Molecular Detection of Coxiella burnetii, Rickettsia africae and Anaplasma Species in Ticks from Domestic Animals in Lesotho
Abstract
:1. Introduction
2. Results
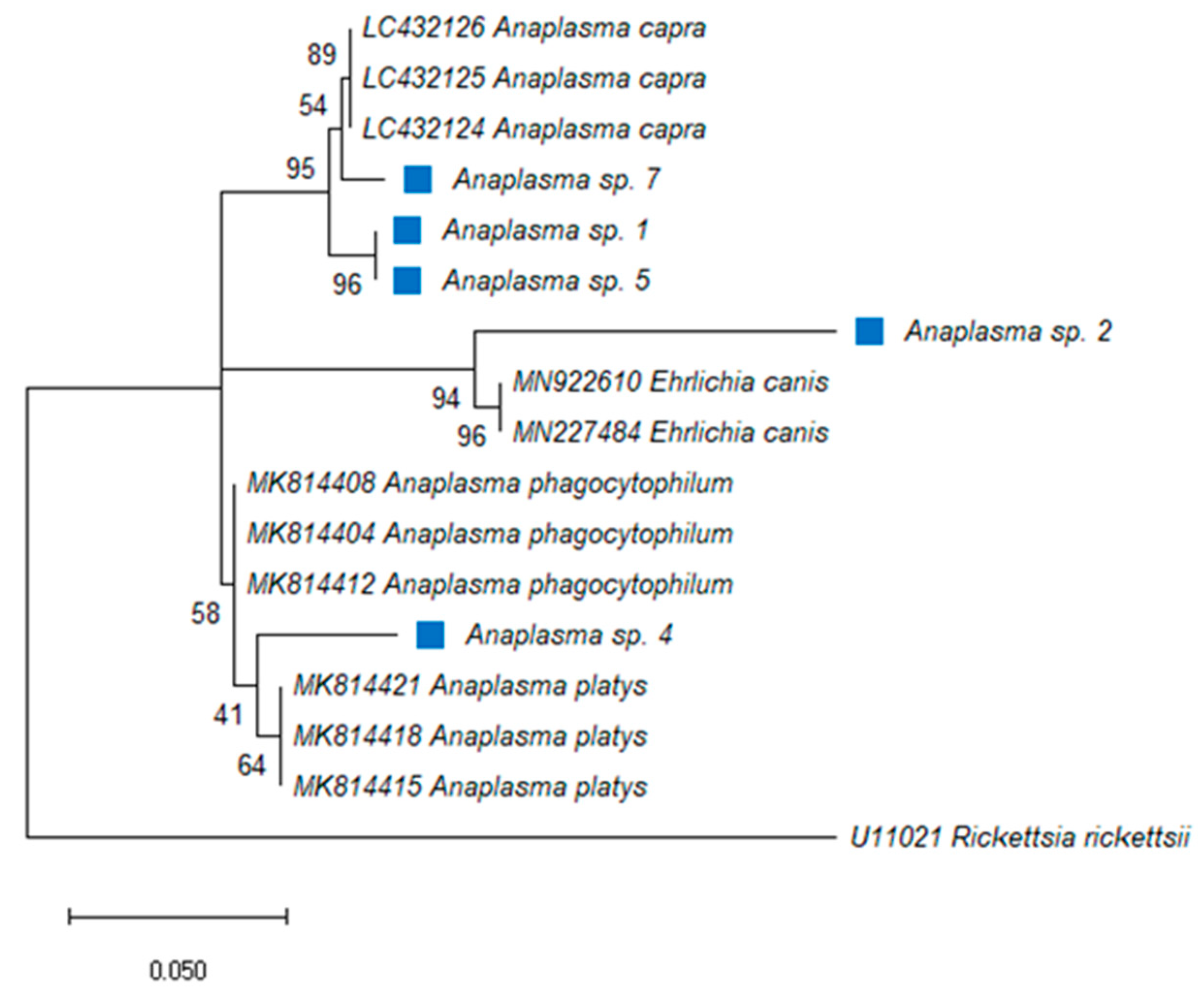
2.1. Anaplasma Species
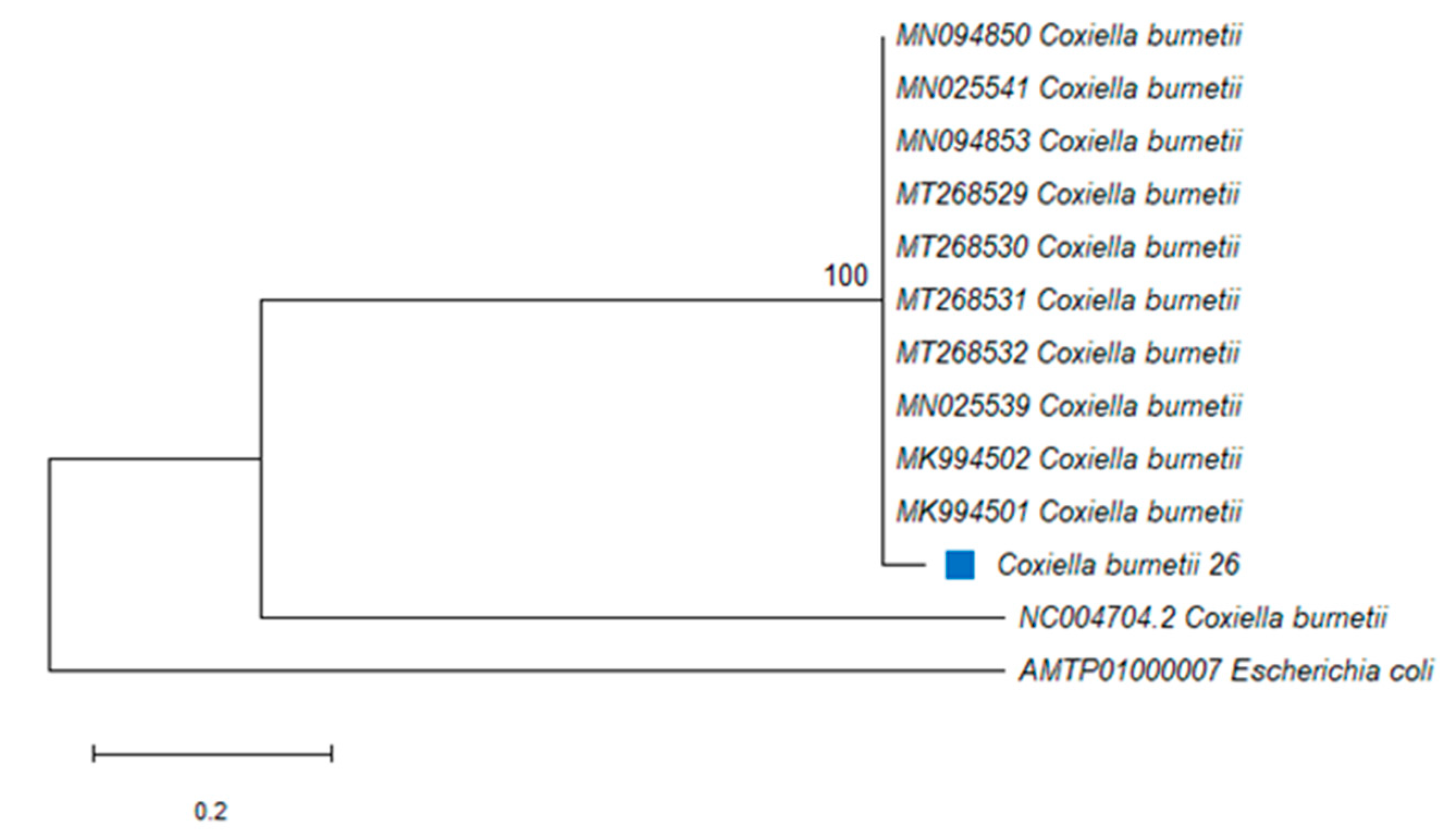
2.2. Coxiella burnetii
2.3. Rickettsia africae
3. Discussion
4. Materials and Methods
4.1. Tick Samples and Identification
4.2. DNA Extraction from Ticks
4.3. Detection of Zoonotic Pathogens DNA by PCR
4.4. Sequencing, Basic Local Alignment Search Tool (BLAST) and Phylogenetic Analysis
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Ashker, M.; Hotzel, H.; Gwida, M. Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR assays, gene sequence analysis and a novel DNA microarray. Vet. Parasitol. 2015, 207, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Bilgic, H.B.; Karagenc, T.; Simuunza, M.; Shiels, B.; Tait, A.; Eren, H.; Weir, W. Development of a multiplex PCR assay for simultaneous detection of Theileria annulata, Babesia bovis and Anaplasma marginale in cattle. Exp. Parasitol. 2013, 133, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Chitanga, S.; Gaff, H.; Mukaratirwa, S. Tick-borne pathogens of potential zoonotic importance in the southern African Region. J. S. Afr. Vet. Assoc. 2014, 85, 1084. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, I.; de Almeida, A.M.; Ventosa, M.; Pruneau, L.; Meyer, D.F.; Martinez, D.; Lefrancois, T.; Vachiery, N.; Coelho, A.V. Tick-borne diseases in cattle: Applications of proteomics to develop new generation vaccines. J. Proteom. 2012, 75, 4232–4250. [Google Scholar] [CrossRef] [PubMed]
- Mtshali, K.; Khumalo, Z.; Nakao, R.; Grab, D.J.; Sugimoto, C.; Thekisoe, O. Molecular detection of zoonotic tick-borne pathogens from ticks collected from ruminants in four South African provinces. J. Bacteriol. 2015, 77, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Lorusso, V.; Wijnveld, M.; Majekodunmi, A.O.; Dongkum, C.; Fajinmi, A.; Dogo, A.G.; Thrusfield, M.; Mugenyi, A.; Vaumourin, E.; Igweh, A.C.; et al. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasites Vectors 2016, 9, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mtshali, K.; Nakao, R.; Sugimoto, C.; Thekisoe, O. Occurrence of Coxiella burnetii, Ehrlichia canis, Rickettsia species and Anaplasma phagocytophilum-like bacterium in ticks collected from dogs and cats in South Africa. J. S. Afr. Vet. Assoc. 2017, 88, e1–e6. [Google Scholar] [CrossRef] [Green Version]
- Aktas, M.; Özübek, S. Bovine anaplasmosis in Turkey: First laboratory confirmed clinical cases caused by Anaplasma phagocytophilum. Vet. Microbiol. 2015, 178, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Choi, K.-S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human Granulocytic Anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef]
- Adaszek, L.; Winiarczyk, S. Identification of Anaplasma spp. Rickettsia isolated from horses from clinical disease cases in Poland. Zoonoses Public Health 2011, 58, 514–518. [Google Scholar] [CrossRef]
- Rymaszewska, A.; Grenda, S. Bacteria of the genus Anaplasma—Characteristics of Anaplasma and their vectors: A review. Vet. Med. 2008, 53, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Inokuma, H.; Oyamada, M.; Kelly, P.J.; Jacobson, L.A.; Fournier, P.E.; Itamoto, K.; Okuda, M.; Brouqui, P. Molecular detection of a new Anaplasma species closely related to Anaplasma phagocytophilum in canine blood from South Africa. J. Clin. Microbiol. 2005, 43, 2934–2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salifu, S.P.; Bukari, A.A.; Frangoulidis, D.; Wheelhouse, N. Current perspectives on the transmission of Q fever: Highlighting the need for a systematic molecular approach for a neglected disease in Africa. Acta Trop. 2019, 193, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mediannikov, O.; Fenollar, F.; Socolovschi, C.; Diatta, G.; Bassene, H.; Molez, J.F.; Sokhna, C.; Trape, J.F.; Raoult, D. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e654. [Google Scholar] [CrossRef] [Green Version]
- Rodolakis, A. Q Fever in Dairy Animals. Ann. N. Y. Acad. Sci. 2009, 1166, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Raoult, D. Q Fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guatteo, R.; Seegers, H.; Taurel, A.F.; Joly, A.; Beaudeau, F. Prevalence of Coxiella burnetii infection in domestic ruminants: A critical review. Vet. Microbiol. 2011, 149, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Moodie, C.E.; Thompson, H.A.; Meltzer, M.I.; Swerdlow, D.L. Prophylaxis after exposure to Coxiella burnetii. Emerg. Infect. Dis. 2008, 14, 1558–1566. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Hadjichristodoulou, C.; Loukaides, F.; Soteriades, E.; Konstantinidis, A.; Papastergiou, P.; Ioannidou, M.C.; Tselentis, Y. Epidemiological study of Q fever in humans, ruminant animals, and ticks in Cyprus using a geographical information system. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 576–586. [Google Scholar] [CrossRef]
- Cazorla, C.; Socolovschi, C.; Jensenius, M.; Parola, P. Tick-borne diseases: Tick-borne spotted fever rickettsioses in Africa. Infect. Dis. Clin. N. Am. 2008, 22, 531–544. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, Y.M.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althaus, F.; Greub, G.; Raoult, D.; Genton, B. African tick-bite fever: A new entity in the differential diagnosis of multiple eschars in travelers. Description of five cases imported from South Africa to Switzerland. Int. J. Infect. Dis. 2010, 14S, e274–e276. [Google Scholar] [CrossRef] [Green Version]
- Ndip, L.M.; Titanji, V.P.K.; Ndip, R.N.; McBride, J.W.; Bouyer, D.H.; Walker, D.H.; Fokam, E.B. Detection of Rickettsia africae in patients and ticks along the coastal region of Cameroon. Am. J. Trop. Med. Hyg. 2004, 71, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, J.S.; Macaluso, K.R.; Smith, N.; Zaki, S.R.; Paddock, C.D.; Davis, J.; Peterson, N.; Azad, A.F.; Rosenburg, R. Fatal Spotted Fever Rickettsiosis, Kenya. Emerg. Infect. Dis. 2004, 10, 910–913. [Google Scholar] [CrossRef]
- Portillo, A.; Pérez-Martínez, L.; Santibáñez, S.; Blanco, J.R.; Ibarra, V.; Oteo, J.A. Short Report: Detection of Rickettsia africae in Rhipicephalus (Boophilus) decoloratus ticks from the Republic of Botswana, South Africa. Am. J. Trop. Med. Hyg. 2007, 77, 376–377. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Zasio, C.; Guzzo, F.; Granata, C.; Mondardini, V.; Guerra, E.; Macri, E.; Benedetti, P. Outbreak of African tick-bite fever in six Italian tourists returning from South Africa. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 133–136. [Google Scholar] [CrossRef]
- Horak, I.G.; Heyne, H.; Halajian, A.; Booysen, S.; Smit, W.J. Parasites of domestic and wild animals in South Africa. L. Ixodid ticks infesting horses and donkeys. Onderstepoort J. Vet. Res. 2017, 84, e1–e6. [Google Scholar] [CrossRef]
- Spickett, A.M.; Heyne, I.H.; Williams, R. Survey of the livestock ticks of the North West province, South Africa. Onderstepoort J. Vet. Res. 2011, 78, 305. [Google Scholar] [CrossRef] [Green Version]
- Horak, I.G.; Nyangiwe, N.; de Matos, C.; Neves, L. Species composition and geographic distribution of ticks infesting cattle, goats and dogs in a temperate and in a subtropical region of south-east Africa. Onderstepoort J. Vet. Res. 2009, 76, 263–276. [Google Scholar] [CrossRef]
- Yawa, M.; Nyangiwe, N.; Kadzere, C.T.; Muchenje, V.; Mpendulo, T.C.; Marufu, M.C. In search of the Rhipicephalus (Boophilus) microplus in the western-central regions of the Eastern Cape Province, South Africa. Ticks Tick Borne Dis. 2019, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Horak, I.G.; Jordaan, A.J.; Nel, P.J.; van Heerden, J.; Heyne, H.; van Dalen, E.M. Distribution of endemic and introduced tick species in Free State Province, South Africa. J. S. Afr. Vet. Assoc. 2015, 86, 1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, S.; van der Merwe, N.A.; Maritz-Olivier, C. The genetic relationship between R. microplus and R. decoloratus ticks in South Africa and their population structure. Mol. Phylogenet. Evol. 2018, 129, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Barker, S.C.; Walker, A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 2014, 1, 1–144. [Google Scholar] [CrossRef]
- Matjila, P.T.; Leisewitz, A.L.; Jongejan, F.; Penzhorn, B.L. Molecular detection of tick-borne protozoal and ehrlichial infections in domestic dogs in South Africa. Vet. Parasitol. 2008, 155, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Halajian, A.; Palomar, A.M.; Portillo, A.; Heyne, H.; Luus-Powell, W.J.; Oteo, J.A. Investigation of Rickettsia, Coxiella burnetii and Bartonella in ticks from animals in South Africa. Ticks Tick Borne Dis. 2016, 7, 361–366. [Google Scholar] [CrossRef]
- Khoo, J.J.; Lim, F.S.; Chen, F.; Phoon, W.H.; Khor, C.S.; Pike, B.L.; Chang, L.Y.; AbuBakar, S. Coxiella detection in ticks from wildlife and livestock in Malaysia. Vector Borne Zoonotic Dis. 2016, 16, 744–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gummow, B.; Poerstamper, N.; Herr, S. The incidence of Coxiella burnetii antibodies in cattle in the Transvaal. Onderstepoort J. Vet. Res. 1987, 54, 569–571. [Google Scholar] [PubMed]
- Qiu, Y.; Nakao, R.; Namangala, B.; Sugimoto, C. First genetic detection of Coxiella burnetii in Zambian livestock. Am. J. Trop. Med. Hyg. 2013, 89, 518–519. [Google Scholar] [CrossRef] [Green Version]
- Vanderburg, S.; Rubach, M.P.; Halliday, J.E.; Cleaveland, S.; Reddy, E.A.; Crump, J.A. Epidemiology of Coxiella burnetii infection in Africa: A One Health systematic review. PLoS Negl. Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.G.; Lee, S.H.; Ouh, I.O.; Lee, G.H.; Goo, Y.K.; Kim, S.; Kwon, O.D.; Kwak, D. Molecular detection and genotyping of Coxiella-like endosymbionts in ticks that infest horses in south Korea. PLoS ONE 2016, 11, e0165784. [Google Scholar] [CrossRef]
- Frean, J.; Grayson, W. South African Tick Bite Fever: An Overview. Dermatopathology 2019, 6, 70–76. [Google Scholar] [CrossRef]
- Roch, N.; Epauland, O.; Pelloux, I.; Pavese, P.; Brion, J.-P.; Raoult, D.; Maurin, M. African tick bite fever in elderly patients: 8 cases in French tourists returning from South Africa. Clin. Infect. Dis. 2008, 47, e28–e35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angerami, R.N.; Krawczak, F.S.; Nieri-Bastos, F.A.; Santos, F.; Medorima, C.; Resende, M.R.; Labruna, M.B. First report of African tick-bite fever in a South American traveler. SAGE Open Med. Case Rep. 2018, 6, 2050313X18775301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macaluso, K.R.; Davis, J.; Alam, U.; Korman, A.; Rutherford, J.S.; Rosenburg, R.; Azad, A.F. Spotted fever group rickettsiae in ticks from the Masai Mara region of Kenya. Am. J. Trop. Med. Hyg. 2003, 68, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, 2872. [Google Scholar] [CrossRef]
- Latif, A.A. Illustrated Guide to Identification of African Tick Species. Ticks and Tick-Borne Diseases Monograph, 2nd ed.; Agri Connect (PTY) Ltd.: Pretoria, South Africa, 2013; Volume 2, p. 79. [Google Scholar]
- Madder, M.; Horak, I.G.; Stoltsz, H. Ticks: Tick Identification. South Africa: Creatives Commons Attribution Lisence. 58. 2013. [Google Scholar]
- Mlangeni, M.A. Molecular Epidemiology of Dourine, Equine Piroplasmosis and Ehrlichiosis from Donkeys and Horses in South Africa. Master’s Thesis, Unit of Environmental Science and Management, North-West Univeristy, Vanderbijlpark, South Africa, September 2016. [Google Scholar]
- Walker, A.R.; Bouattour, A.; Camicas, J.-L.; Estrada-Pena, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003; p. 227. [Google Scholar]
- Mahlobo, S.I.; Zishiri, O.T. A descriptive study of parasites detected in ticks of domestic animals in Lesotho. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100611. [Google Scholar]
- Hodzic, E.; Fish, D.; Maretziki, C.M.; de Silva, A.M.; Feng, S.; Barthold, S.W. Acquisition and Transmission of the Agent of Human Granulocytic Ehrlichiosis by Ixodes scapularis Ticks. J. Clin. Microbiol. 1998, 36, 3574–3578. [Google Scholar] [CrossRef] [Green Version]
- Mares-Guia, M.A.; Rozental, T.; Guterres, A.; Gomes, R.; Almeida, D.N.; Moreira, N.S.; Barreira, J.D.; Favacho, A.R.; Santana, A.L.; Lemos, E.R. Molecular identification of the agent of Q fever—Coxiella burnetii—In domestic animals in State of Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2014, 47, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; McBride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]


| Tick Species | Number of Ticks per District | Total No. of Ticks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Berea (%) | Butha-Buthe (%) | Leribe (%) | Mafeteng (%) | Maseru (%) | Mohale’s Hoek (%) | Mokhotlong (%) | Qacha’s Nek (%) | Quthing (%) | Thaba Tseka (%) | ||
| Haemaphysalis elliptica | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 2 |
| Hyalomma rufipes | 3 (3.4) | 0 (0) | 0 (0) | 0 (0) | 8 (9.2) | 7 (8.0) | 0 (0) | 66 (75.9) | 3 (3.4) | 0 (0) | 87 |
| Hyalomma truncatum | 0 (0) | 0 (0) | 13 (31.7) | 0 (0) | 6 (14.6) | 0 (0) | 0 (0) | 22 (53.7) | 0 (0) | 0 (0) | 41 |
| Otobius megnini | 0 (0) | 0 (0) | 0 (0) | 38 (8.4) | 48 (10.6) | 76 (16.9) | 0 (0) | 289 (64.1) | 0 (0) | 0 (0) | 451 |
| Rhipicephalus appendiculatus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 |
| Rhipicephalus decoloratus | 0 (0) | 4 (1.3) | 43 (14.0) | 24 (7.8) | 190 (61.7) | 0 (0) | 33 (10.7) | 14 (4.5) | 0 (0) | 0 (0) | 308 |
| Rhipicephalus e. evertsi | 161 (7.5) | 168 (7.8) | 647 (30.0) | 13 (0.6) | 244 (11.3) | 190 (9.0) | 27 (1.3) | 694 (32.2) | 5 (0.2) | 7 (0.3) | 2156 |
| Rhipicephalus glabroscutatus | 0 (0) | 8 (18.6) | 0 (0) | 0 (0) | 3 (7.0) | 0 (0) | 0 (0) | 32 (74.4) | 0 (0) | 0 (0) | 43 |
| Rhipicephalus microplus | 0 (0) | 111 (52.7) | 43 (19.5) | 0 (0) | 16 (7.3) | 0 (0) | 0 (0) | 45 (20.5) | 0 (0) | 5 (2.3) | 220 |
| Total no. per district | 164 (5.0) | 291 (6.6) | 746 (22.5) | 75 (2.3) | 518 (15.6) | 273 (8.2) | 60 (1.8) | 1164 (35.2) | 8 (0.2) | 12 (0.4) | 3311 |
| Ticks | Total No. of Tick Pools Screened (n) | Anaplasma spp. | Coxiella burnetii | Rickettsia africae | Total No. of (+ve) Tick Pools for Pathogens |
|---|---|---|---|---|---|
| Hyalomma rufipes | 6 | 2 | *- | 3 | 5 |
| Otobius megnini | 8 | 1 | - | - | 1 |
| Rhipicephalus appendiculatus | 1 | - | - | - | - |
| Rhipicephalus decoloratus | 20 | 2 | 1 | - | 3 |
| Rhipicephalus e. evertsi | 280 | 106 | 1 | 3 | 110 |
| Rhipicephalus microplus | 7 | 2 | - | - | 2 |
| Total no. of (+ve) tick samples | 322 | 113 | 2 | 6 | 121 |
| Hosts | Total No. of Tick Pools (n) | Anaplasma spp. (%) | Coxiella burnetii (%) | Rickettsia africae (%) | Total No. of Tick Species of Domestic Animals (%) |
|---|---|---|---|---|---|
| Cattle | 73 | 16 (22) | 2 (3) | 1 (1) | 19 (26) |
| Donkeys | 5 | *- | - | - | - |
| Goats | 116 | 59 (51) | - | 2 (2) | 61 (52) |
| Horses | 9 | 2 (22) | - | 2 (22) | 4 (44) |
| Sheep | 113 | 34 (30) | - | 1 (1) | 35 (31) |
| Vegetation | 6 | 2 (33) | - | - | 2 (33) |
| Zoonotic pathogens overall infection rate (%) | 322 | 113 (35) | 2 (1) | 6 (2) | 121 (37) |
| Study Group | Anaplasma spp. | Berea | Butha-Buthe | Leribe | Maseru | Mohale’ s Hoek | Qacha’s Nek | Quthing | Total Screened (+ve) Samples per Host and Overall % |
|---|---|---|---|---|---|---|---|---|---|
| Cattle | Total tested | 0 | 45 | 2 | 17 | 4 | 0 | 0 | 68 |
| No. of (+ve) | 0 | 11 | *- | 5 | - | 0 | 0 | 16 | |
| % | 0 | 24 | - | 29 | - | 0 | 0 | 24 | |
| Goats | Total tested | 16 | 1 | 50 | 0 | 4 | 45 | 0 | 116 |
| No. of (+ve) | 10 | 1 | 30 | 0 | - | 18 | 0 | 59 | |
| % | 63 | 100 | 60 | 0 | - | 40 | 0 | 51 | |
| Sheep | Total tested | 24 | 6 | 50 | 0 | 6 | 27 | 0 | 113 |
| No. of (+ve) | 10 | - | 19 | 0 | - | 5 | 0 | 34 | |
| % | 42 | - | 38 | 0 | - | 19 | 0 | 30 | |
| Horses | Total tested | 0 | 3 | 0 | 0 | 1 | 0 | 5 | 9 |
| No. of (+ve) | 0 | - | 0 | 0 | - | 0 | 2 | 2 | |
| % | 0 | - | 0 | 0 | - | 0 | 40 | 22 | |
| Donkeys | Total tested | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 |
| No. of (+ve) | 0 | 0 | 0 | 0 | - | 0 | 0 | - | |
| % | 0 | 0 | 0 | 0 | - | 0 | 0 | - | |
| Vegetation | Total tested | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 |
| No. of (+ve) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | |
| % | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 33 | |
| Total screened | 40 | 55 | 102 | 23 | 20 | 72 | 5 | 317 | |
| (+ve) samples and | 20 | 12 | 49 | 7 | - | 23 | 2 | 113 | |
| overall % per district | 50 | 22 | 48 | 30 | - | 32 | 40 | 36 |
| Sampling Site | Studied Animals | Coxiella burnetii | ||
|---|---|---|---|---|
| District | Study Group | Positive (+ve) | Total Screened | Overall (%) |
| Butha-Buthe | Cattle | 1 | 45 | 2 |
| Mohale’s Hoek | 1 | 4 | 25 | |
| Overall prevalence | 2 | 49 | 4 | |
| Study Group | Rickettsia africae | Berea | Butha-Buthe | Leribe | Maseru | Mohaleshoek | Qacha’s Nek | Quthing | Overall % (+ve) per Population |
|---|---|---|---|---|---|---|---|---|---|
| Cattle | Total tested | 0 | 45 | 2 | 17 | 4 | 0 | 0 | 68 |
| No. of (+ve) | 0 | 0 | *- | 1 | - | 0 | 0 | 1 | |
| % | 0 | 0 | - | 6 | - | 0 | 0 | 1 | |
| Goats | Total tested | 16 | 1 | 50 | 0 | 4 | 45 | 0 | 116 |
| No. of (+ve) | 1 | - | - | 0 | - | 1 | 0 | 2 | |
| % | 6 | - | - | 0 | - | 2 | 0 | 2 | |
| Sheep | Total tested | 24 | 6 | 50 | 0 | 6 | 27 | 0 | 113 |
| No. of (+ve) | 1 | - | - | 0 | - | - | 0 | 1 | |
| % | 4 | - | - | 0 | - | - | 0 | 1 | |
| Horses | Total tested | 0 | 3 | 0 | 0 | 1 | 0 | 5 | 9 |
| No. of (+ve) | 0 | - | 0 | 0 | - | 0 | 2 | 2 | |
| % | 0 | - | 0 | 0 | - | 0 | 40 | 22 | |
| Total screened | 40 | 55 | 102 | 17 | 15 | 72 | 5 | 306 | |
| Overall (+ve) samples | 2 | - | - | 1 | - | 1 | 2 | 6 | |
| Overall % per district | 5 | - | - | 3 | - | 1 | 40 | 2 |
| Coinfections | Pathogens | Prevalence N (%) | |||
|---|---|---|---|---|---|
| Cattle | Goats | Horses | Vegetation | ||
| Two pathogens | Anaplasma spp. + R. africae | *- | 1 (1%) | 1 (11%) | |
| Anaplasma spp. + Babesia bigemina | 5 (7%) | - | - | 1 (17%) | |
| Three pathogens | Anaplasma spp. + Babesia motasi + B. ovis | - | 2 (2%) | - | - |
| Country | Districts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesotho | Hosts | Berea | Butha-Buthe | Leribe | Mafeteng | Maseru | Mohale’s Hoek | Mokhotlong | Qacha’s Nek | Quthing | Thaba Tseka | Total per Host |
| Cattle | *- | 257 | 177 | 75 | 333 | 97 | 43 | 331 | - | 9 | 1322 | |
| Dogs | - | - | 3 | - | - | - | - | 70 | - | - | 73 | |
| Donkeys | - | - | - | - | - | 12 | - | - | - | - | 12 | |
| Goats | 67 | 18 | 219 | - | - | 15 | 7 | 141 | - | 3 | 470 | |
| Horses | - | - | 3 | - | - | 30 | - | 88 | 8 | - | 129 | |
| Sheep | 97 | 12 | 335 | - | - | 119 | 10 | 170 | - | - | 743 | |
| Vegetation | - | 4 | 9 | - | 185 | - | - | 364 | - | - | 562 | |
| Total per district | 164 | 291 | 746 | 75 | 518 | 273 | 60 | 1164 | 8 | 12 | 3311 | |
| Gender and Stage | Berea | Butha-Buthe | Leribe | Mafeteng | Maseru | Mohale’s Hoek | Mokhotlong | Qacha’s Nek | Quthing | Thaba Tseka | Total (Gender and Stage) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male Adult | 101 | 118 | 232 | 12 | 116 | 81 | 23 | 239 | 5 | 7 | 934 |
| Female Adult | 63 | 119 | 442 | 14 | 205 | 94 | 29 | 424 | 3 | 5 | 1398 |
| Nymphs | 0 | 54 | 72 | 49 | 116 | 98 | 8 | 501 | 0 | 0 | 898 |
| Total (district) | 164 | 291 | 746 | 75 | 518 | 273 | 60 | 1164 | 8 | 12 | 3311 |
| Pathogen | Target Genes | Primer Sequences | Product Size (bp) | Annealing Temp (°C) | Reference |
|---|---|---|---|---|---|
| Anaplasma phagocytophilum | 16S rRNA | EHR521F: 5′-TGTAGGCGGTTCGGTAAGTTAAAG-3′ EHR747R: 5′-GCACTCATCGTTTACAGCGTG-3′ | 250 | 60 | [51] |
| Coxiella burnetii | IS1111 transposase | Trans1-F: 5′-TATGTATCCACCGTAGCCAGTC-3′ Trans2-R: 5′-CCCAACAACACCTCCTTATTC-3′ | 687 | 60 | [52] |
| Rickettsia africae | gltA | CS-78: 5′-GCAAGTATCGGTGAGGATGTAAT-3′ CS-323: 5′-GCTTCCTTAAAATTCAATAAATCAGGAT-3′ | 401 | 55 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlobo-Shwabede, S.I.C.; Zishiri, O.T.; Thekisoe, O.M.M.; Makalo, M.J.R. Molecular Detection of Coxiella burnetii, Rickettsia africae and Anaplasma Species in Ticks from Domestic Animals in Lesotho. Pathogens 2021, 10, 1186. https://doi.org/10.3390/pathogens10091186
Mahlobo-Shwabede SIC, Zishiri OT, Thekisoe OMM, Makalo MJR. Molecular Detection of Coxiella burnetii, Rickettsia africae and Anaplasma Species in Ticks from Domestic Animals in Lesotho. Pathogens. 2021; 10(9):1186. https://doi.org/10.3390/pathogens10091186
Chicago/Turabian StyleMahlobo-Shwabede, Sibonginhlanhla I. C., Oliver T. Zishiri, Oriel M. M. Thekisoe, and Mabusetsa J. R. Makalo. 2021. "Molecular Detection of Coxiella burnetii, Rickettsia africae and Anaplasma Species in Ticks from Domestic Animals in Lesotho" Pathogens 10, no. 9: 1186. https://doi.org/10.3390/pathogens10091186






