Influence of Climatic Factors on Human Hantavirus Infections in Latin America and the Caribbean: A Systematic Review
Abstract
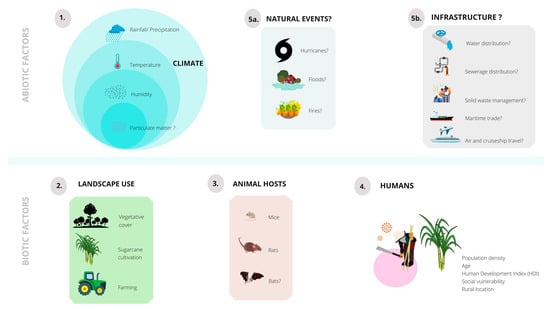
:1. Introduction
2. Results
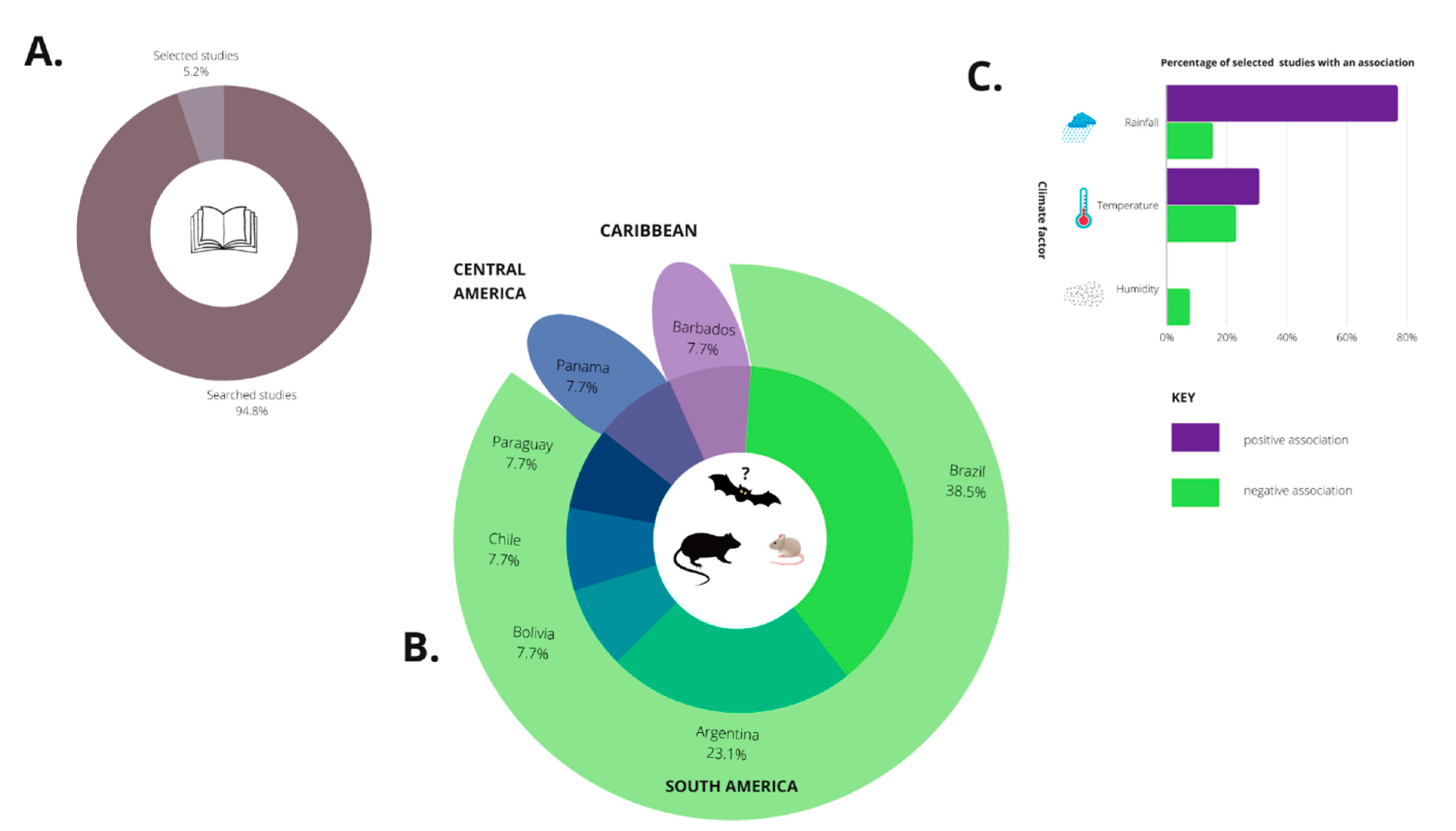
2.1. Bibliographic Search
2.2. Selected Studies
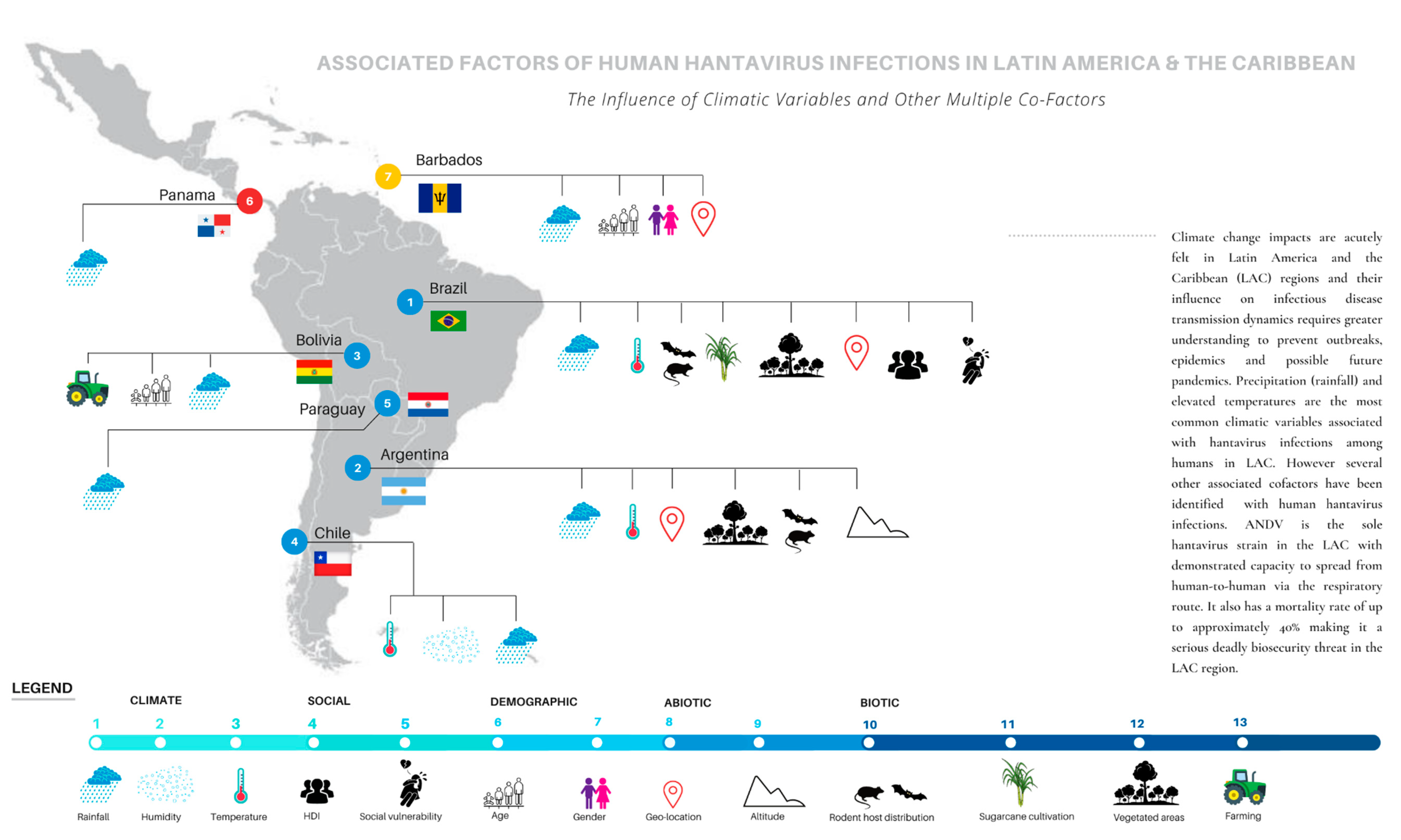
2.2.1. Brazil
2.2.2. Argentina
2.2.3. Chile
2.2.4. Bolivia
2.2.5. Paraguay
2.2.6. Panama
2.2.7. Barbados (Caribbean)
3. Discussion
3.1. Rainfall or Precipitation Factor
3.2. Temperature Factor
3.3. Humidity Factor
3.4. Multiple Co-Factors
3.5. Temporal and Spatial Modelling Approaches
3.6. Selected Studies: Limitations
3.7. Knowledge Gaps & Future Research
4. Materials and Methods
4.1. Bibliographic Search
4.2. Selection Criteria
4.3. Search String
4.4. Study Selection and Quality Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- A.
- Validity of Study Results
- Was a clear focus issue addressed?—Yes/Unsure/NoFollow on and ask some of these other questions to evaluate the rating.
- Which study design was utilized?
- Was the appropriate method used to answer the question posed?
- Was/Were the climate exposure factor(s) accurately measured to minimize bias?
- Was the outcome accurately measured to minimize bias?
- Were all the important confounding factors identified?
- Are these confounding factors addressed in the design and or analysis?
- B.
- What are the Study Results?
- What are the results of this study?—Yes/Unsure/NoFollow and ask some of these other questions to evaluate the rating.
- What is the level of precision of the results? What is the level of precision for the risk estimate used?
- Are the results believable based on statistics?
- How was this study funded?
- C.
- Applicability of Study Results to This Systematic Review
- Does this study answer the question posed by this systematic review?—Yes/Unsure/NoFollow on and ask some of these other questions to evaluate the rating.
- What is the level of precision of the results? What is the level of precision for the risk estimate used?
- Are the results believable?
References
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current classification and future perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lednicky, J.A. Hantaviruses. a short review. Arch. Pathol. Lab. Med. 2003, 127, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Vapalahti, O.; Mustonen, J.; Lundkvist, Å.; Henttonen, H.; Plyusnin, A.; Vaheri, A. Hantavirus infections in Europe. Lancet Infect. Dis. 2003, 3, 653–661. [Google Scholar] [CrossRef]
- Schmaljohn, C. Bunyaviridae: The viruses and their replication. In Field’s Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1996; Volume 1, pp. 1447–1471. [Google Scholar]
- Muyangwa, M.; Martynova, E.V.; Khaiboullina, S.F.; Morzunov, S.P.; Rizvanov, A.A. Hantaviral Proteins: Structure, Functions, and Role in Hantavirus Infection. Front. Microbiol. 2015, 6, 1326. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, C.B.; Figueiredo, L.T.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef] [Green Version]
- Manigold, T.; Vial, P. Human hantavirus infections: Epidemiology, clinical features, pathogenesis and immunology. Swiss. Med. Wkly. 2014, 144, w13937. [Google Scholar] [CrossRef] [PubMed]
- Roda Gracia, J.; Schumann, B.; Seidler, A. Climate variability and the occurrence of human puumala hantavirus infections in Europe: A systematic review. Zoonoses Public Health 2015, 62, 465–478. [Google Scholar] [CrossRef]
- Groen, J.; Koraka, P.; Edwards, C.N.; Branch, S.L.; Douglas, K.O.; Osterhaus, A.D.; Levett, P.N. Serological evidence of hantavirus in humans and rodents in Barbados. J. Infect. 2002, 45, 109–110. [Google Scholar] [CrossRef]
- Kumar, A.; Krishnamurthy, K.; Nielsen, A.L. Hantavirus infection among children hospitalized for febrile illness suspected to be dengue in Barbados. J. Infect. Public Health 2015, 9, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Keenan, L.M.; Chikweto, A.; Sharma, R.N.; Stone, D.M. Seroprevalence of Hantavirus in Rattus norvegicus in Grenada, West Indies. West. Indian Vet. J. 2008, 8, 67–71. [Google Scholar]
- Adesiyun, A.; Dookeran, S.; Stewart-Johnson, A.; Rahaman, S.; Bissessar, S.; Thompson, N. Serological evidence of hantavirus infection in farm and abattoir workers in Trinidad—A preliminary study. J. Agromed. 2011, 16, 194–199. [Google Scholar] [CrossRef]
- Rovida, F.; Percivalle, E.; Sarasini, A.; Chichino, G.; Baldanti, F. Imported hantavirus cardiopulmonary syndrome in an Italian traveller returning from Cuba. New Microbiol. 2013, 36, 103–105. [Google Scholar] [PubMed]
- Padula, P.; Edelstein, A.; Miguel, S.; Lopez, N.; Rossi, C.; Rabinovich, R. Hantavirus pulmonary syndrome outbreak in Argentina: Molecular evidence for person-to-person transmission of Andes virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, R.M.; Estani, S.S.; Yadon, Z.E.; Enria, D.; Padula, P.; Pini, N.; Mills, J.N.; Peters, C.J.; Segura, E.L. An unusual hantavirus outbreak in southern Argentina: Person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia. Emerg. Infect. Dis. 1997, 3, 171. [Google Scholar] [CrossRef]
- Martinez-Valdebenito, C.; Calvo, M.; Vial, C.; Mansilla, R.; Marco, C.; Palma, R.E.; Vial, P.A.; Valdivieso, F.; Mertz, G.; Ferrés, M. Person-to-person household and nosocomial transmission of Andes hantavirus, Southern Chile, 2011. Emerg. Infect. Dis. 2014, 20, 1629. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, R.L.; Bovendorp, R.S.; Sabino-Santos, G., Jr.; Prist, P.R.; Melo, G.L.; Priante, C.d.F.; Wilkinson, D.A.; Ribeiro, M.C.; Hayman, D.T. Hantavirus host assemblages and human disease in the Atlantic Forest. PLoS Negl. Trop. Dis. 2019, 13, e0007655. [Google Scholar] [CrossRef] [Green Version]
- Firth, C.; Tokarz, R.; Simith, D.B.; Nunes, M.R.; Bhat, M.; Rosa, E.S.; Medeiros, D.B.; Palacios, G.; Vasconcelos, P.F.; Lipkin, W.I. Diversity and distribution of hantaviruses in South America. J. Virol. 2012, 86, 13756–13766. [Google Scholar] [CrossRef] [Green Version]
- Kleiman, D.G.; Geist, V.; McDade, M.C. Grzimek’s animal life encyclopedia. In Mammals I–IV; Gale: Detroit, MI, USA, 2003. [Google Scholar]
- D’Elía, G.; Pardinas, U.F. Putting Names to the Phylogenetic Diversity of Neotropical Sigmodontine Rodents: New Genera for Known Species; De Gruyter: Berlin, Germany, 2007. [Google Scholar]
- Gheler-Costa, C.; Vettorazzi, C.A.; Pardini, R.; Verdade, L.M. The Distribution and Abundance of Small Mammals in Agroecosystems of Southeastern Brazil; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Milholland, M.T.; Castro-Arellano, I.; Suzán, G.; Garcia-Peña, G.E.; Lee, T.E.; Rohde, R.E.; Aguirre, A.A.; Mills, J.N. Global diversity and distribution of hantaviruses and their hosts. Ecohealth 2018, 15, 163–208. [Google Scholar] [CrossRef]
- Mejía-Fontecha, I.Y.; Velásquez-Guarín, D.; Tapie, K.D.P.; Ossa-Lopez, P.A.; Rivera-Páez, F.A.; Ramírez-Chaves, H.E. First record of the Northern Ecuadorian Shrew, Cryptotis niausa Moreno Cárdenas & Albuja, 2014 (Eulipotyphla, Soricidae), in Colombia. Check List 2021, 17, 1345. [Google Scholar]
- Sabino-Santos, G., Jr.; Maia, F.G.M.; Martins, R.B.; Gagliardi, T.B.; De Souza, W.M.; Muylaert, R.L.; de Souza Luna, L.K.; Melo, D.M.; de Souza Cardoso, R.; da Silva Barbosa, N. Natural infection of Neotropical bats with hantavirus in Brazil. Sci. Rep. 2018, 8, 8–18. [Google Scholar] [CrossRef] [Green Version]
- de Araujo, J.; Thomazelli, L.M.; Henriques, D.A.; Lautenschalager, D.; Ometto, T.; Dutra, L.M.; Aires, C.C.; Favorito, S.; Durigon, E.L. Detection of hantavirus in bats from remaining rain forest in São Paulo, Brazil. BMC Res. Notes 2012, 5, 690. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, R.; Chang, P. Interaction between tropical Atlantic variability and El Nino-Southern Oscillation. J. Clim. 2000, 13, 2177–2194. [Google Scholar] [CrossRef]
- Klotzbach, P.J. The Influence of El Nino-Southern Oscillation and the Atlantic Multidecadal Oscillation on Caribbean Tropical Cyclone Activity. J. Clim. 2011, 24, 721–731. [Google Scholar] [CrossRef]
- Jury, M.; Malmgren, B.A.; Winter, A. Subregional precipitation climate of the Caribbean and relationships with ENSO and NAO. J. Geophys Res.-Atmos. 2007, 112, D16107. [Google Scholar] [CrossRef]
- Curtis, S.; Gamble, D.W. Regional variations of the Caribbean mid-summer drought. Theor. Appl. Climatol. 2008, 94, 25–34. [Google Scholar] [CrossRef]
- Ayala, J.J.H.; Heslar, M. Examining the spatiotemporal characteristics of droughts in the Caribbean using the standardized precipitation index (SPI). Clim. Res. 2019, 78, 103–116. [Google Scholar] [CrossRef]
- Gamble, D.W.; Parnell, D.B.; Curtis, S. Spatial variability of the Caribbean mid-summer drought and relation to north Atlantic high circulation. Int. J. Climatol. 2008, 28, 343–350. [Google Scholar] [CrossRef]
- Herrera, D.A.; Ault, T.R.; Carrillo, C.M.; Fasullo, J.T.; Li, X.L.; Evans, C.P.; Alessi, M.J.; Mahowald, N.M. Dynamical Characteristics of Drought in the Caribbean from Observations and Simulations. J. Clim. 2020, 33, 10773–10797. [Google Scholar] [CrossRef]
- Herrera, D.; Ault, T. Insights from a New High-Resolution Drought Atlas for the Caribbean Spanning 1950–2016. J. Clim. 2017, 30, 7801–7825. [Google Scholar] [CrossRef]
- Herrera, D.A.; Ault, T.R.; Fasullo, J.T.; Coats, S.J.; Carrillo, C.M.; Cook, B.I.; Williams, A.P. Exacerbation of the 2013-2016 Pan-Caribbean Drought by Anthropogenic Warming. Geophys. Res. Lett. 2018, 45, 10619–10626. [Google Scholar] [CrossRef] [Green Version]
- Klempa, B. Hantaviruse and climate change. Clin. Microbiol. Infect. 2009, 15, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Schmaljohn, C.; Hjelle, B. Hantaviruses: A global disease problem. Emerg. Infect. Dis. 1997, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Engelthaler, D.M.; Mosley, D.G.; Cheek, J.E.; Levy, C.E.; Komatsu, K.K.; Ettestad, P.; Davis, T.; Tanda, D.T.; Miller, L.; Frampton, J.W. Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners region, United States. Emerg. Infect. Dis. 1999, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Reiter, P.; Ebi, K.L.; Yap, W.; Nasci, R.; Patz, J.A. Climate variability and change in the United States: Potential impacts on vector- and rodent-borne diseases. Env. Health Perspect. 2001, 109 (Suppl. 2), 223–233. [Google Scholar] [CrossRef] [Green Version]
- Khaiboullina, S.F.; Morzunov, S.; St Jeor, S.C. Hantaviruses: Molecular biology, evolution and pathogenesis. Curr. Mol. Med. 2005, 5, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.; Ramaekers, B.; Grimm, S.; Worthy, G.; Fayter, D.; Armstrong, N.; Buksnys, T.; Ross, J.; Joore, M.; Kleijnen, J. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. In High-Sensitivity Troponin Assays for Early Rule-Out of Acute Myocardial Infarction in People with Acute Chest Pain: A Systematic Review and Economic Evaluation; NIHR Journals Library: Southampton, UK, 2021. [Google Scholar]
- Prist, P.R.; Uriarte, M.; Fernandes, K.; Metzger, J.P. Climate change and sugarcane expansion increase Hantavirus infection risk. PLoS Negl. Trop. Dis. 2017, 11, e0005705. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, L.R.; Zaki, S.R.; Goldoft, M.J.; Todd, R.L.; Khan, A.S.; Khabbaz, R.F.; Ksiazek, T.G.; Peters, C. Hantavirus pulmonary syndrome associated with entering or cleaning rarely used, rodent-infested structures. J. Infect. Dis. 1995, 172, 1166. [Google Scholar] [CrossRef]
- Douglass, R.J.; Kuenzi, A.J.; Williams, C.Y.; Douglass, S.J.; Mills, J.N. Removing deer mice from buildings and the risk for human exposure to Sin Nombre virus. Emerg. Infect. Dis. 2003, 9, 390. [Google Scholar] [CrossRef]
- Donalisio, M.R.; Vasconcelos, C.H.; Pereira, L.E.; Avila, A.M.; Katz, G. Climatic aspects in hantavirus transmission areas in Sao Paulo State, Brazil. Cad. Saude Publica 2008, 24, 1141–1150. [Google Scholar] [CrossRef]
- Donalisio, M.R.; Peterson, A.T. Environmental factors affecting transmission risk for hantaviruses in forested portions of southern Brazil. Acta Trop. 2011, 119, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Bayard, V.; Kitsutani, P.T.; Barria, E.O.; Ruedas, L.A.; Tinnin, D.S.; Muñoz, C.; De Mosca, I.B.; Guerrero, G.; Kant, R.; Garcia, A. Outbreak of hantavirus pulmonary syndrome, Los Santos, Panama, 1999–2000. Emerg. Infect. Dis. 2004, 10, 1635. [Google Scholar] [CrossRef]
- Montgomery, J.M.; Blair, P.J.; Carroll, D.S.; Mills, J.N.; Gianella, A.; Iihoshi, N.; Briggiler, A.M.; Felices, V.; Salazar, M.; Olson, J.G. Hantavirus pulmonary syndrome in Santa Cruz, Bolivia: Outbreak investigation and antibody prevalence study. PLoS Negl. Trop. Dis. 2012, 6, e1840. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Bryan, R.T.; Mills, J.N.; Palma, R.E.; Vera, I.; De Velasquez, F.; Baez, E.; Schmidt, W.E.; Figueroa, R.E.; Peters, C.J. An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am. J. Trop. Med. Hyg. 1997, 57, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, R.L.; Sabino-Santos, G.; Prist, P.R.; Oshima, J.E.F.; Niebuhr, B.B.; Sobral-Souza, T.; de Oliveira, S.V.; Bovendorp, R.S.; Marshall, J.C.; Hayman, D.T.S.; et al. Spatiotemporal Dynamics of Hantavirus Cardiopulmonary Syndrome Transmission Risk in Brazil. Viruses 2019, 11, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreo, V.; Neteler, M.; Rocchini, D.; Provensal, C.; Levis, S.; Porcasi, X.; Rizzoli, A.; Lanfri, M.; Scavuzzo, M.; Pini, N. Estimating Hantavirus risk in southern Argentina: A GIS-based approach combining human cases and host distribution. Viruses 2014, 6, 201–222. [Google Scholar] [CrossRef] [Green Version]
- Vadell, M.V.; Carbajo, A.E.; Massa, C.; Cueto, G.R.; Villafane, I.E.G. Hantavirus pulmonary syndrome risk in Entre Ríos, Argentina. EcoHealth 2019, 16, 558–569. [Google Scholar] [CrossRef]
- Ferro, I.; Bellomo, C.M.; López, W.; Coelho, R.; Alonso, D.; Bruno, A.; Córdoba, F.E.; Martinez, V.P. Hantavirus pulmonary syndrome outbreaks associated with climate variability in Northwestern Argentina, 1997–2017. PLoS Negl. Trop. Dis. 2020, 14, e0008786. [Google Scholar] [CrossRef]
- Nsoesie, E.O.; Mekaru, S.R.; Ramakrishnan, N.; Marathe, M.V.; Brownstein, J.S. Modeling to predict cases of hantavirus pulmonary syndrome in Chile. PLoS Negl. Trop. Dis. 2014, 8, e2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prist, P.R.; Uriarte, M.; Tambosi, L.R.; Prado, A.; Pardini, R.; D’Andrea, P.S.; Metzger, J.P. Landscape, environmental and social predictors of Hantavirus risk in São Paulo, Brazil. PLoS ONE 2016, 11, e0163459. [Google Scholar]
- He, J.Y.; Wang, Y.; Mu, D.; Xu, Z.W.; Qian, Q.; Chen, G.B.; Wen, L.; Yin, W.W.; Li, S.S.; Zhang, W.Y.; et al. The Impacts of Climatic Factors and Vegetation on Hemorrhagic Fever with Renal Syndrome Transmission in China: A Study of 109 Counties. Int. J. Environ. Res. Public Health 2019, 16, 3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, K.O.; Samuels, T.A.; Iheozor-Ejiofor, R.; Vapalahti, O.; Sironen, T.; Gittens-St Hilaire, M. Serological Evidence of Human Orthohantavirus Infections in Barbados, 2008 to 2016. Pathogens 2021, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.O.; Cayol, C.; Forbes, K.M.; Samuels, T.A.; Vapalahti, O.; Sironen, T.; Gittens-St Hilaire, M. Serological Evidence of Multiple Zoonotic Viral Infections among Wild Rodents in Barbados. Pathogens 2021, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.J.; Yi, S.Y.; Zhang, A.H.; Zhao, Z.H.; Liu, Y.Q.; Zhang, C.; Ye, Z. Epidemiology of hemorrhagic fever with renal syndrome in Tai’an area. Sci. Rep. 2021, 11, 11596. [Google Scholar] [CrossRef] [PubMed]
- Carver, S.; Mills, J.N.; Parmenter, C.A.; Parmenter, R.R.; Richardson, K.S.; Harris, R.L.; Douglass, R.J.; Kuenzi, A.J.; Luis, A.D. Toward a Mechanistic Understanding of Environmentally Forced Zoonotic Disease Emergence: Sin Nombre Hantavirus. Bioscience 2015, 65, 651–666. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.Y.; Yu, P.B.; Cazelles, B.; Xu, L.; Tan, H.; Yang, J.; Huang, S.Q.; Xu, B.; Cai, J.; Ma, C.F.; et al. Interannual cycles of Hantaan virus outbreaks at the human-animal interface in Central China are controlled by temperature and rainfall. Proc. Natl. Acad. Sci. USA 2017, 114, 8041–8046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozada, M.; Guthmann, N.; Baccala, N. Microhabitat selection of five sigmodontine rodents in a forest-steppe transition zone in northwestern Patagonia. Stud. Neotrop. Fauna Environ. 2000, 35, 85–90. [Google Scholar]
- Carbajo, A.E.; Vera, C.; Gonzalez, P.L.M. Hantavirus reservoir Oligoryzomys longicaudatus spatial distribution sensitivity to climate change scenarios in Argentine Patagonia. Int. J. Health Geogr. 2009, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.C.; Gentile, R.; Guterres, A.; Fernandes, J.; Teixeira, B.R.; Vaz, V.; Valdez, F.P.; Vicente, L.H.B.; da Costa-Neto, S.F.; Bonvicino, C.; et al. Ecological study of hantavirus infection in wild rodents in an endemic area in Brazil. Acta Trop. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Piudo, L.; Monteverde, M.; Gonzalez Capria, S.; Padula, P.; Carmanchahi, P. Distribution and abundance of sigmodontine rodents in relation to hantavirus in Neuquen, Argentina. J. Vector. Ecol. 2005, 30, 119–125. [Google Scholar]
- Bi, P.; Tong, S.; Donald, K.; Parton, K.; Ni, J. Climatic, reservoir and occupational variables and the transmission of haemorrhagic fever with renal syndrome in China. Int. J. Epidemiol. 2002, 31, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Tersago, K.; Verhagen, R.; Servais, A.; Heyman, P.; Ducoffre, G.; Leirs, H. Hantavirus disease (nephropathia epidemica) in Belgium: Effects of tree seed production and climate. Epidemiol. Infect. 2009, 137, 250–256. [Google Scholar] [CrossRef]
- Mills, J.N.; Ksiazek, T.G.; Peters, C.; Childs, J.E. Long-term studies of hantavirus reservoir populations in the southwestern United States: A synthesis. Emerg. Infect. Dis. 1999, 5, 135. [Google Scholar] [CrossRef]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Dechet, A.M.; Parsons, M.; Rambaran, M.; Mohamed-Rambaran, P.; Florendo-Cumbermack, A.; Persaud, S.; Baboolal, S.; Ari, M.D.; Shadomy, S.V.; Zaki, S.R. Leptospirosis outbreak following severe flooding: A rapid assessment and mass prophylaxis campaign; Guyana, January–February 2005. PLoS ONE 2012, 7, e39672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.D.; Taylor, M.A.; Bezanilla-Morlot, A.; Stephenson, T.S.; Centella-Artola, A.; Clarke, L.A.; Stephenson, K.A. Generating Projections for the Caribbean at 1.5, 2.0, and 2.5 °C from a High-Resolution Ensemble. Atmosphere 2021, 12, 328. [Google Scholar] [CrossRef]
- Jiang, G.; Zhao, T.; Liu, J.; Xu, L.; Yu, G.; He, H.; Krebs, C.; Zhang, Z. Effects of ENSO-linked climate and vegetation on population dynamics of sympatric rodent species in semiarid grasslands of Inner Mongolia, China. Can. J. Zool. 2011, 89, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Calisher, C.H.; Mills, J.N.; Sweeney, W.P.; Root, J.J.; Reeder, S.A.; Jentes, E.S.; Wagoner, K.; Beaty, B.J. Population dynamics of a diverse rodent assemblage in mixed grass-shrub habitat, southeastern Colorado, 1995–2000. J. Wildl. Dis. 2005, 41, 12–28. [Google Scholar] [CrossRef] [Green Version]
- Zeier, M.; Handermann, M.; Bahr, U.; Rensch, B.; Müller, S.; Kehm, R.; Muranyi, W.; Darai, G. New ecological aspects of hantavirus infection: A change of a paradigm and a challenge of prevention-a review. Virus Genes 2005, 30, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Kallio, E.R.; Klingström, J.; Gustafsson, E.; Manni, T.; Vaheri, A.; Henttonen, H.; Vapalahti, O.; Lundkvist, Å. Prolonged survival of Puumala hantavirus outside the host: Evidence for indirect transmission via the environment. J. Gen. Virol. 2006, 87, 2127–2134. [Google Scholar] [CrossRef]
- Jones, P.D.; Harpham, C.; Harris, I.; Goodess, C.M.; Burton, A.; Centella-Artola, A.; Taylor, M.A.; Bezanilla-Morlot, A.; Campbell, J.D.; Stephenson, T.S. Long-term trends in precipitation and temperature across the Caribbean. Int. J. Climatol. 2016, 36, 3314–3333. [Google Scholar] [CrossRef] [Green Version]
- Peters, E.J. The 2009/2010 Caribbean drought: A case study. Disasters 2015, 39, 738–761. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; French, G.; Lee, P.; Baek, L.; Tsuchiya, K. Observations on Natural and Laboratory Infection of Rodents with the Etiologic Agent of Korean Hemorrhagic Fever; Korea University Seoul Inst for Viral Diseases: Seoul, Korea, 1981. [Google Scholar]
- Vapalahti, K.; Virtala, A.-M.; Vaheri, A.; Vapalahti, O. Case-control study on Puumala virus infection: Smoking is a risk factor. Epidemiol. Infect. 2010, 138, 576–584. [Google Scholar] [CrossRef]
- Mallet, M.; Tulet, P.; Serça, D.; Solmon, F.; Dubovik, O.; Pelon, J.; Pont, V.; Thouron, O. Impact of dust aerosols on the radiative budget, surface heat fluxes, heating rate profiles and convective activity over West Africa during March 2006. Atmos. Chem. Phys. 2009, 9, 7143–7160. [Google Scholar] [CrossRef] [Green Version]
- Vieira, E.; Baumgarten, L.; Paise, G.; Becker, R. Seasonal patterns and influence of temperature on the daily activity of the diurnal neotropical rodent Necromys lasiurus. Can. J. Zool. 2010, 88, 259–265. [Google Scholar] [CrossRef]
- Paise, G.; Vieira, E.M. Daily activity of a neotropical rodent (Oxymycterus nasutus): Seasonal changes and influence of environmental factors. J. Mammal. 2006, 87, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Bittencourt, E.B.; Vera, C.; Conde, C.; Rocha, C.; Bergallo, H. Activity patterns of small mammals in an Atlantic forest area of southeastern Brazil. Cienc. E Cult. (Sao Paulo) 1999, 51, 126–132. [Google Scholar]
- Keppel-Aleks, G.; Wolf, A.S.; Mu, M.; Doney, S.C.; Morton, D.C.; Kasibhatla, P.S.; Miller, J.B.; Dlugokencky, E.J.; Randerson, J.T. Separating the influence of temperature, drought, and fire on interannual variability in atmospheric CO2. Glob. Biogeochem. Cycles 2014, 28, 1295–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boruff, B.J. A Multiple Hazards Assessment of Two Caribbean Nations: Barbados and St. Vincent; University of South Carolina: Columbia, SC, USA, 2005. [Google Scholar]
- Cook, S.F. The effects of fire on a population of small rodents. Ecology 1959, 40, 102–108. [Google Scholar] [CrossRef]
- Richardson, B.C. Igniting the Caribbean’s Past: Fire in British West. Indian History; University of North Carolina Press: Chapel Hill, NC, USA, 2004. [Google Scholar]
- Richardson, K.S.; Kuenzi, A.; Douglass, R.J.; Hart, J.; Carver, S. Human exposure to particulate matter potentially contaminated with Sin Nombre virus. Ecohealth 2013, 10, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.S.; Kim, S.; Choi, Y.; Kim, S.; Kim, Y.S. Air pollution and hemorrhagic fever with renal syndrome in South Korea: An ecological correlation study. BMC Public Health 2013, 13, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, C., Jr.; Hurd, F.K.; Corbett, W.J. Aerosol size and relative humidity. J. Colloid Sci. 1958, 13, 472–482. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Fu, P.; Jiang, Q.; Yang, T.; Li, J.; Ge, X. The impact of relative humidity on aerosol composition and evolution processes during wintertime in Beijing, China. Atmos. Environ. 2013, 77, 927–934. [Google Scholar] [CrossRef]
- Sisterson, D.; Johnson, S.; Kumar, R. The influence of humidity on fine-particle aerosol dynamics and precipitation scavenging. Aerosol Sci. Technol. 1985, 4, 287–300. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Guo, W.-D.; Fang, L.-Q.; Li, C.-P.; Bi, P.; Glass, G.E.; Jiang, J.-F.; Sun, S.-H.; Qian, Q.; Liu, W. Climate variability and hemorrhagic fever with renal syndrome transmission in Northeastern China. Environ. Health Perspect. 2010, 118, 915–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Tian, H.-Y.; Cazelles, B.; Li, X.-J.; Tong, S.-L.; Gao, L.-D.; Qin, J.-X.; Lin, X.-L.; Liu, H.-N.; Zhang, X.-X. Atmospheric moisture variability and transmission of hemorrhagic fever with renal syndrome in Changsha City, Mainland China, 1991–2010. PLoS Negl. Trop. Dis. 2013, 7, e2260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.L.; Yang, X.D.; Liu, H.J.; Hu, Y.X.; He, M.F.; Huang, B.; Yao, L.S.; Li, N.; Zhou, G.; Yin, Y.; et al. Effects of climate factors on hemorrhagic fever with renal syndrome in Changchun, 2013 to 2017. Medicine 2019, 98, e14640. [Google Scholar] [CrossRef] [PubMed]
- Evander, M.; Ahlm, C. Milder winters in northern Scandinavia may contribute to larger outbreaks of haemorrhagic fever virus. Glob. Health Action 2009, 2, 98–102. [Google Scholar] [CrossRef]
- Grantz, D. Plant response to atmospheric humidity. Plant. Cell Environ. 1990, 13, 667–679. [Google Scholar] [CrossRef]
- Roriz, M.; Carvalho, S.M.; Vasconcelos, M.W. High relative air humidity influences mineral accumulation and growth in iron deficient soybean plants. Front. Plant. Sci. 2014, 5, 726. [Google Scholar] [CrossRef]
- de Brito, M.G.; de Oliveira, T.M.; Ferraz, M.L.; Duarte, R.M.; Frankó, M.d.A.L.; Amâncio, F.F.; de Araújo Diniz, S.; Silva, M.X. Association between hantavirus cardiopulmonary syndrome in humans and landscape configuration in the Cerrado region of Minas Gerais, Brazil. Acta Vet. Bras. 2020, 14, 201–208. [Google Scholar] [CrossRef]
- Oscarsson, K.B.; Brorstad, A.; Baudin, M.; Lindberg, A.; Forssén, A.; Evander, M.; Eriksson, M.; Ahlm, C. Human Puumala hantavirus infection in northern Sweden; increased seroprevalence and association to risk and health factors. BMC Infect. Dis. 2016, 16, 566. [Google Scholar]
- Jameson, L.J.; Newton, A.; Coole, L.; Newman, E.N.; Carroll, M.W.; Beeching, N.J.; Hewson, R.; Christley, R.M. Prevalence of antibodies against hantaviruses in serum and saliva of adults living or working on farms in Yorkshire, United Kingdom. Viruses 2014, 6, 524–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Pérez, F.; Navarrete-Droguett, J.; Aldunate, R.; Yates, T.L.; Mertz, G.J.; Vial, P.A.; Ferrés, M.; Marquet, P.A.; Palma, R.E. Peridomestic small mammals associated with confirmed cases of human hantavirus disease in southcentral Chile. Am. J. Trop. Med. Hyg. 2004, 70, 305–309. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.V.; Fonseca, L.X.; de Araújo Vilges, K.M.; Maniglia, F.V.P.; Pereira, S.V.C.; de Caldas, E.P.; Tauil, P.L.; Gurgel-Gonçalves, R. Vulnerability of Brazilian municipalities to hantavirus infections based on multi-criteria decision analysis. Emerg. Themes Epidemiol. 2015, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Vieira, E.; Palma, A. Pequenos mamíferos do Cerrado: Distribuição dos gêneros e estrutura das comunidades nos diferentes habitats. In Cerrado: Ecologia, Biodiversidade e Conservação; Ministério do Meio Ambiente: Brasilia, Brazil, 2005; pp. 265–282. [Google Scholar]
- Orrock, J.L.; Allan, B.F.; Drost, C.A. Biogeographic and ecological regulation of disease: Prevalence of Sin Nombre virus in island mice is related to island area, precipitation, and predator richness. Am. Nat. 2011, 177, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Roques, A. Alien forest insects in a warmer world and a globalised economy: Impacts of changes in trade, tourism and climate on forest biosecurity. N. Z. J. For. Sci. 2010, 40, S77–S94. [Google Scholar]
- Yashina, L.N.; Hay, J.; Smetannikova, N.A.; Kushnareva, T.V.; Iunikhina, O.V.; Kompanets, G.G. Hemorrhagic Fever With Renal Syndrome in Vladivostok City, Russia. Front. Public Health 2021, 9, 620279. [Google Scholar] [CrossRef]
- Dupinay, T.; Pounder, K.C.; Ayral, F.; Laaberki, M.-H.; Marston, D.A.; Lacôte, S.; Rey, C.; Barbet, F.; Voller, K.; Nazaret, N. Detection and genetic characterization of Seoul Virus from commensal brown rats in France. Virol. J. 2014, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Clement, J.; Maes, P.; Van Ranst, M. Hemorrhagic Fever with Renal Syndrome in the New, and Hantavirus Pulmonary Syndrome in the Old World: Paradi(se)gm lost or regained? Virus Res. 2014, 187, 55–58. [Google Scholar] [CrossRef]
- Blasdell, K.R.; Morand, S.; Perera, D.; Firth, C. Association of rodent-borne Leptospira spp. with urban environments in Malaysian Borneo. PLoS Negl. Trop. Dis. 2019, 13, e0007141. [Google Scholar] [CrossRef]
- Byers, K.A.; Lee, M.J.; Patrick, D.M.; Himsworth, C.G. Rats about town: A systematic review of rat movement in urban ecosystems. Front. Ecol. Evol. 2019, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Pépin, M.; Dupinay, T.; Zilber, A.-L.; McElhinney, L.M. Of rats and pathogens: Pathogens transmitted by urban rats with an emphasis on hantaviruses. CAB Rev. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Atkinson, B.; Jameson, L.J.; Bovill, B.A.; Aarons, E.J.; Clewlow, J.; Lumley, S.; Latham, J.; Jenkins, M.H.; MacGowan, A.P.; Simpson, A.J.; et al. A non-fatal case of hantavirus cardiopulmonary syndrome imported into the UK (ex Panama), July 2014. J. Clin. Virol. 2015, 67, 52–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puca, E.; Qato, M.; Pipero, P.; Akshija, I.; Kote, M.; Kraja, D. Two cases of imported hemorrhagic fever with renal syndrome and systematic review of literature. Travel Med. Infect. Dis. 2019, 28, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Shengmei, Y.; Huyin, H.; Yili, Z.; Baofa, Y.; Le, Z.; Wanhong, W. Effects of railway traffic on the community structure of rodents in warm steppe along the Qinghai-Tibet Railway. Acta Theriol. Sin. 2006, 26, 267. [Google Scholar]
- Bao, C.; Liu, W.; Zhu, Y.; Liu, W.; Hu, J.; Liang, Q.; Cheng, Y.; Wu, Y.; Yu, R.; Zhou, M. The spatial analysis on hemorrhagic fever with renal syndrome in Jiangsu province, China based on geographic information system. PLoS ONE 2014, 9, e83848. [Google Scholar] [CrossRef] [Green Version]
- Prospero, J.M.; Bonatti, E.; Schubert, C.; Carlson, T.N. Dust in the Caribbean atmosphere traced to an African dust storm. Earth Planet. Sci. Lett. 1970, 9, 287–293. [Google Scholar] [CrossRef]
- Prospero, J.M.; Blades, E.; Mathison, G.; Naidu, R. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia 2005, 21, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Akpinar-Elci, M.; Coomansingh, K.; Blando, J.; Mark, L. Household bush burning practice and related respiratory symptoms in Grenada, the Caribbean. J. Air Waste Manag. Assoc. 2015, 65, 1148–1152. [Google Scholar] [CrossRef] [Green Version]
- Shairsingh, K.K.; Jeong, C.-H.; Evans, G.J. Transboundary and traffic influences on air pollution across two Caribbean islands. Sci. Total. Environ. 2019, 653, 1105–1110. [Google Scholar] [CrossRef]
- Ramírez, O.; da Boit, K.; Blanco, E.; Silva, L.F. Hazardous thoracic and ultrafine particles from road dust in a Caribbean industrial city. Urban Clim. 2020, 33, 100655. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Hantavirus Infection, Non-Hantavirus Pulmonary Syndrome. 2015 Case Definition. Available online: https://wwwn.cdc.gov/nndss/conditions/hantavirus-infection-non-hantavirus-pulmonary-syndrome/case-definition/2015/ (accessed on 17 July 2021).
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]

| Study | Quality | Study Location | Study Design | Time Period | Human Cases (n) | Climatic Factors | Outcome | Co-Factors | Statistical Methods | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Donalisio (2008) | + | Brazil | Ecological | 1993–2005 | 80 | Rainfall (mm) | HPS cases | Spatial analysis | None | Higher HPS incidence observed in drier months and increased access to food sources by rodents (Harvesting and grain storage and sugar cane cultivation). |
| Donalisio (2011) | ++ | ENM | 1993–2008 | 288 | Winter precipitation | HPS cases | GIS, topography, men > 14 years old and EVI | None | Winter precipitation increased in the dry season and EVI was associated with HPS disease incidence. | |
| Prist (2016) | ++ | Bayesian model | 1993–2012 | 207 | Rainfall and temperature | HCPS risk | HDI, men > 14 years old, sugarcane, population at risk and landscape | t-test, one-way ANOVA, and Tukey’s MCT | HDI and sugarcane cultivation were associated with HCPS cases in the Cerrado whilst males > 14 years old, HDI, sugarcane cultivation and temperature were associated with HPS cases in the Atlantic Forest. | |
| Prist (2017) | + | Bayesian model | 2000–2010 | ? | Temperature, precipitation, RCP4.5 and RCP8.5 | HCPS risk & HCPS cases | Sugarcane cultivation | Moran’s I, Queen’s case neighbourhood relation | A positive association was observed with increased temperatures & sugarcane cultivation and HCPS risk and HCPS cases. | |
| Muyalert (2019) | ++ | Zero inflated model and temporal term (rw2) + Besag Intrinsic Conditional Auto-Regressive (ICAR) spatial model | 1993–2016 | 1758 | Temperature and precipitation | HCPS risk & HCPS cases | Host diversity, social vulnerability, and land use change (sugarcane) | INLA | A positive effect was observed with size of population at risk, social vulnerability, rainfall, host diversity, rural workers, land use (sugarcane, maize, and forest cover) on HCPS risk and HCPS cases whilst a negative effect was observed with temperature. | |
| Andreo (2014) | ++ | Argentina | GLM and MaxEnt models | 1995–2009 | 149 | Climate and precipitation related factors | HPS cases | Altitude, rodent host, vegetation, & GIS | Kruskal-Wallis and pairwise Pearson correlation | A positive association of precipitation, forested and scrubland habitats with HPS cases was observed. |
| Vadell (2019) | ++ | GLM | 2004–2015 | 60 | Rainfall and temperature | HPS cases | Area distance from roads, vegetation, topography, number of human inhabitants and reservoir host population | Kappa index, VIF analysis, bootstrap procedure, and residual plots | A positive association with of HPS cases with areas with high percentage of tree cover and locations near rivers was observed. No association with annual precipitation and mean annual temperature was observed and human hantavirus cases. | |
| Ferro (2020) | ++ | Argentina | ARIMA & dynamic regression models | 1997–2017 | 902 | Rainfall and temperature | HPS cases | None | Augmented Dickey-Fuller Test, AICc, RMSE & Ljund-Box test | A positive association of HPS cases with rainfall and temperature was observed with notable delay or lags ranging from two to six months. |
| Montgomery (2012) | - | Bolivia | Epidemiology | 2002 | 45 | Rainfall | HPS cases | Age and farming | None mentioned | A possible association of HPS cases with monthly precipitation levels exists. |
| Nsoesie (2014) | ++ | Chile | ARIMA and regression with ARIMA errors | 2001–2012 | 667 | Precipitation, temperature & humidity | HPS cases | None | R2 and RMSE | Positive associations of HPS cases with peaks in temperatures and HPS troughs with precipitation and humidity levels were observed. |
| Bayard (2004) | − | Panama | Outbreak investigation | 1999–2000 | 9 | Precipitation | HPS cases | None | None mentioned | A positive association of HPS cases with increased rainfall (two to threefold) during September/October 1999 (outbreak year) was observed. |
| Williams (1997) | − | Paraguay | Outbreak investigation | 1995–1996 | 17 | Precipitation | HPS cases | None | None mentioned | A positive association of HPS cases with increased rainfall (10-fold) during the 1995 (outbreak year) was observed. |
| Douglas (2021) | + | Barbados | Cross-sectional epidemiology | 2008–2016 | 862 | Rainfall (seasonality) | Hantavirus cases * | Gender, age and urban location | 95% CI | A positive association of human hantavirus infections with rainy season, age, gender, and geospatial location was observed. |
| Study | Quality | Study Location | Study Design | Statistical Methods | Metric | Best Model Value | Worst Model Value |
|---|---|---|---|---|---|---|---|
| Donalisio (2008) | + | Brazil | Ecological | None | N/A | N/A | N/A |
| Donalisio (2011) | ++ | ENM | t-test | p-value | 0.02 | 0.0001 | |
| Prist (2016) | ++ | Bayesian model | t-test, one-way ANOVA, and Tukey’s MCT | p-value | 0.9231 | 0.0626 | |
| Prist (2017) | + | Bayesian model | Moran’s I, Queen’s case neighbourhood relation | SD of risk values | 3.4 | 3.8 | |
| Muyalert (2019) | ++ | Zero inflated model and temporal term (rw2) + Besag ICAR spatial model | INLA, Moran’s I, cross validation procedures | CPO | 0.58 * | 0.36 | |
| Andreo (2014) | ++ | Argentina | GLM and MaxEnt models | Kruskal-Wallis and pairwise Pearson correlation | AIC | 76.07 | 100.42 |
| Vadell (2019) | ++ | GLM | Kappa index, VIF analysis, bootstrap procedure, and residual plotsAugmented Dickey-Fuller Test, AICc, RMSE & Ljund-Box test | Kappa index | 0.58 | 0.44 | |
| Explained deviance | 70.5% | 61.2% | |||||
| Ferro (2020) | ++ | ARIMA & dynamic regression models | AICc | 97.74 | 291.4 | ||
| RMSE | 0.91 | 0.78 | |||||
| R2adj | 0.71 | 0.54 | |||||
| Montgomery (2012) | - | Bolivia | Epidemiology | None mentioned | N/A | N/A | N/A |
| Nsoesie (2014) | ++ | Chile | ARIMA and regression with ARIMA errors | Coefficient of variation R2 and RMSE | AICc | 437.62 | 444.98 |
| RMSE | 2.421 | 2.774 | |||||
| R2 | 0.594 | 0.551 | |||||
| Bayard (2004) | − | Panama | Outbreak investigation | None mentioned | N/A | N/A | N/A |
| Williams (1997) | − | Paraguay | Outbreak investigation | None mentioned | N/A | N/A | N/A |
| Douglas (2021) | + | Barbados | Cross-sectional epidemiology | 95% confidence intervals (CI) | N/A | N/A | N/A |
| Parameters | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | People with acute hantavirus infection or HPS/HCPS/HFRS | Only animal hantavirus infections |
| Exposure | At least one climate factor | No climate factors |
| Comparators | N/A | |
| Outcomes | Hantavirus case/infection/risk/incidence | Rodent density/distribution |
| Study design | Observational, epidemiological, retrospective, predictive modelling study design | Prospective study design |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douglas, K.O.; Payne, K.; Sabino-Santos, G., Jr.; Agard, J. Influence of Climatic Factors on Human Hantavirus Infections in Latin America and the Caribbean: A Systematic Review. Pathogens 2022, 11, 15. https://doi.org/10.3390/pathogens11010015
Douglas KO, Payne K, Sabino-Santos G Jr., Agard J. Influence of Climatic Factors on Human Hantavirus Infections in Latin America and the Caribbean: A Systematic Review. Pathogens. 2022; 11(1):15. https://doi.org/10.3390/pathogens11010015
Chicago/Turabian StyleDouglas, Kirk Osmond, Karl Payne, Gilberto Sabino-Santos, Jr., and John Agard. 2022. "Influence of Climatic Factors on Human Hantavirus Infections in Latin America and the Caribbean: A Systematic Review" Pathogens 11, no. 1: 15. https://doi.org/10.3390/pathogens11010015