Detection of Circulating VZV-Glycoprotein E-Specific Antibodies by Chemiluminescent Immunoassay (CLIA) for Varicella–Zoster Diagnosis
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics and Sampling
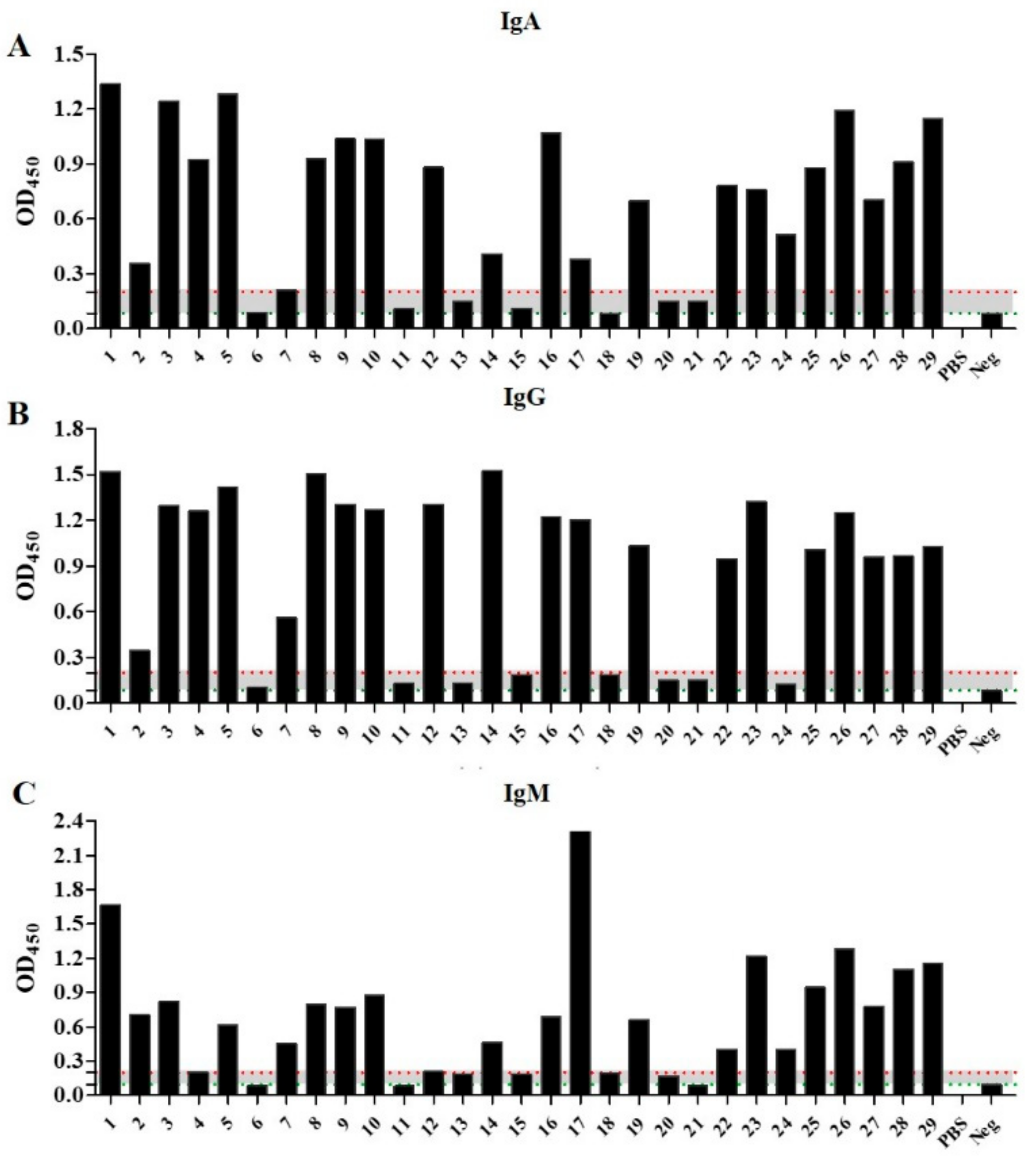
2.2. Patient Samples React with VZV Glycoprotein Depending on Concentration: Cohort Stratification
2.3. Anti-VZV-gE IgA, IgG, and IgM Detection-Based VZV Diagnostic Kit Has High Sensitivity/Accuracy
2.4. Combining IgM to IgG and IgA Detection Improves the Accuracy of the Varicella–Zoster Diagnosis
2.5. Equivocal Sample Analysis Shows Higher Sensitivity/Accuracy for CLIA Than ELISA in Combined Antibody Detection
2.6. Diagnostic Performance of CLIA-Based Immunoglobulin Diagnosis Regarding Equivocal Patients
2.7. Antibody Titer Analysis in VZV Patients Suggests a Primary Infection in Equivocal Patients
2.8. VZV-gE-Specific IgM Titer Negatively Correlates with Age
3. Discussion
4. Materials and Methods
4.1. Patient and Clinical Samples
4.2. Enzyme-Linked Immunosorbent Assay Tests
4.3. VZV Glycoprotein E Antigen Preparation
4.3.1. Cell Cultures
4.3.2. Molecular Cloning, Expression, and Purification of VZV-gE Protein
4.4. Preparation and Validation of the CLIA-Based Diagnostic Kit
4.5. Statistical Analysis
- Sensitivity (%) = 100 × [True Positive/(True Positive + False Negative)];
- Specificity (%) = 100 × [True Negative/(True Negative + False Positive)];
- Overall agreement (%) = (True Positive + True Negative)/Total Tests.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arvin, A.M. Varicella-zoster virus. Clin. Microbiol. Rev. 1996, 9, 361–381. [Google Scholar] [CrossRef]
- Baird, N.L.; Zhu, S.; Pearce, C.M.; Viejo-Borbolla, A. Current In Vitro Models to Study Varicella Zoster Virus Latency and Reactivation. Viruses 2019, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers 2015, 1, 15016. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.G.E.; Gershon, A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, A.M.; Sarmast, S.T.; Jahan, N. Viral Infections of the Central Nervous System in Children: A Systematic Review. Cureus 2020, 12, e11174. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Ma, L.; Cao, S. Zoster sine herpete: A review. Korean J. Pain 2020, 33, 208–215. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. Neurological complications of varicella zoster virus reactivation. Curr. Opin. Neurol. 2014, 27, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Nichols, R.A.; Averbeck, K.T.; Poulsen, A.G.; Al Bassam, M.M.; Cabral, F.; Aaby, P.; Breuer, J. Household size is critical to varicella-zoster virus transmission in the tropics despite lower viral infectivity. Epidemics 2011, 3, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Heywood, A.E.; Macartney, K.K.; MacIntyre, C.R.; McIntyre, P.B. Current developments in varicella-zoster virus disease prevention. A report on the varicella-zoster virus workshop convened by the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases on 16–17 November 2006. Commun. Dis. Intell. Q. Rep. 2007, 31, 303–310. [Google Scholar]
- Bonmarin, I.; Santa-Olalla, P.; Levy-Bruhl, D. Modelling the impact of vaccination on the epidemiology of varicella zoster virus. Rev. Epidemiol. Sante Publique 2008, 56, 323–331. [Google Scholar] [CrossRef]
- De Donno, A.; Kuhdari, P.; Guido, M.; Rota, M.C.; Bella, A.; Brignole, G.; Lupi, S.; Idolo, A.; Stefanati, A.; Del Manso, M.; et al. Has VZV epidemiology changed in Italy? Results of a seroprevalence study. Hum. Vaccin. Immunother. 2017, 13, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, J.; Harpaz, R.; Baughman, A.L.; Heath, K.; Loparev, V.; Vazquez, M.; Watson, B.M.; Schmid, D.S. Evaluation of Laboratory Methods for Diagnosis of Varicella. Clin. Infect. Dis. 2010, 51, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Adriana Lopez, J.L.; Scott Schmid, M.M. Manual for the Surveillance of Vaccine-Preventable Diseases: Chapter 7—Varicella; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018. [Google Scholar]
- Senders, S.D.; Bundick, N.D.; Li, J.; Zecca, C.; Helmond, F.A. Evaluation of immunogenicity and safety of VARIVAX New Seed Process (NSP) in children. Hum. Vaccin. Immunother. 2018, 14, 442–449. [Google Scholar] [CrossRef]
- Sauerbrei, A.; Wutzler, P. Serological detection of varicella-zoster virus-specific immunoglobulin G by an enzyme-linked immunosorbent assay using glycoprotein antigen. J. Clin. Microbiol. 2006, 44, 3094–3097. [Google Scholar] [CrossRef] [Green Version]
- Edmunds, W.J.; Brisson, M. The effect of vaccination on the epidemiology of varicella zoster virus. J. Infect. 2002, 44, 211–219. [Google Scholar] [CrossRef]
- Hope-Simpson, R.E. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc. R. Soc. Med. 1965, 58, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.S.; Wang, X.; Liu, J.Y.; Chen, Y.F.; Zhou, Q.; Wang, Y.; Sha, J.D.; Xuan, Z.L.; Zhang, L.W.; Yan, L.; et al. Varicella outbreak trends in school settings during the voluntary single-dose vaccine era from 2006 to 2017 in Shanghai, China. Int. J. Infect. Dis. 2019, 89, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Li, Y.; Wang, Y.; Liu, Y.; Cao, L.; Zhu, X.; Martin, K.; Wang, H.; An, Z. The varicella vaccination pattern among children under 5 years old in selected areas in china. Oncotarget 2017, 8, 45612–45618. [Google Scholar] [CrossRef]
- Luan, L.; Shen, X.; Qiu, J.; Jing, Y.; Zhang, J.; Wang, J.; Zhang, J.; Dong, C. Seroprevalence and molecular characteristics of varicella-zoster virus infection in Chinese children. BMC Infect. Dis. 2019, 19, 643. [Google Scholar] [CrossRef] [PubMed]
- Haugnes, H.; Flem, E.; Wisloff, T. Healthcare costs associated with varicella and herpes zoster in Norway. Vaccine 2019, 37, 3779–3784. [Google Scholar] [CrossRef]
- Marin, M.; Guris, D.; Chaves, S.S.; Schmid, S.; Seward, J.F.; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007, 56, 1–40. [Google Scholar]
- Provost, P.J.; Krah, D.L.; Kuter, B.J.; Morton, D.H.; Schofield, T.L.; Wasmuth, E.H.; White, C.J.; Miller, W.J.; Ellis, R.W. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine 1991, 9, 111–116. [Google Scholar] [CrossRef]
- Nikolic, D.; Kohn, D.; Yen-Lieberman, B.; Procop, G.W. Detection of Herpes Simplex Virus and Varicella-Zoster Virus by Traditional and Multiplex Molecular Methods. Am. J. Clin. Pathol. 2019, 151, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Yen-Lieberman, B.; Schindler, S.; Asamoto, K.; Schold, J.D.; Procop, G.W. Should varicella-zoster virus culture be eliminated? A comparison of direct immunofluorescence antigen detection, culture, and PCR, with a historical review. J. Clin. Microbiol. 2012, 50, 4120–4122. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Aoyama, Y. Detection of multinucleated giant cells in differentiated keratinocytes with herpes simplex virus and varicella zoster virus infections by modified Tzanck smear method. J. Dermatol. 2021, 48, 21–27. [Google Scholar] [CrossRef]
- Maple, P.A.; Breuer, J.; Quinlivan, M.; Kafatos, G.; Brown, K.E. Comparison of a commercial Varicella Zoster glycoprotein IgG enzyme immunoassay with a reference time resolved fluorescence immunoassay (VZV TRFIA) for measuring VZV IgG in sera from pregnant women, sera sent for confirmatory testing and pre and post vOka vaccination sera from healthcare workers. J. Clin. Virol. 2012, 53, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Schmutzhard, J.; Merete Riedel, H.; Zweygberg Wirgart, B.; Grillner, L. Detection of herpes simplex virus type 1, herpes simplex virus type 2 and varicella-zoster virus in skin lesions. Comparison of real-time PCR, nested PCR and virus isolation. J. Clin. Virol. 2004, 29, 120–126. [Google Scholar] [CrossRef]
- Kennedy, P.G. Issues in the Treatment of Neurological Conditions Caused by Reactivation of Varicella Zoster Virus (VZV). Neurotherapeutics 2016, 13, 509–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, M.A.; Gilden, D. Update on varicella zoster virus vasculopathy. Curr. Infect. Dis. Rep. 2014, 16, 407. [Google Scholar] [CrossRef] [Green Version]
- Majumder, P.; Shetty, A.K. Comparison between ELISA and chemiluminescence immunoassay for the detection of Hepatitis C virus antibody. Indian J. Microbiol. Res. 2017, 4, 353–357. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Xu, Y.; Shen, T.; Cheng, G.; Huang, B.; Ruan, X.; Wang, C. Comparison of chemiluminescence immunoassay, enzyme-linked immunosorbent assay and passive agglutination for diagnosis of Mycoplasma pneumoniae infection. Ther. Clin. Risk Manag. 2018, 14, 1091–1097. [Google Scholar] [CrossRef] [Green Version]
- de Ory, F.; Minguito, T.; Balfagon, P.; Sanz, J.C. Comparison of chemiluminescent immunoassay and ELISA for measles IgG and IgM. APMIS 2015, 123, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Yang, Y.; Jiang, D.; Qi, P.Y.; He, W.; Zhao, C.; Yi, R.; et al. COVID-19 diagnosis and study of serum SARS-CoV-2 specific IgA, IgM and IgG by chemiluminescence immunoanalysis. medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.04.17.20064907v2 (accessed on 22 November 2021). [CrossRef] [Green Version]
- Nicol, T.; Lefeuvre, C.; Serri, O.; Pivert, A.; Joubaud, F.; Dubee, V.; Kouatchet, A.; Ducancelle, A.; Lunel-Fabiani, F.; Le Guillou-Guillemette, H. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J. Clin. Virol. 2020, 129, 104511. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaewpoowat, Q.; Salazar, L.; Aguilera, E.; Wootton, S.H.; Hasbun, R. Herpes simplex and varicella zoster CNS infections: Clinical presentations, treatments and outcomes. Infection 2016, 44, 337–345. [Google Scholar] [CrossRef]
- Bunyaratavej, S.; Prasertyothin, S.; Leeyaphan, C. Atypical Presentation of Recurrent Varicella Zoster Virus Infection: A Case Report and Review of the Literature. Southeast Asian J. Trop. Med. Public Health 2015, 46, 27–29. [Google Scholar] [PubMed]
- Freer, G.; Pistello, M. Varicella-zoster virus infection: Natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018, 41, 95–105. [Google Scholar]
- van Loon, A.M.; van der Logt, J.T.; Heessen, F.W.; Heeren, M.C.; Zoll, J. Antibody-capture enzyme-linked immunosorbent assays that use enzyme-labelled antigen for detection of virus-specific immunoglobulin M, A and G in patients with varicella or herpes zoster. Epidemiol. Infect. 1992, 108, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Microbiol. 2014, 12, 197–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, S.L.; Yang, E.; Arvin, A.M. Varicella-Zoster Virus Glycoproteins: Entry, Replication, and Pathogenesis. Curr. Clin. Microbiol. Rep. 2016, 3, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Grahn, A.; Studahl, M.; Nilsson, S.; Thomsson, E.; Backstrom, M.; Bergstrom, T. Varicella-zoster virus (VZV) glycoprotein E is a serological antigen for detection of intrathecal antibodies to VZV in central nervous system infections, without cross-reaction to herpes simplex virus 1. Clin. Vaccine Immunol. 2011, 18, 1336–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, S.W.; Kim, Y.S.; Nahm, F.S.; Yoo, D.H.; Choi, E.; Lee, P.B.; Choo, H.; Park, Z.Y.; Yang, C.S. The positive duration of varicella zoster immunoglobulin M antibody test in herpes zoster. Medicine 2016, 95, e4616. [Google Scholar] [CrossRef] [PubMed]
- Enders, G. Serodiagnosis of Varicella-Zoster virus infection in pregnancy and standardization of the ELISA IgG and IgM antibody tests. Dev. Biol. Stand. 1982, 52, 221–236. [Google Scholar] [PubMed]
- Iltis, J.P.; Achilli, G.; Madden, D.L.; Sever, J.L. Serologic study by enzyme-linked immunosorbent assay of the IgM antibody response in the patas monkey following experimental simian varicella virus infection. Diagn. Immunol. 1984, 2, 137–142. [Google Scholar]
- Dobec, M.; Bossart, W.; Kaeppeli, F.; Mueller-Schoop, J. Serology and serum DNA detection in shingles. Swiss Med. Wkly. 2008, 138, 47–51. [Google Scholar]
- Medic, S.; Petrovic, V.; Milosevic, V.; Lozanov-Crvenkovic, Z.; Brkic, S.; Andrews, N.; de Ory, F.; Anastassopoulou, C. Seroepidemiology of varicella zoster virus infection in Vojvodina, Serbia. Epidemiol. Infect. 2018, 146, 1593–1601. [Google Scholar] [CrossRef] [Green Version]
- Hellstern, P.; Solheim, B.G. The Use of Solvent/Detergent Treatment in Pathogen Reduction of Plasma. Transfus. Med. Hemoth. 2011, 38, 65–70. [Google Scholar] [CrossRef] [PubMed]




| Antibody Type | Sensitivity | Specificity | Overall Agreement | |||||
|---|---|---|---|---|---|---|---|---|
| n/Total | % | IC95% | n/Total | % | IC95% | n/Total | % | |
| IgA # | 40/42 | 95.2 | 76.2–99.9 | 444/453 | 98.0 | 96.3–99.1 | 484/495 | 97.8 |
| IgG # | 40/42 | 95.2 | 83.8–99.4 | 453/453 | 100 | 99.2–100 | 493/495 | 99.6 |
| IgM # | 41/42 | 97.6 | 87.4–99.9 | 448/453 | 98.9 | 97.4–99.6 | 489/495 | 98.8 |
| IgA # & IgG # | 38/42 | 90.5 | NA | 440/453 | 97.2 | NA | 480/495 | 97.0 |
| IgG # & IgM # | 40/42 | 95.2 | NA | 448/453 | 98.9 | NA | 488/495 | 98.6 |
| IgA # & IgM # | 40/42 | 95.2 | NA | 440/453 | 97.2 | NA | 480/495 | 97.0 |
| IgA # & IgG # & IgM # | 39/42 | 92.9 | NA | 440/453 | 97.2 | NA | 480/495 | 97.0 |
| IgA # or IgG # | 40/42 | 95.2 | NA | 453/453 | 100 | NA | 493/495 | 99.6 |
| IgG # or IgM # | 41/42 | 97.6 | NA | 453/453 | 100 | NA | 494/495 | 99.8 |
| IgA # or IgM # | 42/42 | 100 | NA | 452/453 | 99.8 | NA | 494/495 | 99.8 |
| IgA # or IgG # or IgM # | 41/42 | 97.6 | NA | 453/453 | 100 | NA | 494/495 | 99.8 |
| IgA * | 46/62 | 74.2 | 61.5–84.5 | 435/453 | 96.0 | 93.8–97.6 | 481/515 | 93.4 |
| IgG * | 43/62 | 69.4 | 56.3–80.4 | 452/453 | 99.8 | 98.0–100 | 495/515 | 96.1 |
| IgM * | 58/62 | 93.6 | 84.3–98.2 | 444/453 | 98.0 | 96.3–99.1 | 502/515 | 97.5 |
| IgA * & IgG * | 40/62 | 64.5 | NA | 434/453 | 95.8 | NA | 474/515 | 92.0 |
| IgG * & IgM * | 42/62 | 67.7 | NA | 443/453 | 97.8 | NA | 485/515 | 95.2 |
| IgA * & IgM * | 43/62 | 69.4 | NA | 427/453 | 94.3 | NA | 470/515 | 91.3 |
| IgA * & IgG * & IgM * | 39/62 | 62.9 | NA | 426/453 | 94.0 | NA | 465/515 | 90.3 |
| IgA * or IgG * | 49/62 | 79.0 | NA | 453/453 | 100 | NA | 502/515 | 97.5 |
| IgG * or IgM * | 59/62 | 95.2 | NA | 453/453 | 100 | NA | 512/515 | 99.4 |
| IgA * or IgM * | 61/62 | 98.4 | NA | 452/453 | 99.8 | NA | 513/515 | 99.6 |
| IgA * or IgG * or IgM * | 61/62 | 98.4 | NA | 453/453 | 100 | NA | 514/515 | 99.8 |
| Pat. | n° | RLU (OD450) Values | CLIA (ELISA) Results | Agreement Positivity (IgA or IgG or IgM) | ||||
|---|---|---|---|---|---|---|---|---|
| IgA | IgG | IgM | IgA | IgG | IgM | |||
| 6 | 1 | 34000 (0.099) | 14883 (0.131) | 795717 (0.09) | N (N) | N (E) | P (N) | P (E) |
| 2 | 19610 (0.076) | 18775 (0.076) | 932321 (0.076) | N (N) | N (N) | P (N) | ||
| 3 | 14062 (0.089) | 8502 (0.103) | 1011533 (0.083) | N (N) | N (E) | P (N) | ||
| 11 | 1 | 2042 (0.136) | 15398 (0.182) | 717862 (0.073) | N (E) | N (E) | P (N) | P (E) |
| 2 | 8306 (0.089) | 18254 (0.188) | 816205 (0.088) | N (N) | N (E) | P (N) | ||
| 13 | 1 | 39269 (0.198) | 23450 (0.159) | 221901 (0.192) | N (E) | P (E) | P (E) | P (E) |
| 2 | 20987 (0.097) | 47135 (0.097) | 873343 (0.187) | N (N) | P (N) | P (E) | ||
| 3 | 33650 (0.147) | 27064 (0.129) | 415041 (0.191) | N (E) | P (E) | P (N) | ||
| 15 | 1 | 69084 (0.157) | 18366 (0.191) | 995029 (0.169) | N (E) | N (E) | P (E) | P (E) |
| 2 | 34439 (0.069) | 13626 (0.187) | 854559 (0.188) | N (N) | N (E) | P (E) | ||
| 3 | 56329 (0.11) | 12408 (0.185) | 1017070 (0.192) | N (E) | N (E) | P (E) | ||
| 18 | 1 | 34252 (0.071) | 8940 (0.091) | 64208 (0.111) | N (N) | N (N) | N (E) | N (E) |
| 2 | 26172 (0.091) | 10172 (0.188) | 78205 (0.191) | N (N) | N (E) | N (E) | ||
| 20 | 1 | 102290 (0.081) | 7684 (0.198) | 103009 (0.236) | P (N) | N (N) | P (P) | P (P) |
| 2 | 102454 (0.069) | 8749 (0.099) | 94009 (0.068) | P (N) | N (E) | P (N) | ||
| 3 | 187560 (0.146) | 11790 (0.151) | 100154 0.197) | P (E) | N (E) | P (E) | ||
| 21 | 1 | 25117 (0.198) | 3836 (0.075) | 30841 (0.062) | N (E) | N (N) | N (N) | N (E) |
| 2 | 78099 (0.084) | 15208 (0.153) | 88775 (0.074) | N (N) | N (E) | N (N) | ||
| 24 | 1 | 3365 (0.513) | 27405 (0.189) | 571206 (0.401) | N (P) | P (E) | P (P) | P (P) |
| 2 | 69084 (0.601) | 13048 (0.089) | 605542 (0.285) | N (P) | N (N) | P (P) | ||
| Total | - | - | 3(2)/20 | 4(1)/20 | 16(3)/20 | 6(2)/8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kombe Kombe, A.J.; Xie, J.; Zahid, A.; Ma, H.; Xu, G.; Deng, Y.; Nsole Biteghe, F.A.; Mohammed, A.; Dan, Z.; Yang, Y.; et al. Detection of Circulating VZV-Glycoprotein E-Specific Antibodies by Chemiluminescent Immunoassay (CLIA) for Varicella–Zoster Diagnosis. Pathogens 2022, 11, 66. https://doi.org/10.3390/pathogens11010066
Kombe Kombe AJ, Xie J, Zahid A, Ma H, Xu G, Deng Y, Nsole Biteghe FA, Mohammed A, Dan Z, Yang Y, et al. Detection of Circulating VZV-Glycoprotein E-Specific Antibodies by Chemiluminescent Immunoassay (CLIA) for Varicella–Zoster Diagnosis. Pathogens. 2022; 11(1):66. https://doi.org/10.3390/pathogens11010066
Chicago/Turabian StyleKombe Kombe, Arnaud John, Jiajia Xie, Ayesha Zahid, Huan Ma, Guangtao Xu, Yiyu Deng, Fleury Augustin Nsole Biteghe, Ahmed Mohammed, Zhao Dan, Yunru Yang, and et al. 2022. "Detection of Circulating VZV-Glycoprotein E-Specific Antibodies by Chemiluminescent Immunoassay (CLIA) for Varicella–Zoster Diagnosis" Pathogens 11, no. 1: 66. https://doi.org/10.3390/pathogens11010066
APA StyleKombe Kombe, A. J., Xie, J., Zahid, A., Ma, H., Xu, G., Deng, Y., Nsole Biteghe, F. A., Mohammed, A., Dan, Z., Yang, Y., Feng, C., Zeng, W., Chang, R., Zhu, K., Zhang, S., & Jin, T. (2022). Detection of Circulating VZV-Glycoprotein E-Specific Antibodies by Chemiluminescent Immunoassay (CLIA) for Varicella–Zoster Diagnosis. Pathogens, 11(1), 66. https://doi.org/10.3390/pathogens11010066








