-10 ratio in macrophages infected with antimony-resistant Leishmania donovani and clears organ parasites in experimental infection. J. Immunol. 2014, 193, 4083–4094. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, B.; Mukhopadhyay, R.; Bannerjee, B.; Chowdhury, S.; Mukherjee, S.; Naskar, K.; Allam, U.S.; Chakravortty, D.; Sundar, S.; Dujardin, J.C.; et al. Antimony-resistant but not antimony-sensitive Leishmania donovani up-regulates host IL-10 to overexpress multidrug-resistant protein 1. Proc. Natl. Acad. Sci. USA 2013, 110, E575–E582. [Google Scholar] [CrossRef] [Green Version]
- Guha, R.; Das, S.; Ghosh, J.; Sundar, S.; Dujardin, J.C.; Roy, S. Antimony resistant Leishmania donovani but not sensitive ones drives greater frequency of potent T-regulatory cells upon interaction with human PBMCs: Role of IL-10 and TGF-beta in early immune response. PLoS Negl. Trop. Dis. 2014, 8, e2995. [Google Scholar] [CrossRef]
- Mukherjee, B.; Mukherjee, K.; Nanda, P.; Mukhopadhayay, R.; Ravichandiran, V.; Bhattacharyya, S.N.; Roy, S. Probing the molecular mechanism of aggressive infection by antimony resistant Leishmania donovani. Cytokine 2020, 145, 155245. [Google Scholar] [CrossRef]
- Sacks, D.L.; Scott, P.A.; Asofsky, R.; Sher, F.A. Cutaneous leishmaniasis in anti-IgM-treated mice: Enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J. Immunol. 1984, 132, 2072–2077. [Google Scholar]
- Smelt, S.C.; Cotterell, S.E.; Engwerda, C.R.; Kaye, P.M. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J. Immunol. 2000, 164, 3681–3688. [Google Scholar] [CrossRef] [Green Version]
- Baumgarth, N. The double life of a B-1 cell: Self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011, 11, 34–46. [Google Scholar] [CrossRef]
- Allman, D.; Lindsley, R.C.; DeMuth, W.; Rudd, K.; Shinton, S.A.; Hardy, R.R. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 2001, 167, 6834–6840. [Google Scholar] [CrossRef]
- Loder, F.; Mutschler, B.; Ray, R.J.; Paige, C.J.; Sideras, P.; Torres, R.; Lamers, M.C.; Carsetti, R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J. Exp. Med. 1999, 190, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.F.; Flores-Langarica, A.; Bobat, S.; Medina, C.C.D.; Cook, C.N.; Ross, E.A.; Lopez-Macias, C.; Henderson, I.R. B1b cells recognize protective antigens after natural infection and vaccination. Front. Immunol. 2014, 5, 535. [Google Scholar] [CrossRef] [Green Version]
- Youinou, P.; Taher, T.E.; Pers, J.O.; Mageed, R.A.; Renaudineau, Y. B lymphocyte cytokines and rheumatic autoimmune disease. Arthritis Rheum. 2009, 60, 1873–1880. [Google Scholar] [CrossRef]
- Silva-Barrios, S.; Smans, M.; Duerr, C.U.; Qureshi, S.T.; Fritz, J.H.; Descoteaux, A.; Stager, S. Innate Immune B Cell Activation by Leishmania donovani Exacerbates Disease and Mediates Hypergammaglobulinemia. Cell Rep. 2016, 15, 2427–2437. [Google Scholar] [CrossRef] [Green Version]
- Arcanjo, A.F.; Nico, D.; de Castro, G.M.M.; da Silva Fontes, Y.; Saltarelli, P.; Decote-Ricardo, D.; Nunes, M.P.; Ferreira-Pereira, A.; Palatnik-de-Sousa, C.B.; Freire-de-Lima, C.G.; et al. Dependency of B-1 Cells in the Maintenance of Splenic Interleukin-10 Producing Cells and Impairment of Macrophage Resistance in Visceral Leishmaniasis. Front. Microbiol. 2017, 8, 978. [Google Scholar] [CrossRef] [Green Version]
- Mangan, N.E.; Fallon, R.E.; Smith, P.; van Rooijen, N.; McKenzie, A.N.; Fallon, P.G. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J. Immunol. 2004, 173, 6346–6356. [Google Scholar] [CrossRef] [Green Version]
- Gillan, V.; Lawrence, R.A.; Devaney, E. B cells play a regulatory role in mice infected with the L3 of Brugia pahangi. Int. Immunol. 2005, 17, 373–382. [Google Scholar] [CrossRef]
- Candando, K.M.; Lykken, J.M.; Tedder, T.F. B10 cell regulation of health and disease. Immunol. Rev. 2014, 259, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Nishizumi, H.; Taniuchi, I.; Yamanashi, Y.; Kitamura, D.; Ilic, D.; Mori, S.; Watanabe, T.; Yamamoto, T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity 1995, 3, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Scapini, P.; Lamagna, C.; Hu, Y.; Lee, K.; Tang, Q.; DeFranco, A.L.; Lowell, C.A. B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc. Natl. Acad. Sci. USA 2011, 108, E823–E832. [Google Scholar] [CrossRef] [Green Version]
- Argov, S.; Jaffe, C.L.; Krupp, M.; Slor, H.; Shoenfeld, Y. Autoantibody production by patients infected with Leishmania. Clin. Exp. Immunol. 1989, 76, 190–197. [Google Scholar] [PubMed]
- Deak, E.; Jayakumar, A.; Cho, K.W.; Goldsmith-Pestana, K.; Dondji, B.; Lambris, J.D.; McMahon-Pratt, D. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur. J. Immunol. 2010, 40, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Schaut, R.G.; Lamb, I.M.; Toepp, A.J.; Scott, B.; Mendes-Aguiar, C.O.; Coutinho, J.F.; Jeronimo, S.M.; Wilson, M.E.; Harty, J.T.; Waldschmidt, T.J.; et al. Regulatory IgDhi B Cells Suppress T Cell Function via IL-10 and PD-L1 during Progressive Visceral Leishmaniasis. J. Immunol. 2016, 196, 4100–4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squires, K.E.; Kirsch, M.; Silverstein, S.C.; Acosta, A.; McElrath, M.J.; Murray, H.W. Defect in the tissue cellular immune response: Experimental visceral leishmaniasis in euthymic C57BL/6 ep/ep mice. Infect. Immun. 1990, 58, 3893–3898. [Google Scholar] [CrossRef] [Green Version]
- Ronet, C.; Hauyon-La Torre, Y.; Revaz-Breton, M.; Mastelic, B.; Tacchini-Cottier, F.; Louis, J.; Launois, P. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J. Immunol. 2010, 184, 886–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamagna, C.; Hu, Y.; DeFranco, A.L.; Lowell, C.A. B cell-specific loss of Lyn kinase leads to autoimmunity. J. Immunol. 2014, 192, 919–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzel, D.M.; Rhodes, E.L.; Li, S.; McMahon-Pratt, D.; Koleske, A.J. The Src kinases Hck, Fgr and Lyn activate Arg to facilitate IgG-mediated phagocytosis and Leishmania infection. J. Cell Sci. 2016, 129, 3130–3143. [Google Scholar] [CrossRef] [Green Version]
- Teague, B.N.; Pan, Y.; Mudd, P.A.; Nakken, B.; Zhang, Q.; Szodoray, P.; Kim-Howard, X.; Wilson, P.C.; Farris, A.D. Cutting edge: Transitional T3 B cells do not give rise to mature B cells, have undergone selection, and are reduced in murine lupus. J. Immunol. 2007, 178, 7511–7515. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cabezas, B.; Cecilio, P.; Gaspar, T.B.; Gartner, F.; Vasconcellos, R.; Cordeiro-da-Silva, A. Understanding Resistance vs. Susceptibility in Visceral Leishmaniasis Using Mouse Models of Leishmania infantum Infection. Front. Cell. Infect. Microbiol. 2019, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.B.; Sater, R.A.; Fields, M.L.; Erikson, J.; Monroe, J.G. CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. Int. Immunol. 2002, 14, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Cyster, J.G.; Allen, C.D.C. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, Y.; Han, J.; Yang, M.; Zhu, J.; Jin, T. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J. Transl. Med. 2020, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.M.; Svensson, M.; Ato, M.; Maroof, A.; Polley, R.; Stager, S.; Zubairi, S.; Engwerda, C.R. The immunopathology of experimental visceral leishmaniasis. Immunol. Rev. 2004, 201, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.; Kondo, M.; Chen, H.; Kelsoe, G. Effects of acute and chronic inflammation on B-cell development and differentiation. J. Invest. Dermatol. 2009, 129, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Dayakar, A.; Chandrasekaran, S.; Kuchipudi, S.V.; Kalangi, S.K. Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy. Front. Immunol. 2019, 10, 670. [Google Scholar] [CrossRef] [Green Version]
- Gonzaga, W.F.; Xavier, V.; Vivanco, B.C.; Lopes, J.D.; Xander, P. B-1 cells contribute to susceptibility in experimental infection with Leishmania (Leishmania) chagasi. Parasitology 2015, 142, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Passos, L.S.A.; Magalhaes, L.M.D.; Soares, R.P.; Marques, A.F.; Alves, M.L.R.; Giunchetti, R.C.; Nunes, M.; Gollob, K.J.; Dutra, W.O. Activation of Human CD11b(+) B1 B-Cells by Trypanosoma cruzi-Derived Proteins Is Associated With Protective Immune Response in Human Chagas Disease. Front. Immunol. 2018, 9, 3015. [Google Scholar] [CrossRef] [Green Version]
- Bankoti, R.; Gupta, K.; Levchenko, A.; Stager, S. Marginal zone B cells regulate antigen-specific T cell responses during infection. J. Immunol. 2012, 188, 3961–3971. [Google Scholar] [CrossRef] [Green Version]
- Andreani, G.; Ouellet, M.; Menasria, R.; Gomez, A.M.; Barat, C.; Tremblay, M.J. Leishmania infantum amastigotes trigger a subpopulation of human B cells with an immunoregulatory phenotype. PLoS Negl. Trop. Dis. 2015, 9, e0003543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, H.W. Accelerated control of visceral Leishmania donovani infection in interleukin-6-deficient mice. Infect. Immun. 2008, 76, 4088–4091. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, F.M.C.; Dupin, T.V.; Toledo, M.D.S.; Reis, N.; Ribeiro, K.; Cronemberger-Andrade, A.; Rugani, J.N.; De Lorenzo, B.H.P.; Novaes, E.B.R.R.; Soares, R.P.; et al. Extracellular Vesicles Released by Leishmania (Leishmania) amazonensis Promote Disease Progression and Induce the Production of Different Cytokines in Macrophages and B-1 Cells. Front. Microbiol. 2018, 9, 3056. [Google Scholar] [CrossRef]
- Ansari, N.A.; Saluja, S.; Salotra, P. Elevated levels of interferon-gamma, interleukin-10, and interleukin-6 during active disease in Indian kala azar. Clin. Immunol. 2006, 119, 339–345. [Google Scholar] [CrossRef]
- Barr, T.A.; Shen, P.; Brown, S.; Lampropoulou, V.; Roch, T.; Lawrie, S.; Fan, B.; O’Connor, R.A.; Anderton, S.M.; Bar-Or, A.; et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 2012, 209, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Guha, R.; Gupta, D.; Rastogi, R.; Vikram, R.; Krishnamurthy, G.; Bimal, S.; Roy, S.; Mukhopadhyay, A. Vaccination with leishmania hemoglobin receptor-encoding DNA protects against visceral leishmaniasis. Sci. Transl. Med. 2013, 5, 202ra121. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, K.; Das, S.; Naskar, K.; Roy, S. Modulation of Splenic B Cell Subsets during Experimental Leishmania donovani Infection in BALB/c Mice. Pathogens 2021, 10, 814. https://doi.org/10.3390/pathogens10070814
Mondal K, Das S, Naskar K, Roy S. Modulation of Splenic B Cell Subsets during Experimental Leishmania donovani Infection in BALB/c Mice. Pathogens. 2021; 10(7):814. https://doi.org/10.3390/pathogens10070814
Chicago/Turabian StyleMondal, Koushik, Shantanabha Das, Khudiram Naskar, and Syamal Roy. 2021. "Modulation of Splenic B Cell Subsets during Experimental Leishmania donovani Infection in BALB/c Mice" Pathogens 10, no. 7: 814. https://doi.org/10.3390/pathogens10070814






