Undiagnosed Cases of Human Pneumonia Following Exposure to Chlamydia psittaci from an Infected Rosella Parrot
Abstract
:1. Introduction
2. Case Presentation
3. Follow Up Investigation in the Adelaide Rosella
3.1. DNA Extraction and C. psittaci-Specific qPCR
3.2. Molecular Characterisation
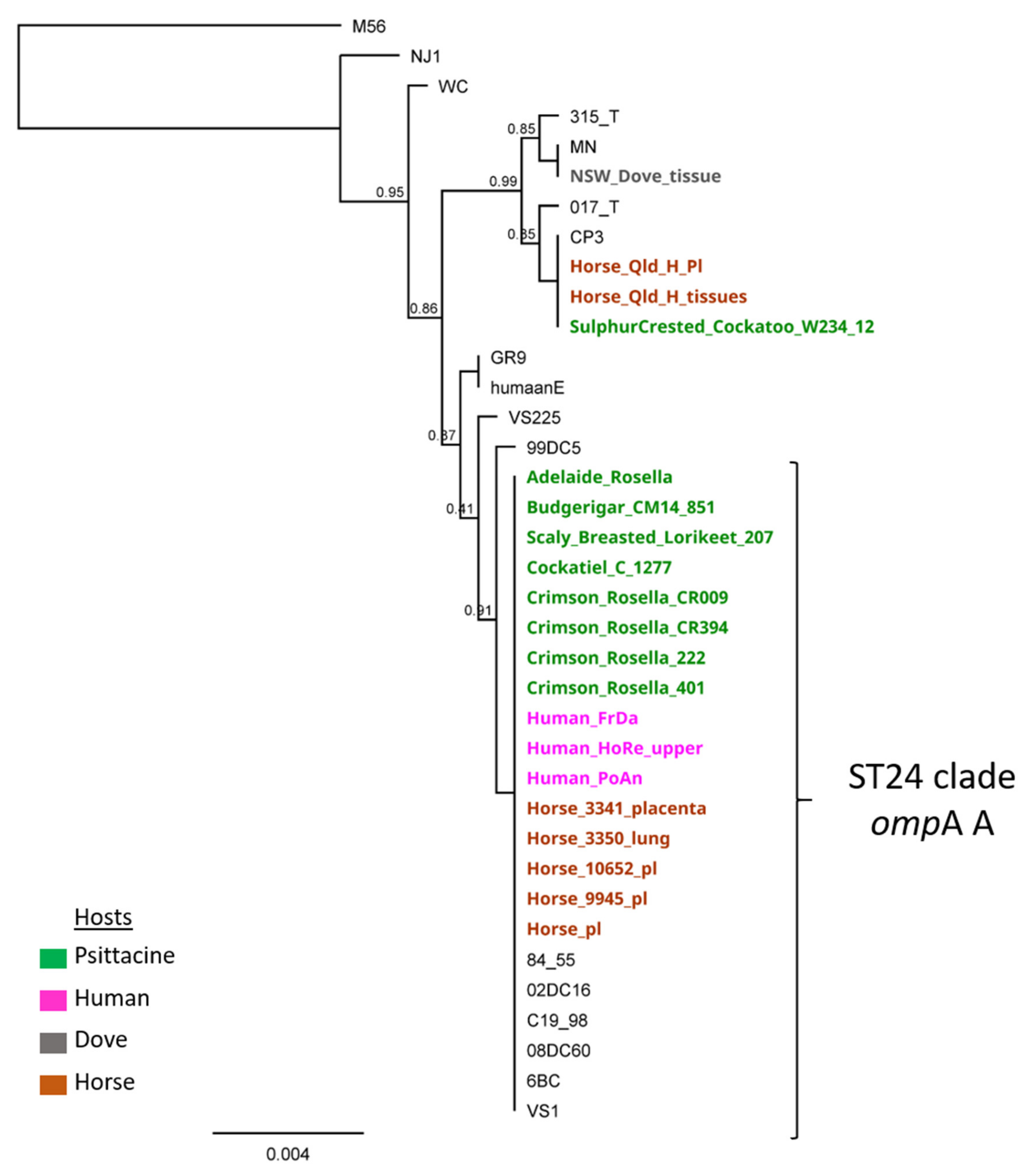
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knittler, M.R.; Sachse, K. Chlamydia psittaci: Update on an underestimated zoonotic agent. Pathog. Dis. 2015, 73, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, J.; Donnelly, T. Clinical Veterinary Advisor, Birds and Exotic Pets, 1: Clinical Veterinary Advisor; Elsevier Health Sciences: St. Louis, MO, USA, 2013. [Google Scholar]
- Radomski, N.; Einenkel, R.; Müller, A.; Knittler, M.R. Chlamydia-host cell interaction not only from a bird’s eye view: Some lessons from Chlamydia psittaci. FEBS Lett. 2016, 590, 3920–3940. [Google Scholar] [CrossRef]
- Cong, W.; Huang, S.Y.; Zhang, X.X.; Zhou, D.H.; Xu, M.J.; Zhao, Q.; Qian, A.D.; Zhu, X.Q. Chlamydia psittaci exposure in pet birds. J. Med Microbiol. 2014, 63, 578–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewardson, A.J.; Grayson, M.L. Psittacosis. Infect Dis Clin. 2010, 24, 7–25. [Google Scholar] [CrossRef]
- Heymann, D. Control of Communicable Diseases Manual: An Official Report of the American Public Health Association, 20th ed.; American Public Health Association: Washington, DC, USA, 2015; p. 2745. [Google Scholar]
- Hogerwerf, L.; Roof, I.; De Jong, M.J.K.; Dijkstra, F.; Van Der Hoek, W. Animal sources for zoonotic transmission of psittacosis: A systematic review. BMC Infect. Dis. 2020, 20, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hulin, V.; Bernard, P.; Vorimore, F.; Aaziz, R.; Cléva, D.; Robineau, J.; Durand, B.; Angelis, L.; Siarkou, V.I.; Laroucau, K. Assessment of Chlamydia psittaci Shedding and Environmental Contamination as Potential Sources of Worker Exposure throughout the Mule Duck Breeding Process. Appl. Environ. Microbiol. 2016, 82, 1504–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laroucau, K.; Aaziz, R.; Meurice, L.; Servas, V.; Chossat, I.; Royer, H.; De Barbeyrac, B.; Vaillant, V.; Moyen, J.L.; Meziani, F.; et al. Outbreak of psittacosis in a group of women exposed to Chlamydia psittaci-infected chickens. Eurosurveillance 2015, 20, 21155. [Google Scholar] [CrossRef] [Green Version]
- Telfer, B.L.; Moberley, S.; Hort, K.P.; Branley, J.M.; Dwyer, D.E.; Muscatello, D.J.; Correll, P.K.; England, J.; McAnulty, J.M. Probable Psittacosis Outbreak Linked to Wild Birds. Emerg. Infect. Dis. 2005, 11, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorne, A.; Weston, K.M.; Branley, J. Recent history of psittacosis in Australia: Expanding our understanding of the epidemiology of this important globally distributed zoonotic disease. Intern. Med. J. 2020, 50, 246–249. [Google Scholar] [CrossRef]
- Australian Government Department of Health. Australian National Notifiable Diseases Surveillance System Reports. Available online: http://www9.health.gov.au/cda/source/cda-index.cfm (accessed on 25 May 2021).
- De Gier, B.; Hogerwerf, L.; Dijkstra, F.; Van der Hoek, W. Disease burden of psittacosis in the Netherlands. Epidemiol Infect. 2018, 146, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Balsamo, G.; Maxted, A.M.; Midla, J.W.; Murphy, J.M.; Wohrle, R.; Edling, T.M.; Fish, P.H.; Flammer, K.; Hyde, D.; Kutty, P.K.; et al. Compendium of Measures to ControlChlamydia psittaciInfection Among Humans (Psittacosis) and Pet Birds (Avian Chlamydiosis), 2017. J. Avian Med. Surg. 2017, 31, 262–282. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-C.; Jackson, L.A.; Campbell, L.A.; Grayston, J.T. Chlamydia pneumoniae (TWAR). Clin. Microbiol. Rev. 1995, 8, 451–461. [Google Scholar] [CrossRef]
- Jelocnik, M.; Islam, M.M.; Madden, D.; Jenkins, C.; Branley, J.; Carver, S. Development and evaluation of rapid novel isothermal amplification assays for important veterinary pathogens: Chlamydia psittaci and Chlamydia pecorum. PeerJ 2017, 5, e3799. [Google Scholar] [CrossRef] [Green Version]
- Jelocnik, M.; Jenkins, C.; O’Rourke, B.; Barnwell, J.; Polkinghorne, A. Molecular evidence to suggest pigeon-type Chlamydia psittaci in association with an equine foal loss. Transbound. Emerg. Dis. 2018, 65, 911–915. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar]
- Kaleta, E.F.; Taday, E.M.A. Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathol. 2003, 32, 435–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelocnik, M. Chlamydiae from Down Under: The Curious Cases of Chlamydial Infections in Australia. Microorganisms 2019, 7, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wobeser, G.; Brand, C.J. Chlamydiosis in 2 biologists investigating disease occurrences in wild waterfowl. Wildl. Soc. Bull. 1982, 10, 170–172. [Google Scholar]
- Deschuyffeleer, T.P.; Tyberghien, L.F.; Dickx, V.L.; Geens, T.; Saelen, J.M.; Vanrompay, D.C.; Braeckman, L.A. Risk Assessment and Management of Chlamydia psittaci in Poultry Processing Plants. Ann. Occup. Hyg. 2012, 56, 340–349. [Google Scholar]
- Andersen, A.A.; Vanrompay, D. Avian chlamydiosis (psittacosis, ornithosis). Dis. Poult. 2008, 12, 971–986. [Google Scholar]
- Australian Public Health Laboratory Network. Chlamydophila psittaci Laboratory Case Definition (LCD). Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-phlncd-chlamydophila-psittaci.htm (accessed on 23 May 2021).
- Jones, B.; Taylor, K.; Lucas, R.M.; Merritt, T.; Chicken, C.; Heller, J.; Carrick, J.; Givney, R.; Durrheim, D.N. Challenges in using serological methods to explore historical transmission risk of Chlamydia psittaci in a workforce with high exposure to equine chlamydiosis. Commun. Dis. Intell. 2019, 43. [Google Scholar] [CrossRef]
- De Boeck, C.; Dehollogne, C.; Dumont, A.; Spierenburg, M.; Heijne, M.; Gyssens, I.; Hilst, J.V.D.; Vanrompay, D. Managing a cluster outbreak of psittacosis in Belgium linked to a pet shop visit in The Netherlands. Epidemiol. Infect. 2016, 144, 1710–1716. [Google Scholar] [CrossRef]
- Rybarczyk, J.; Versteele, C.; Lernout, T.; Vanrompay, D. Human psittacosis: A review with emphasis on surveillance in Belgium. Acta Clin. Belg. 2019, 75, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, S.; Rybarczyk, J.; Schauwvlieghe, P.-P.; Accou, G.; Abeele, A.-M.V.D.; Vanrompay, D. A Bird’s-Eye View of Chronic Unilateral Conjunctivitis: Remember about Chlamydia psittaci. Microorganisms 2019, 7, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rane, V.; Khailin, K.; Williams, J.; Francis, M.; Kotsanas, D.; Korman, T.M.; Graham, M. Underdiagnosis of Chlamydia trachomatis and Chlamydia psittaci revealed by introduction of respiratory multiplex PCR assay with Chlamydiaceae family primers. Diagn. Microbiol. Infect. Dis. 2018, 90, 163–166. [Google Scholar] [CrossRef]
- Stokes, H.S.; Martens, J.M.; Walder, K.; Segal, Y.; Berg, M.L.; Bennett, A.T.D. Species, sex and geographic variation in chlamydial prevalence in abundant wild Australian parrots. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Branley, J.; Bachmann, N.L.; Jelocnik, M.; Myers, G.S.A.; Polkinghorne, A. Australian human and parrot Chlamydia psittaci strains cluster within the highly virulent 6BC clade of this important zoonotic pathogen. Sci. Rep. 2016, 6, 30019. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaber, A.-L.; Jelocnik, M.; Woolford, L. Undiagnosed Cases of Human Pneumonia Following Exposure to Chlamydia psittaci from an Infected Rosella Parrot. Pathogens 2021, 10, 968. https://doi.org/10.3390/pathogens10080968
Chaber A-L, Jelocnik M, Woolford L. Undiagnosed Cases of Human Pneumonia Following Exposure to Chlamydia psittaci from an Infected Rosella Parrot. Pathogens. 2021; 10(8):968. https://doi.org/10.3390/pathogens10080968
Chicago/Turabian StyleChaber, Anne-Lise, Martina Jelocnik, and Lucy Woolford. 2021. "Undiagnosed Cases of Human Pneumonia Following Exposure to Chlamydia psittaci from an Infected Rosella Parrot" Pathogens 10, no. 8: 968. https://doi.org/10.3390/pathogens10080968





