Antigenic Diversity of Human Norovirus Capsid Proteins Based on the Cross-Reactivities of Their Antisera
Abstract
:1. Introduction
2. Results
2.1. Phylogenetic Analysis of 23 HuNoV Strains
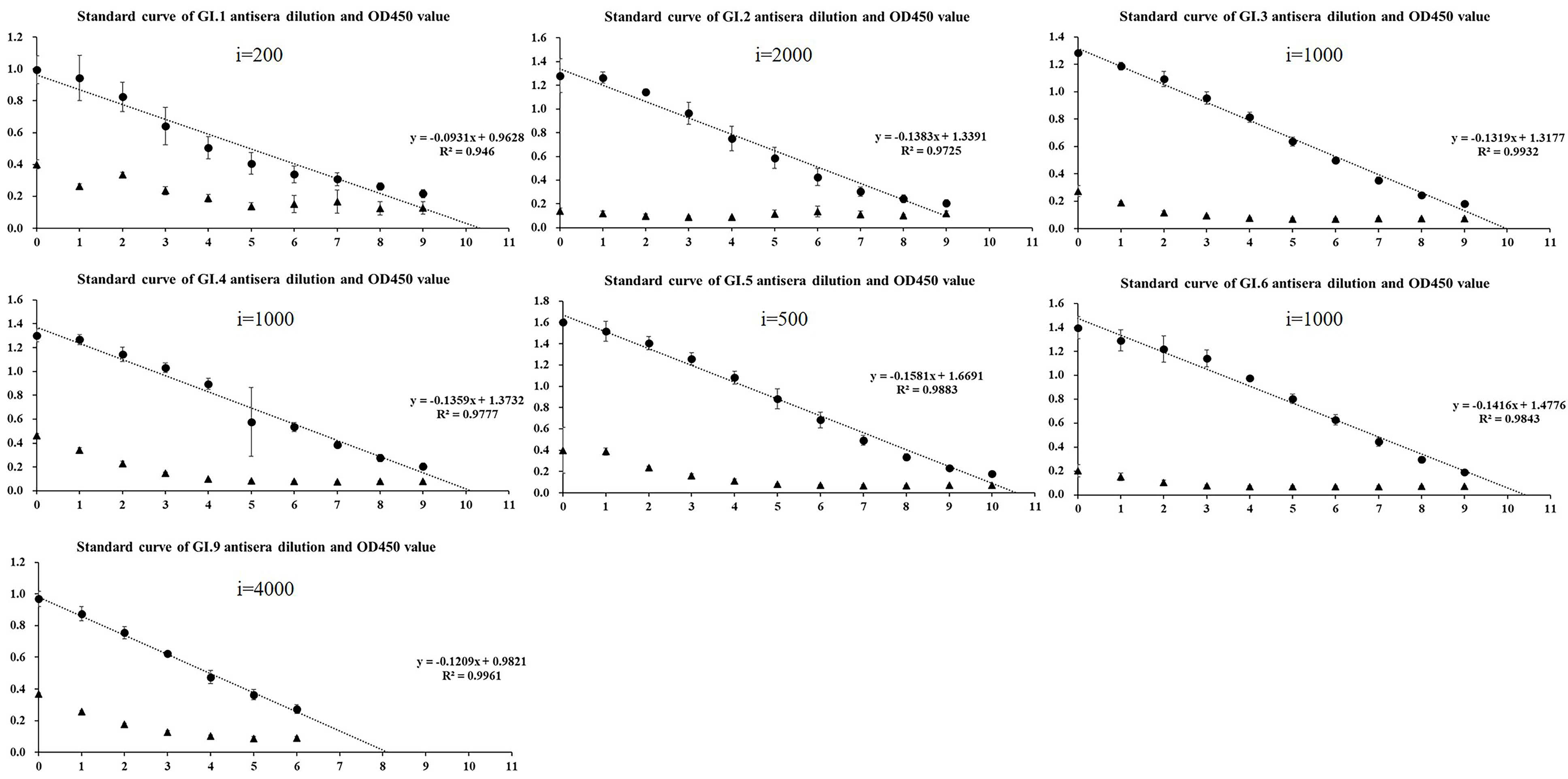
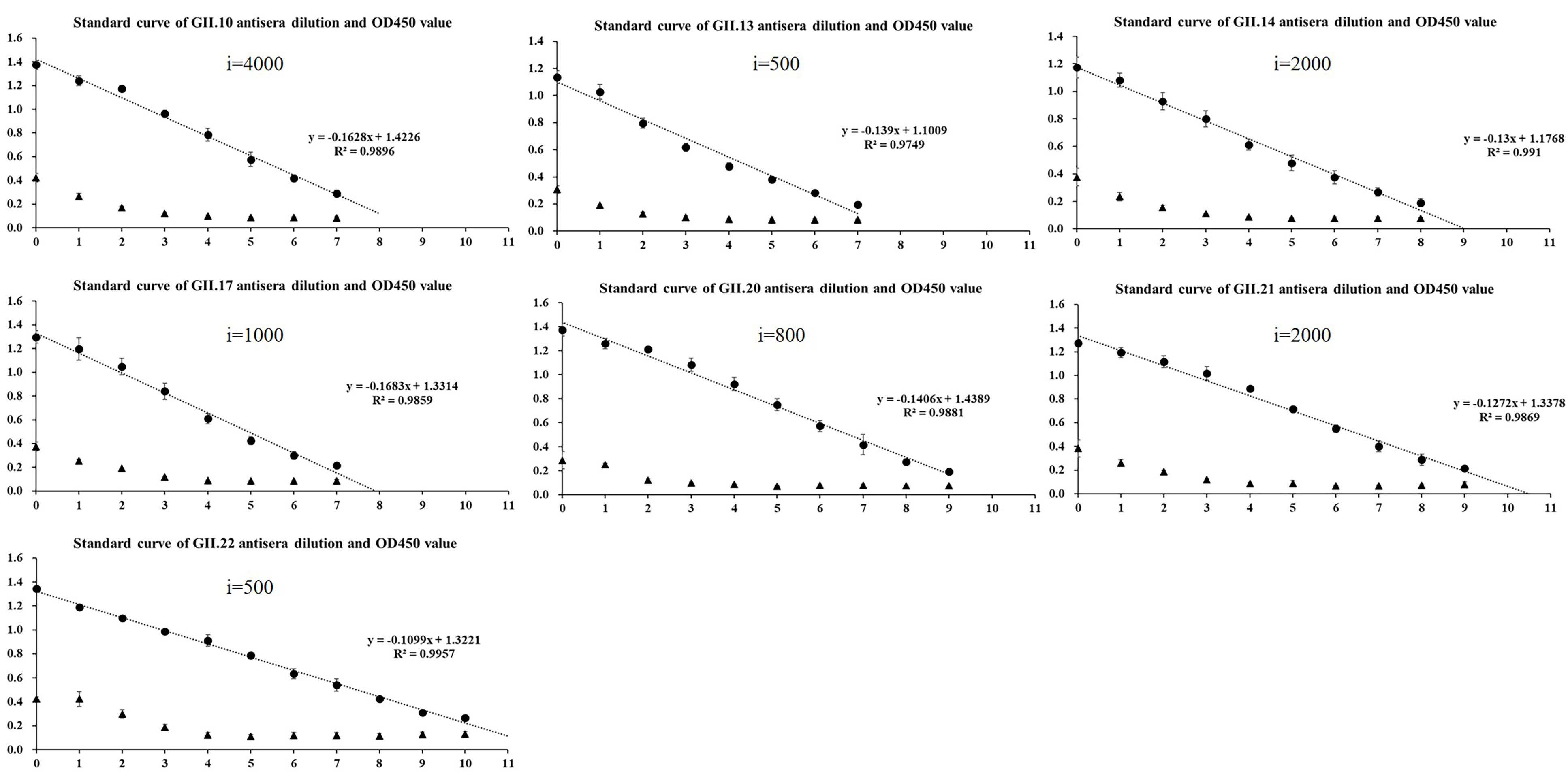
2.2. Homologous Antigenic Analysis
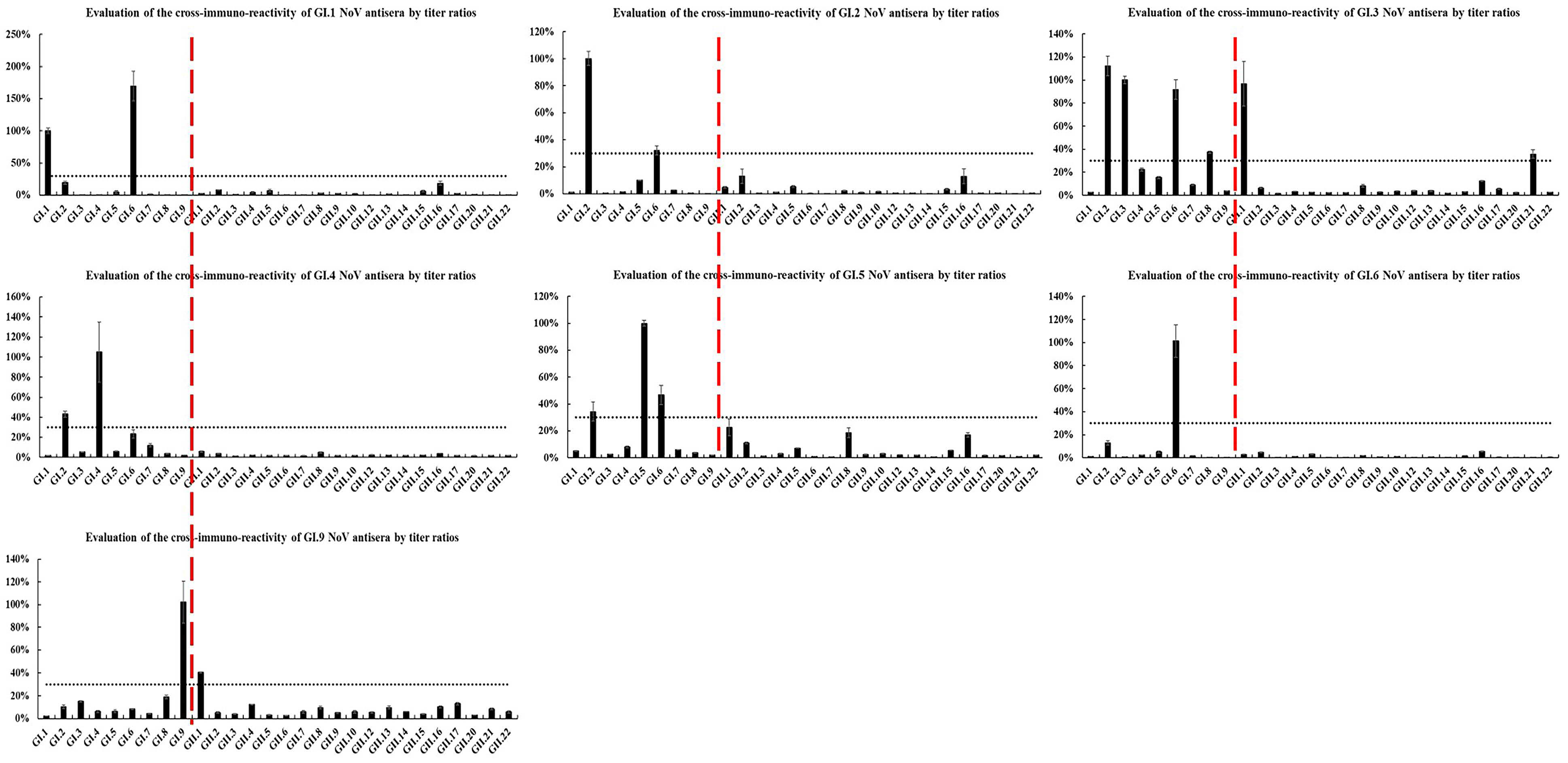
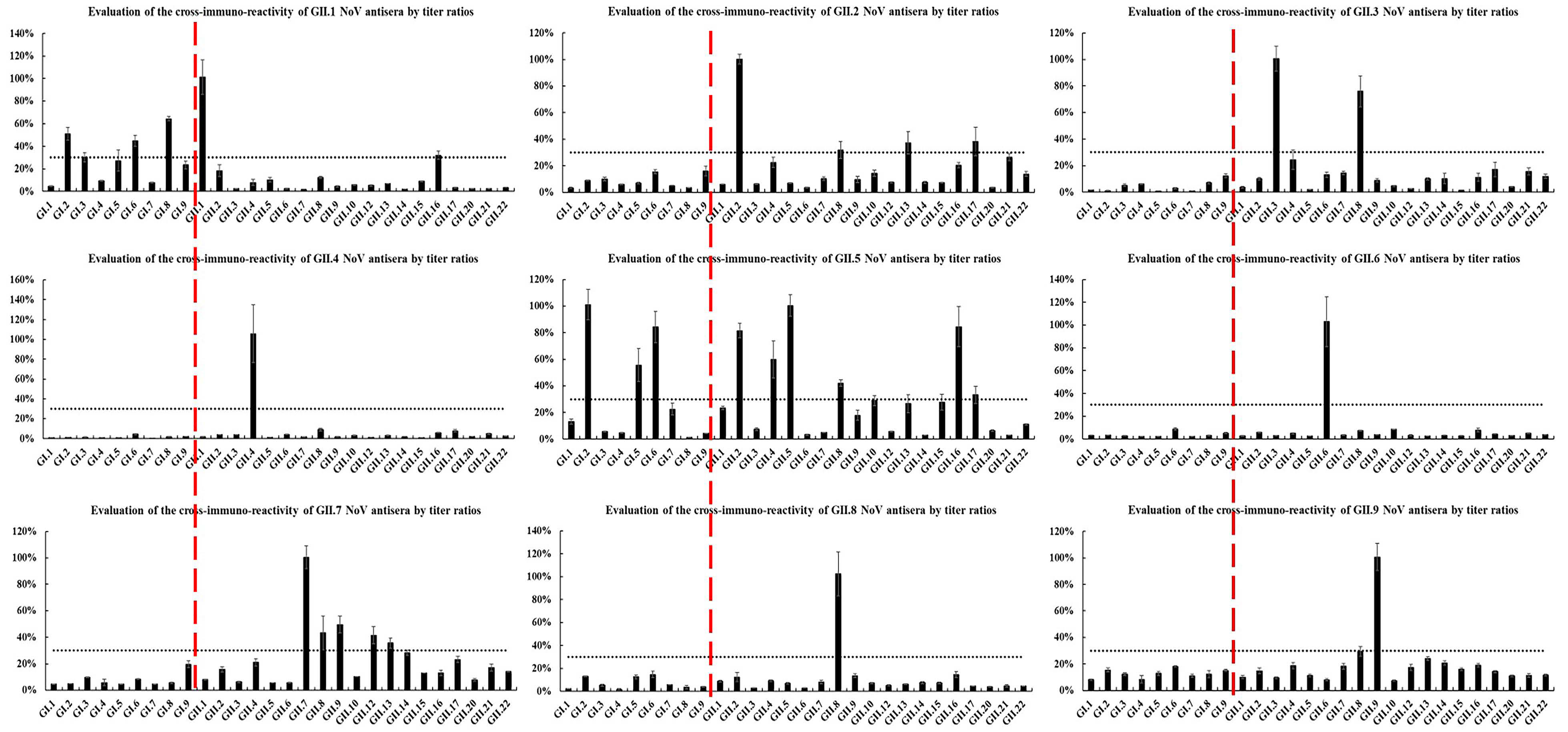
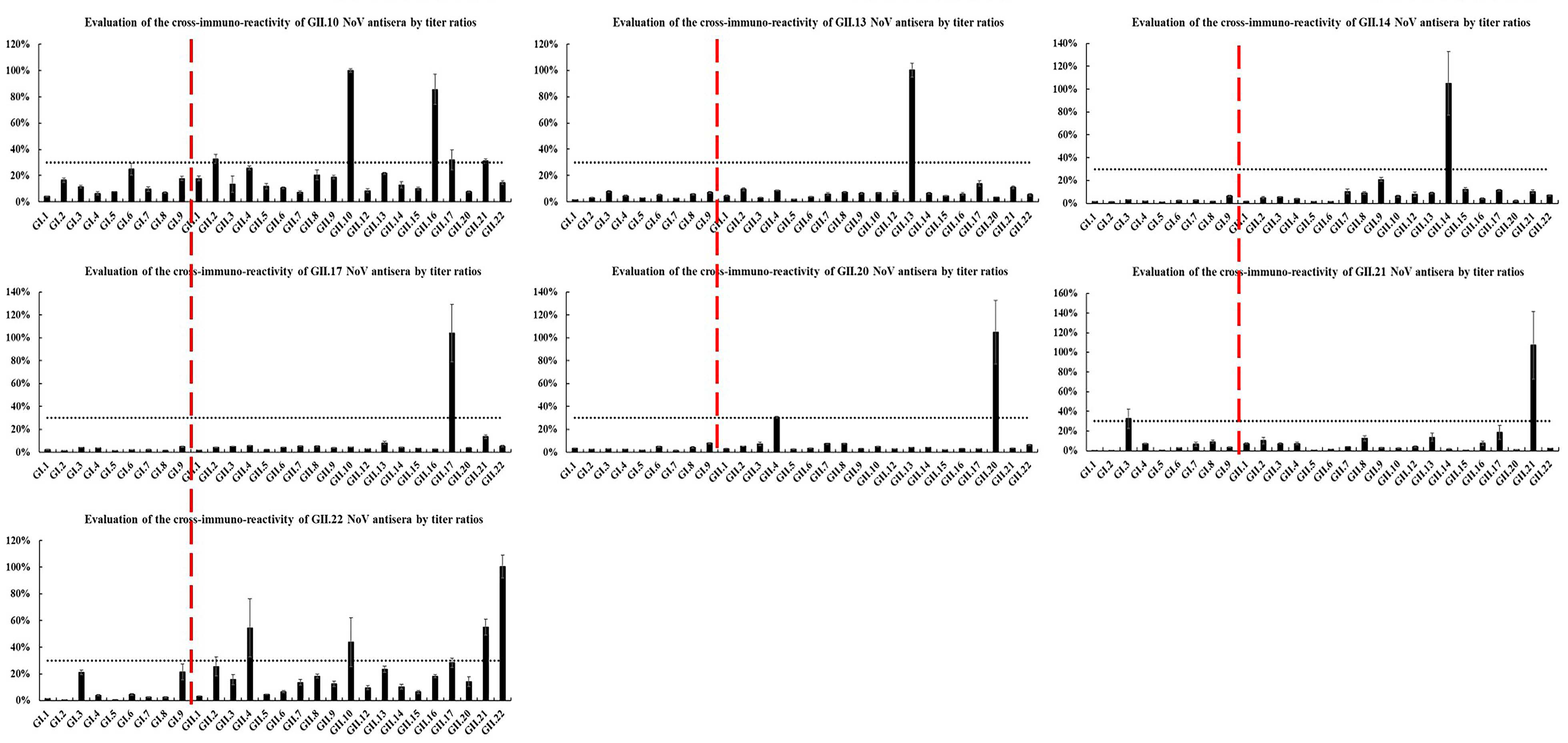
2.3. Heterologous Antigenic Analysis
2.4. Antigenic Epitope Analysis
3. Discussion
4. Materials and Methods
4.1. HuNoV Strains
4.2. Expression of P particles
4.3. Immunization and ELISA
4.4. Immune Cross-Reactivity
4.5. Phylogenetic Analysis
4.6. Homology Modeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucardo, F.; Reyes, Y.; Svensson, L.; Nordgren, J. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS ONE 2014, 9, e98201. [Google Scholar] [CrossRef] [Green Version]
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The vast and varied global burden of norovirus: Prospects for prevention and control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.R.; Arias, C.; Domínguez, A.; Bartolomé, R.; Muntada, J.M. Foodborne outbreak of gastroenteritis due to norovirus and vibrio parahaemolyticus. Epidemiol. Infect. 2009, 137, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Franck, K.T.; Nielsen, R.T.; Holzknecht, B.J.; Ersboll, A.K.; Fischer, T.K.; Bottiger, B. Norovirus genotypes in hospital settings: Differences between nosocomial and community-acquired infections. J. Infect. Dis. 2015, 212, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Goller, J.L.; Dimitriadis, A.; Tan, A.; Kelly, H.; Marshall, J.A. Long-term features of norovirus gastroenteritis in the elderly. J. Hosp. Infect. 2004, 58, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, T.K.; Desai, R.; Hall, A.J.; Patel, M.; Parashar, U.D.; Lopman, B.A. Clinical characteristics of norovirus-associated deaths: A systematic literature review. Am. J. Infect. Control 2013, 41, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Mattison, C.P.; Cardemil, C.V.; Hall, A.J. Progress on norovirus vaccine research: Public health considerations and future directions. Expert Rev. Vaccines 2018, 17, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Ramani, S.; Estes, M.K. Human noroviruses: Recent advances in a 50-year history. Curr. Opin. Infect. Dis. 2018, 31, 422–432. [Google Scholar] [CrossRef]
- Xi, J.N.; Graham, D.Y.; Wang, K.N.; Estes, M.K. Norwalk virus genome cloning and characterization. Science 1990, 250, 1580–1583. [Google Scholar] [CrossRef]
- Lambden, P.R.; Caul, E.O.; Ashley, C.R.; Clarke, I.N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science 1993, 259, 516–519. [Google Scholar] [CrossRef]
- Prasad, B.V.V.; Hardy, M.E.; Dokland, T.; Bella, J.; Rossmann, M.G.; Estes, M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science 1999, 286, 287–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, B.V.; Rothnagel, R.; Jiang, X.I.; Estes, M.K. Three-dimensional structure of baculovirus-expressed Norwalk virus capsids. J. Virol. 1994, 68, 5117–5125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bereszczak, J.Z.; Barbu, I.M.; Tan, M.; Xia, M.; Jiang, X.; van Duijn, E.; Heck, A.J. Structure, stability and dynamics of norovirus P domain derived protein complexes studied by native mass spectrometry. J. Struct. Biol. 2012, 177, 273–282. [Google Scholar] [CrossRef]
- Tomé-Amat, J.; Fleischer, L.; Parker, S.A.; Bardliving, C.L.; Batt, C.A. Secreted production of assembled norovirus virus-like particles from pichia pastoris. Microb. Cell Fact. 2014, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koromyslova, A.D.; Morozov, V.A.; Hefele, L.; Hansman, G.S. Human norovirus neutralized by a monoclonal antibody targeting the histo-blood group antigen pocket. J. Virol. 2019, 93, e02174-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Corrigendum: Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2020, 101, 893. [Google Scholar] [CrossRef] [PubMed]
- Vinjé, J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2015, 53, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, J.R.; Costantini, V.P.; Cannon, J.L.; Lin, S.C.; Nascimento, M.S.; Vinjé, J. Presence of antibodies against genogroup VI norovirus in humans. Virol. J. 2013, 10, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramani, S.; Atmar, R.L.; Estes, M.K. Epidemiology of human noroviruses and updates on vaccine development. Curr. Opin. Gastroenterol. 2014, 30, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Wu, Q.P.; Cai, W.C.; Zhang, J.M.; Guo, W.P. Molecular characterization of new emerging GII.17 norovirus strains from South China. Infect. Genet. Evol. 2016, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.L.; Zhen, S.S.; Wang, J.X.; Zhang, C.J.; Qiu, C.; Wang, S.M. Burden of acute gastroenteritis caused by norovirus in china: A systematic review. J. Infect. 2017, 75, 216–224. [Google Scholar] [CrossRef]
- Wang, X.S.; Wei, Z.Q.; Guo, J.Y.; Cai, J.H.; Chang, H.L.; Ge, Y.L.; Zeng, M. Norovirus activity and genotypes in sporadic acute diarrhea in children in Shanghai during 2014–2018. Pediatr. Infect. Dis. J. 2019, 38, 1085–1089. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Liu, B.W.; Yan, H.Q.; Li, W.H.; Jia, L.; Tian, Y.; Chen, Y.W.; Wang, Q.Y.; Pang, X.H. Norovirus outbreaks in Beijing, China, from 2014 to 2017. J. Infect. Dis. 2019, 79, 159–166. [Google Scholar] [CrossRef]
- Chiu, S.C.; Hsu, J.K.; Hu, S.C.; Wu, C.Y.; Lin, J.H. Molecular epidemiology of GI.3 norovirus outbreaks from acute gastroenteritis surveillance system in taiwan, 2015–2019. BioMed Res. Int. 2020, 2020, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Malm, M.; Tamminen, K.; Lappalainen, S.; Uusi-Kerttula, H.; Vesikari, T.; Blazevic, V. Genotype considerations for virus-Like particle-based bivalent norovirus vaccine composition. Clin. Vaccine Immunol. 2015, 22, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, D.I.; Atmar, R.L.; Marshall, L.G.; Treanor, J.J.; Chen, W.H.; Jiang, X.; Jan Vinjé, J.; Gregoricus, N.; Frenck, R.W.; Moe, C.L.; et al. Norovirus vaccine against experimental human GII.4 virus illness: A challenge study in healthy adults. J. Infect. Dis. 2015, 6, 870–878. [Google Scholar] [CrossRef]
- Treanor, J.; Sherwood, J.; Cramer, J.P.; Bouveret, N.L.; Lin, S.; Baehner, F.; Borkowski, A. A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose. Vaccine 2020, 38, 5842–5850. [Google Scholar] [CrossRef]
- Tamminen, K.; Lappalainen, S.; Huhti, L.; Vesikari, T.; Blazevic, V. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS ONE 2013, 8, e70409. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wu, Q.; Kou, X.; Kou, X.; Cai, W.; Zhang, J.; Guo, W. Complete genome analysis of a novel norovirus GII.4 variant identified in China. Virus Genes 2013, 47, 228–234. [Google Scholar] [CrossRef]
- Xue, L.; Cai, W.; Wu, Q.; Kou, X.; Zhang, J.; Guo, W. Comparative genome analysis of a norovirus GII.4 strain GZ2013-L10 isolated from South China. Virus Genes 2016, 52, 14–21. [Google Scholar] [CrossRef]
- Xue, L.; Dong, R.; Wu, Q.; Li, Y.; Cai, W.; Kou, X.; Zhang, J.; Guo, W. Molecular epidemiology of noroviruses associated with sporadic gastroenteritis in Guangzhou, China, 2013–2015. Arch. Virol. 2016, 161, 1377–1384. [Google Scholar] [CrossRef]
- Van, D.J.; Cuvry, A.; Knickmann, J.; Ny, A.; Rakers, S.; Taube, S.; Witte, P.; Neyts, J.; Pereira, J.R. Infection of zebrafish larvae with human norovirus and evaluation of the in vivo efficacy of small-molecule inhibitors. Nat. Protoc. 2021, 16, 1830–1849. [Google Scholar]
- Jones, M.K.; Watanabe, M.; Zhu, S.; Graves, C.L.; Keyes, L.R.; Grau, K.R.; Gonzalez-Hernandez, M.B.; Iovine, N.M.; Wobus, C.E.; Vinjé, J.; et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 2014, 346, 755–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef]
- Siebenga, J.J.; Vennema, H.; Renckens, B.; de Bruin, E.; van der Veer, B.; Siezen, R.J.; Koopmans, M. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J. Virol. 2007, 81, 9932–9941. [Google Scholar] [CrossRef] [Green Version]
- Bull, R.A.; Eden, J.S.; Rawlinson, W.D.; White, P.A. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog. 2010, 6, e1000831. [Google Scholar] [CrossRef]
- Malm, M.; Tamminen, K.; Vesikari, T.; Blazevic, V. Norovirus GII.17 virus-like particles bind to different histo-blood group anti-gens and cross-react with genogroup II-specific mouse sera. Viral Immunol. 2018, 31, 649–657. [Google Scholar] [CrossRef]
- Czakó, R.; Atmar, R.L.; Opekun, A.R.; Gilger, M.A.; Graham, D.Y.; Estes, M.K. Experimental human infection with Norwalk virus elicits a surrogate neutralizing antibody response with cross-genogroup activity. Clin. Vaccine Immunol. 2015, 22, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansman, G.S.; Natori, K.; Shirato-Horikoshi, H.; Ogawa, S.; Oka, T.; Katayama, K.; Tanaka, T.; Miyoshi, T.; Sakae, K.; Kobayashi, S.; et al. Genetic and antigenic diversity among noroviruses. J. Gen. Virol. 2006, 87, 909–919. [Google Scholar] [CrossRef]
- Lindesmith, L.C.; Beltramello, M.; Swanstrom, J.; Jones, T.A.; Corti, D.; Lanzavecchia, A.; Baric, R.S. Serum immunoglobulin a cross-strain blockade of human noroviruses. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2015; Volume 2. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zuo, Y.; Xue, L.; Wang, L.; Liang, Y.; Jiang, Y.; Cai, W.; Meng, L.; Zhang, J.; Ye, Q.; et al. Antigenic Diversity of Human Norovirus Capsid Proteins Based on the Cross-Reactivities of Their Antisera. Pathogens 2021, 10, 986. https://doi.org/10.3390/pathogens10080986
Gao J, Zuo Y, Xue L, Wang L, Liang Y, Jiang Y, Cai W, Meng L, Zhang J, Ye Q, et al. Antigenic Diversity of Human Norovirus Capsid Proteins Based on the Cross-Reactivities of Their Antisera. Pathogens. 2021; 10(8):986. https://doi.org/10.3390/pathogens10080986
Chicago/Turabian StyleGao, Junshan, Yueting Zuo, Liang Xue, Linping Wang, Yanhui Liang, Yueting Jiang, Weicheng Cai, Luobing Meng, Jumei Zhang, Qinghua Ye, and et al. 2021. "Antigenic Diversity of Human Norovirus Capsid Proteins Based on the Cross-Reactivities of Their Antisera" Pathogens 10, no. 8: 986. https://doi.org/10.3390/pathogens10080986





