Genetic Diversity of Coxiella burnetii in Iran by Multi-Spacer Sequence Typing
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction and Quantitative Polymerase Chain Reaction (qPCR)
2.3. Multi-Spacer Sequence Typing (MST)
2.4. Data Analysis
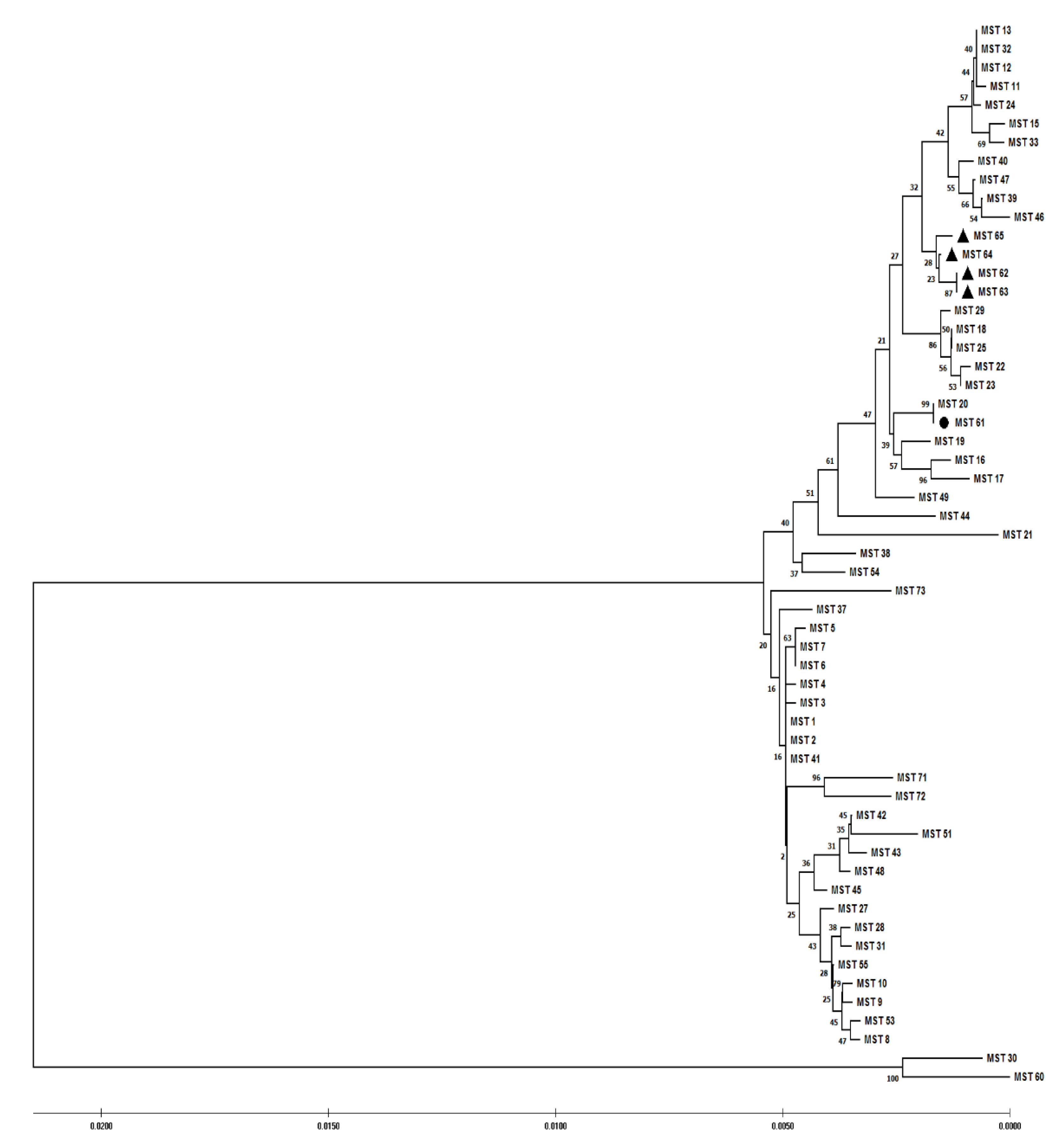
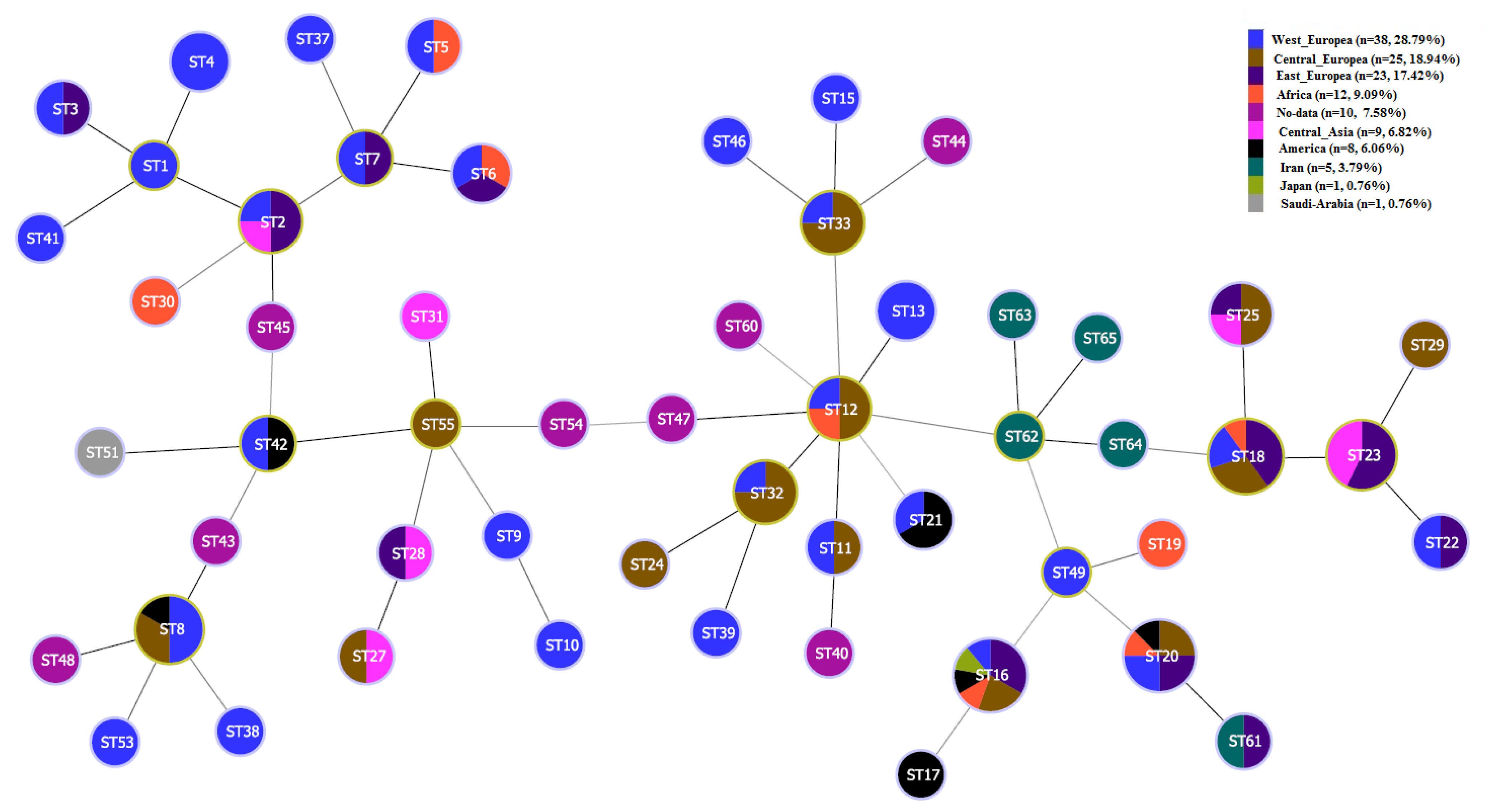
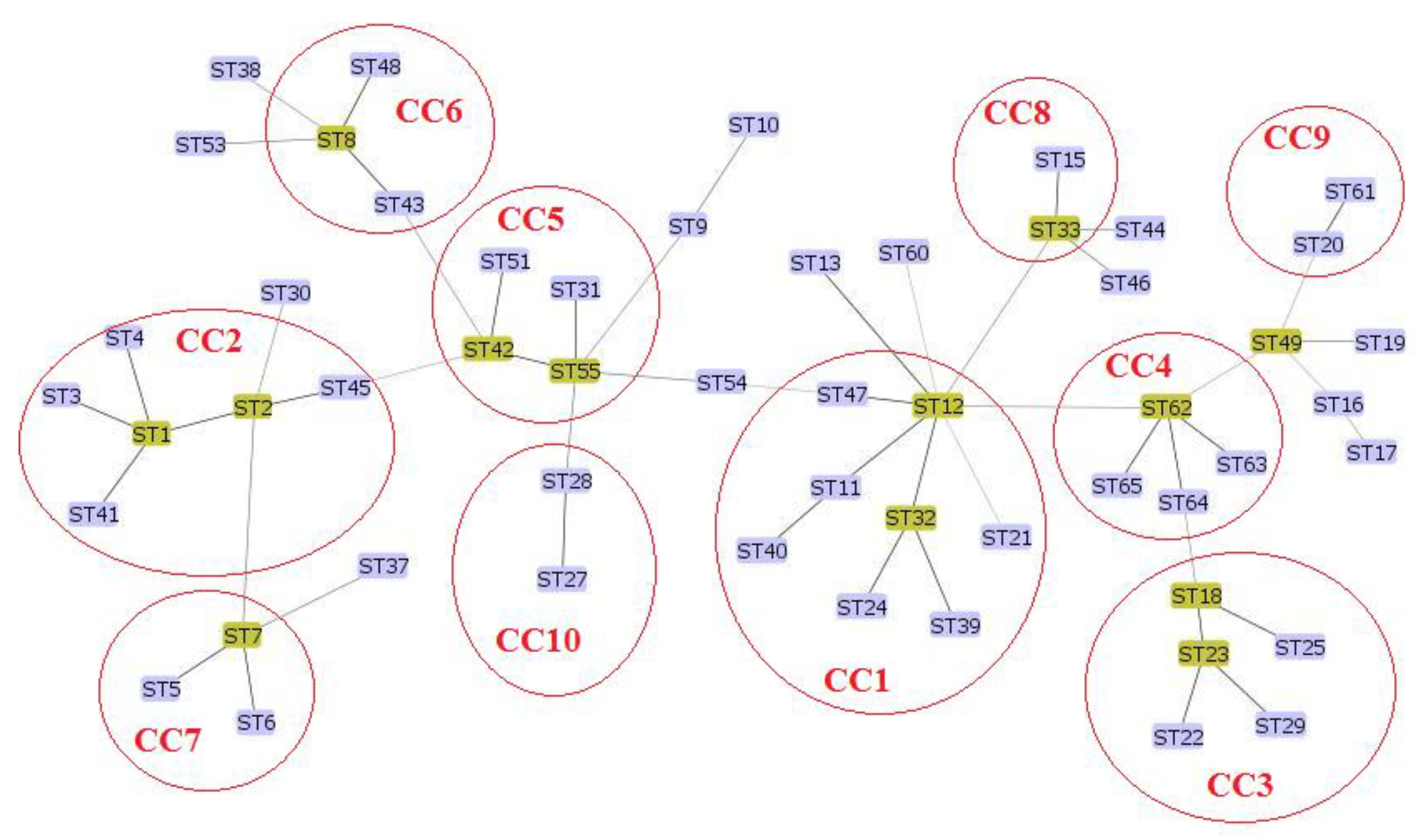
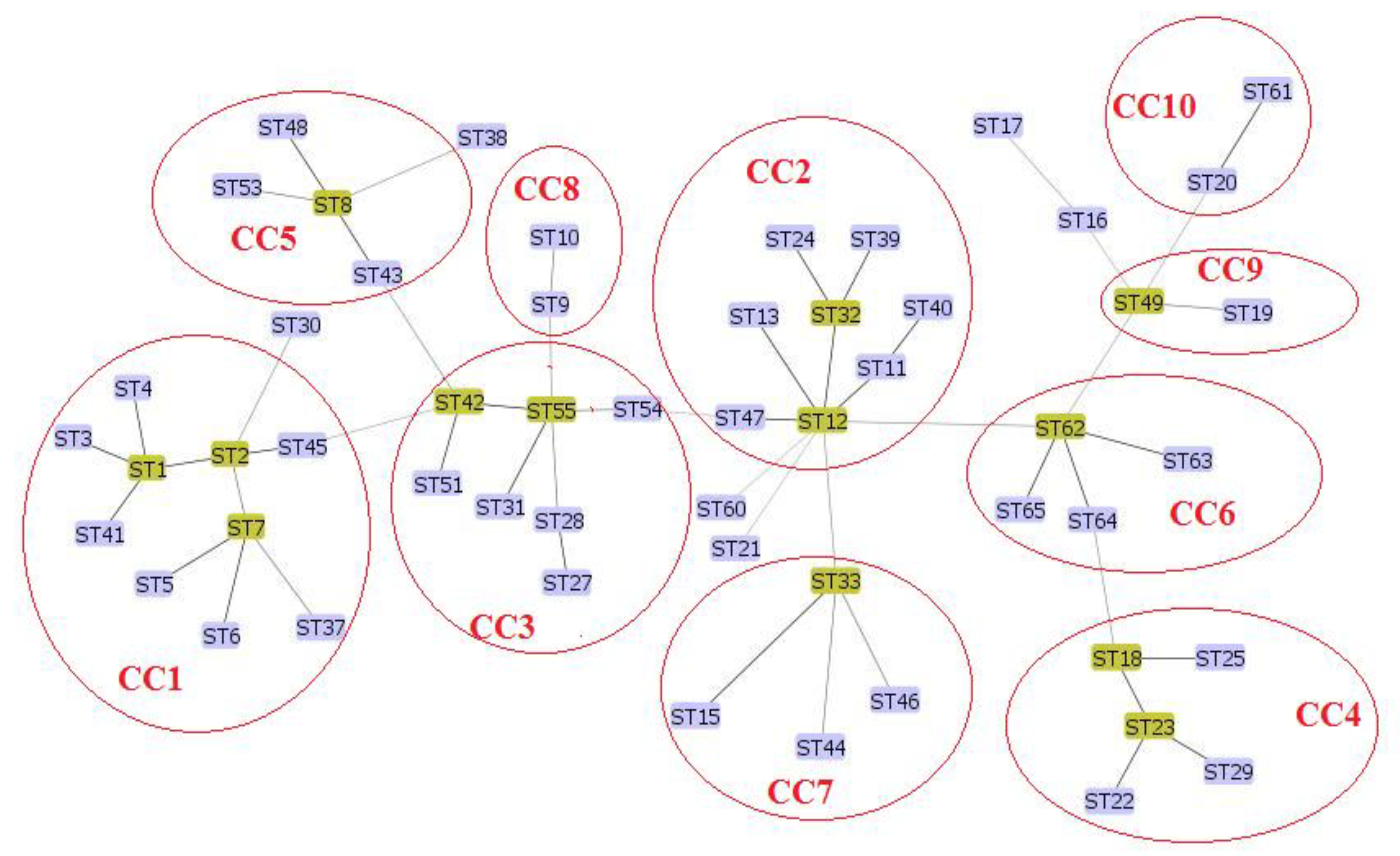
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eldin, C.; Melenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef]
- Massung, R.F.; Cutler, S.J.; Frangoulidis, D. Molecular typing of Coxiella burnetii (Q fever). In Coxiella burnetii: Recent Advances New Perspectives in Research of the Q Fever Bacterium; Toman, R., Heinzen, R., Samuel, J., Mege, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 984, pp. 381–396. [Google Scholar]
- Hemsley, C.M.; Essex-Lopresti, A.; Norville, I.H.; Titball, R.W. Correlating Genotyping Data of Coxiella burnetii with Genomic Groups. Pathogens 2021, 10, 604. [Google Scholar] [CrossRef]
- Larson, C.L.; Martinez, E.; Beare, P.A.; Jeffrey, B.; Heinzen, R.A.; Bonazzi, M. Right on Q: Genetics begin to unravel Coxiella burnetii host cell interactions. Future Microbiol. 2016, 11, 919–939. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Q fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef]
- D’Amato, F.; Eldin, C.; Raoult, D. The contribution of genomics to the study of Q fever. Future Microbiol. 2016, 11, 253–272. [Google Scholar] [CrossRef]
- Boldis, V.; Spitalska, E.; Toman, R. Molecular Typing of Coxiella burnetii: A Review of Available Methods with Major Focus on PCR-Based Techniques. In Molecular Typing in Bacterial Infections; de Filippis, I., McKee, M.L., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 457–469. [Google Scholar]
- Glazunova, O.; Roux, V.; Freylikman, O.; Sekeyova, Z.; Fournous, G.; Tyczka, J.; Tokarevich, N.; Kovacova, E.; Marrie, T.J.; Raoult, D. Coxiella burnetii genotyping. Emerg. Infect. Dis. 2005, 11, 1211–1217. [Google Scholar] [PubMed]
- Mohabbati Mobarez, A.; Bagheri Amiri, F.; Esmaeili, S. Seroprevalence of Q fever among human and animal in Iran; A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005521. [Google Scholar] [CrossRef]
- Moradnejad, P.; Esmaeili, S.; Maleki, M.; Sadeghpour, A.; Kamali, M.; Rohani, M.; Ghasemi, A.; Bagheri Amiri, F.; Pasha, H.R.; Boudagh, S. Q fever endocarditis in iran. Sci. Rep. 2019, 9, 15276. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Mohabati Mobarez, A.; Khalili, M.; Mostafavi, E.; Moradnejad, P. Molecular prevalence of Coxiella burnetii in milk in Iran: A systematic review and meta-analysis. Trop. Anim. Health Prod. 2019, 51, 1345–1355. [Google Scholar] [CrossRef]
- Rahravani, M.; Moravedji, M.; Mostafavi, E.; Mohammadi, M.; Seyfi, H.; Baseri, N.; Mozoun, M.M.; Latifian, M.; Esmaeili, S.J.B.V.R. The epidemiological survey of Coxiella burnetii in small ruminants and their ticks in western Iran. BMC Vet. Res. 2022, 18, 292. [Google Scholar] [CrossRef]
- Mohabati Mobarez, A.; Khalili, M.; Mostafavi, E.; Esmaeili, S. Molecular detection of Coxiella burnetii infection in aborted samples of domestic ruminants in Iran. PLoS ONE 2021, 16, e0250116. [Google Scholar] [CrossRef]
- Esmaeili, S.; Mobarez, A.M.; Khalili, M.; Mostafavi, E. High prevalence and risk factors of Coxiella burnetii in milk of dairy animals with a history of abortion in Iran. Comp. Immunol. Microbiol. Infect. Dis. 2019, 63, 127–130. [Google Scholar] [CrossRef]
- Mobarez, A.M.; Mostafavi, E.; Khalili, M.; Esmaeili, S. Identification of Coxiella burnetii in raw milk of livestock animal in Iran. Int. J. Microbiol. 2021, 2021, 6632036. [Google Scholar] [CrossRef]
- Klee, S.R.; Tyczka, J.; Ellerbrok, H.; Franz, T.; Linke, S.; Baljer, G.; Appel, B. Highly sensitive real-time PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiol. 2006, 6, 2. [Google Scholar] [CrossRef]
- De Bruin, A.; Van der Plaats, R.; De Heer, L.; Paauwe, R.; Schimmer, B.; Vellema, P.; Van Rotterdam, B.; Van Duynhoven, Y.J.A. Detection of Coxiella burnetii DNA on small-ruminant farms during a Q fever outbreak in the Netherlands. Appl. Environ. Microbiol. 2012, 78, 1652–1657. [Google Scholar] [CrossRef]
- Mioni, M.d.S.R.; Sidi-Boumedine, K.; Morales Dalanezi, F.; Fernandes Joaquim, S.; Denadai, R.; Reis Teixeira, W.S.; Bahia Labruna, M.; Megid, J.J.P. New genotypes of Coxiella burnetii circulating in Brazil and Argentina. Pathogens 2019, 9, 30. [Google Scholar] [CrossRef]
- Tomaiuolo, S.; Boarbi, S.; Fancello, T.; Michel, P.; Desqueper, D.; Grégoire, F.; Callens, J.; Fretin, D.; Devriendt, B.; Cox, E.J.F.i.c.; et al. Phylogeography of human and animal Coxiella burnetii strains: Genetic fingerprinting of Q fever in Belgium. Front. Cell. Infect. Microbiol. 2021, 10, 625576. [Google Scholar] [CrossRef]
- Szymańska-Czerwińska, M.; Jodełko, A.; Zaręba-Marchewka, K.; Niemczuk, K.J.P.o. Shedding and genetic diversity of Coxiella burnetii in Polish dairy cattle. PLoS ONE 2019, 14, e0210244. [Google Scholar] [CrossRef]
- Olivas, S.; Hornstra, H.; Priestley, R.; Kaufman, E.; Hepp, C.; Sonderegger, D.; Handady, K.; Massung, R.; Keim, P.; Kersh, G. Massive dispersal of Coxiella burnetii among cattle across the United States. Microb. Genom. 2016, 2, e000068. [Google Scholar] [CrossRef]
- Menadi, S.E.; Chisu, V.; Santucciu, C.; Di Domenico, M.; Curini, V.; Masala, G.J.V.S. Serological, Molecular Prevalence and Genotyping of Coxiella burnetii in Dairy Cattle Herds in Northeastern Algeria. Vet. Sci. 2022, 9, 40. [Google Scholar] [CrossRef]
- Galiero, A.; Fratini, F.; Cammà, C.; Di Domenico, M.; Curini, V.; Baronti, I.; Turchi, B.; Cerri, D.J.I.j.o.f.m. Occurrence of Coxiella burnetii in goat and ewe unpasteurized cheeses: Screening and genotyping. Int. J. Food Microbiol. 2016, 237, 47–54. [Google Scholar] [CrossRef]
- Chochlakis, D.; Santos, A.S.; Giadinis, N.D.; Papadopoulos, D.; Boubaris, L.; Kalaitzakis, E.; Psaroulaki, A.; Kritas, S.K.; Petridou, E.I.J.B.m. Genotyping of Coxiella burnetii in sheep and goat abortion samples. BMC Microbiol. 2018, 18, 204. [Google Scholar] [CrossRef]
- Di Domenico, M.; Curini, V.; Di Lollo, V.; Massimini, M.; Di Gialleonardo, L.; Franco, A.; Caprioli, A.; Battisti, A.; Cammà, C.J.B.v.r. Genetic diversity of Coxiella burnetii in domestic ruminants in central Italy. BMC Vet. Res. 2018, 14, 171. [Google Scholar] [CrossRef]
- Hemsley, C.M.; O’Neill, P.A.; Essex-Lopresti, A.; Norville, I.H.; Atkins, T.P.; Titball, R.W.J.B.g. Extensive genome analysis of Coxiella burnetii reveals limited evolution within genomic groups. BMC Genom. 2019, 20, 441. [Google Scholar] [CrossRef]
- Hornstra, H.M.; Priestley, R.A.; Georgia, S.M.; Kachur, S.; Birdsell, D.N.; Hilsabeck, R.; Gates, L.T.; Samuel, J.E.; Heinzen, R.A.; Kersh, G.J.J.P.o. Rapid typing of Coxiella burnetii. PLoS ONE 2011, 6, e26201. [Google Scholar] [CrossRef]
- Sulyok, K.M.; Kreizinger, Z.; Hornstra, H.M.; Pearson, T.; Szigeti, A.; Dán, Á.; Balla, E.; Keim, P.S.; Gyuranecz, M.J.B.v.r. Genotyping of Coxiella burnetii from domestic ruminants and human in Hungary: Indication of various genotypes. BMC Vet. Res. 2014, 10, 107. [Google Scholar] [CrossRef]
- D’Amato, F.; Robert, C.; Azhar, E.; Fournier, P.-E.; Raoult, D.J.G.A. Draft genome sequence of Coxiella burnetii strain Cb196, an agent of endocarditis in Saudi Arabia. Genome Announc. 2014, 2, e01180-14. [Google Scholar] [CrossRef]
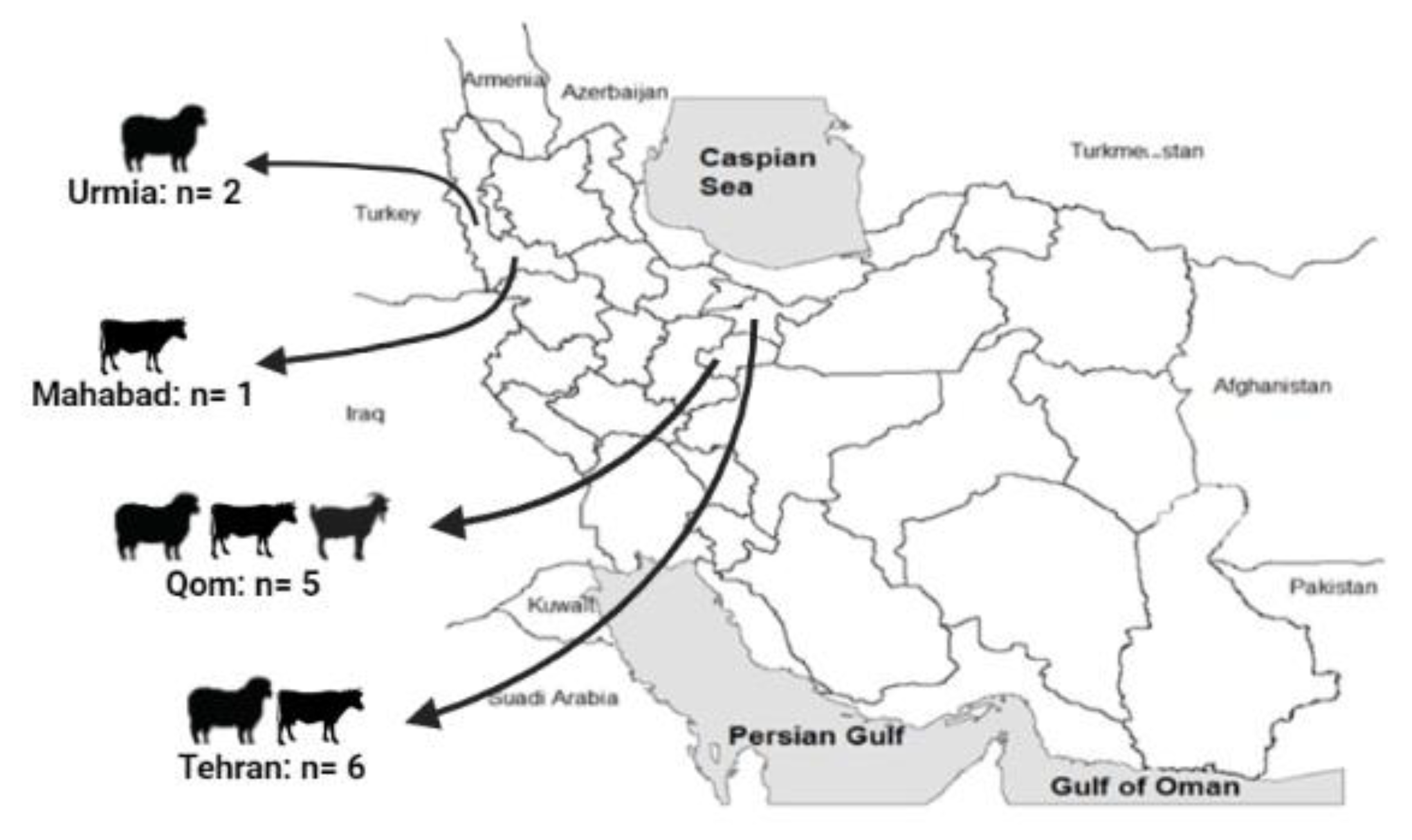
| Sample ID | Host | Source | Region (County) | Cq * |
|---|---|---|---|---|
| MB14 | Cattle | Milk | Qom | 27.32 |
| MB47 | Cattle | Milk | Qom | 25.24 |
| MB92 | Cattle | Milk | Tehran | 29.61 |
| MB98 | Cattle | Milk | Tehran | 27.25 |
| AC3 | Cattle | Aborted Fetus Spleen | Tehran | 29.02 |
| AC11 | Cattle | Aborted Fetus Spleen | Tehran | 27.82 |
| MG101 | Goat | Milk | Qom | 11.6 |
| AG1 | Goat | Aborted Fetal Fluids | Tehran | 17.98 |
| AG4 | Goat | Aborted Fetal Fluids | Mahabad | 31.07 |
| MS45 | Sheep | Milk | Qom | 25.01 |
| MS51 | Sheep | Milk | Qom | 25.46 |
| AS3 | Sheep | Aborted Fetal Fluids | Tehran | 25.5 |
| AS25 | Sheep | Aborted Fetus Spleen | Urmia | 25.91 |
| AS26 | Sheep | Aborted Fetus Spleen | Urmia | 26.13 |
| Spacer | Forward Primer (5´–3´) | Reverse Primer (5´–3´) |
|---|---|---|
| Cox2 | CAACCCTGAATACCCAAGGA | GAAGCTTCTGATAGGCGGGA |
| Cox5 | CAGGAGCAAGCTTGAATGCG | TGGTATGACAACCCGTCATG |
| Cox18 | CGCAGACGAATTAGCCAATC | TTCGATGATCCGATGGCCTT |
| Cox20 | GATATTTATCAGCGTCAAAGCAA | TCTATTATTGCAATGCAAGTGG |
| Cox22 | GGGAATAAGAGAGTTAGCTCA | CGCAAATTTCGGCACAGACC |
| Cox37 | GGCTTGTCTGGTGTAACTGT | ATTCCGGGACCTTCGTTAAC |
| Cox51 | TAACGCCCGAGAGCTCAGAA | GCGAGAACCGAATTGCTATC |
| Cox56 | CAAGCTCTCTGTGCCCAAT | ATGCGCCAGAAACGCATAGG |
| Cox57 | TGGAAATGGAAGGCGGATTC | GGTGGAAGGCGTAAGCCTTT |
| Cox61 | GAAGATAGAGCGGCAAGGAT | GGGATTTCAACTTCCGATAGA |
| Sample ID | Sample Type | Cox 2 | Cox 5 | Cox 18 | Cox 20 | Cox 22 | Cox 37 | Cox 51 | Cox 56 | Cox 57 | Cox 61 | MST Genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB92 | Cattle Milk | 3 | 2 | 6 | 1 | 5 | 10 | 4 | 10 | 6 | 5 | 61 |
| MB98 | Cattle Milk | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 4 | 6 | 11 | 62 |
| AC3 | Cattle Abortion | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 4 | 6 | 11 | 62 |
| MG101 | Goat Milk | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 4 | 6 | 11 | 62 |
| AG4 | Goat Abortion | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 4 | 6 | 11 | 62 |
| MS45 | Sheep Milk | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 4 | 6 | 11 | 62 |
| MS51 | Sheep Milk | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 4 | 6 | 11 | 62 |
| AG1 | Goat Abortion | 3 | 11 * | 1 | 6 | 5 | 4 | 5 | 4 | 6 | 11 | 63 |
| AS3 | Sheep Abortion | 3 | 11 * | 1 | 6 | 5 | 4 | 5 | 4 | 6 | 11 | 63 |
| MB47 | Cattle Milk | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 9 | 6 | 11 | 64 |
| AC11 | Cattle Abortion | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 9 | 6 | 11 | 64 |
| AS25 | Sheep Abortion | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 9 | 6 | 11 | 64 |
| AS26 | Sheep Abortion | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 9 | 6 | 11 | 64 |
| MB14 | Cattle Milk | 3 | 8 | 1 | 6 | 5 | 4 | 5 | 15 ¥ | 6 | 11 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohabati Mobarez, A.; Baseri, N.; Khalili, M.; Mostafavi, E.; Stenos, J.; Esmaeili, S. Genetic Diversity of Coxiella burnetii in Iran by Multi-Spacer Sequence Typing. Pathogens 2022, 11, 1175. https://doi.org/10.3390/pathogens11101175
Mohabati Mobarez A, Baseri N, Khalili M, Mostafavi E, Stenos J, Esmaeili S. Genetic Diversity of Coxiella burnetii in Iran by Multi-Spacer Sequence Typing. Pathogens. 2022; 11(10):1175. https://doi.org/10.3390/pathogens11101175
Chicago/Turabian StyleMohabati Mobarez, Ashraf, Neda Baseri, Mohammad Khalili, Ehsan Mostafavi, John Stenos, and Saber Esmaeili. 2022. "Genetic Diversity of Coxiella burnetii in Iran by Multi-Spacer Sequence Typing" Pathogens 11, no. 10: 1175. https://doi.org/10.3390/pathogens11101175
APA StyleMohabati Mobarez, A., Baseri, N., Khalili, M., Mostafavi, E., Stenos, J., & Esmaeili, S. (2022). Genetic Diversity of Coxiella burnetii in Iran by Multi-Spacer Sequence Typing. Pathogens, 11(10), 1175. https://doi.org/10.3390/pathogens11101175






