Detection of Theileria orientalis Genotypes from Cattle in Kyrgyzstan
Abstract
:1. Introduction
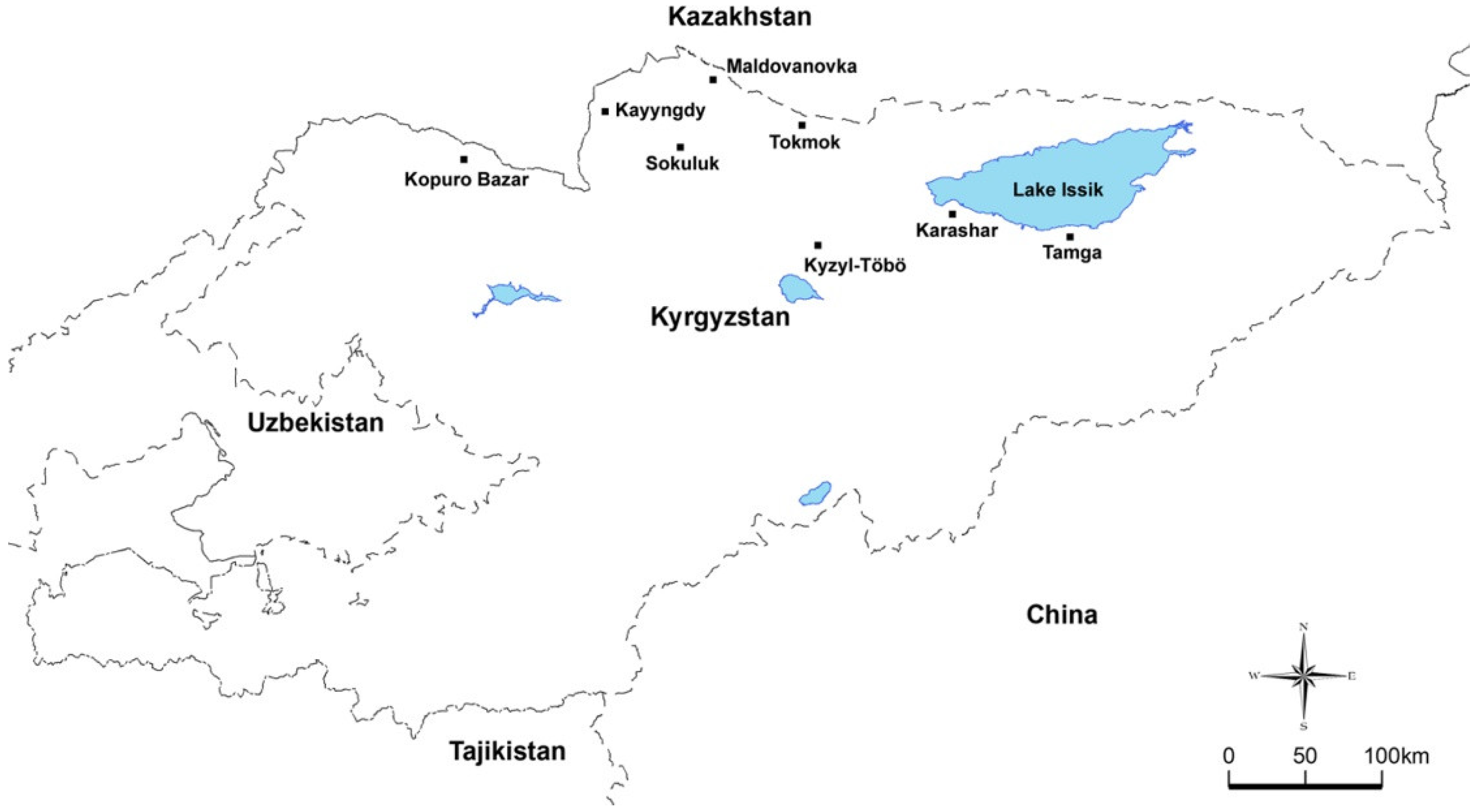
2. Materials and Methods
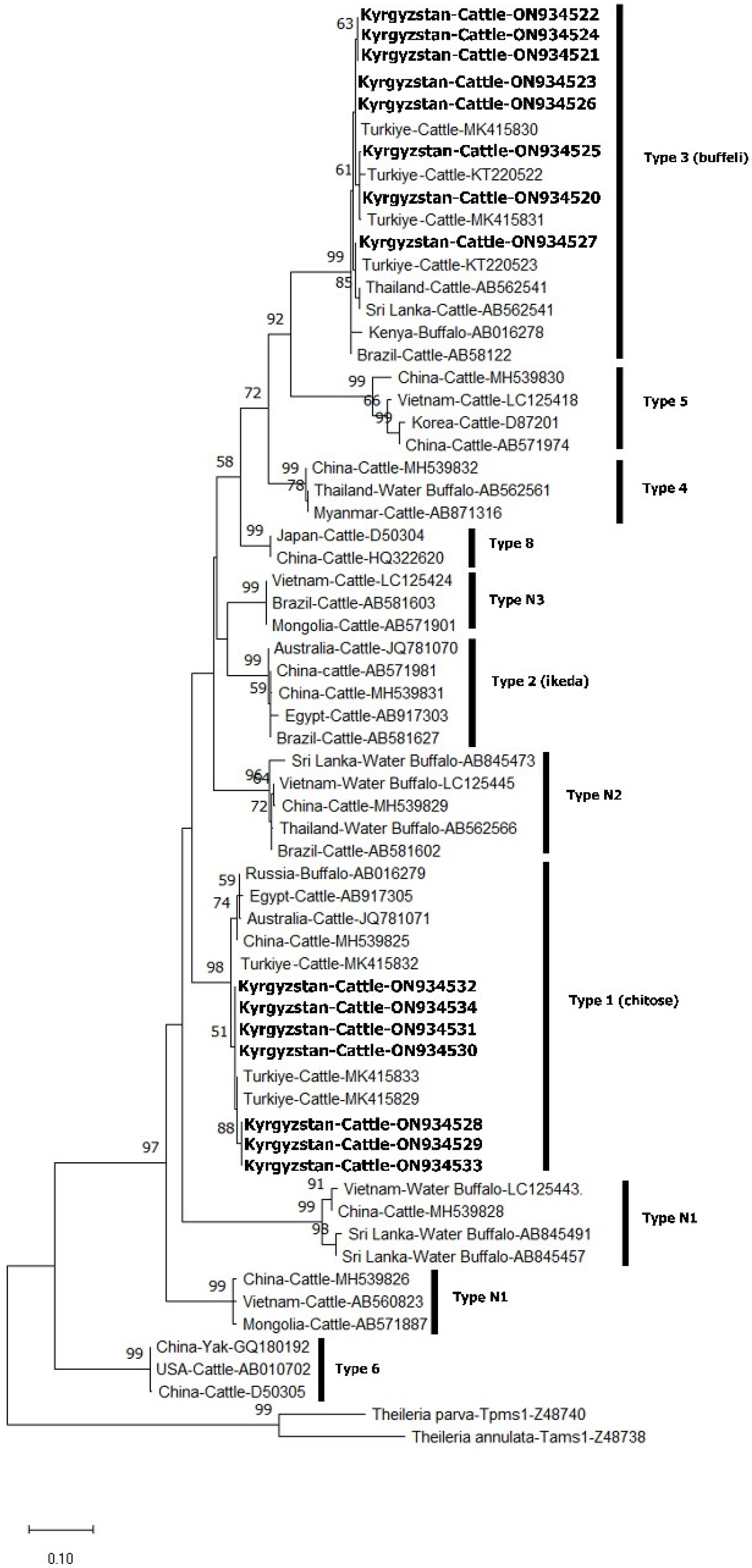
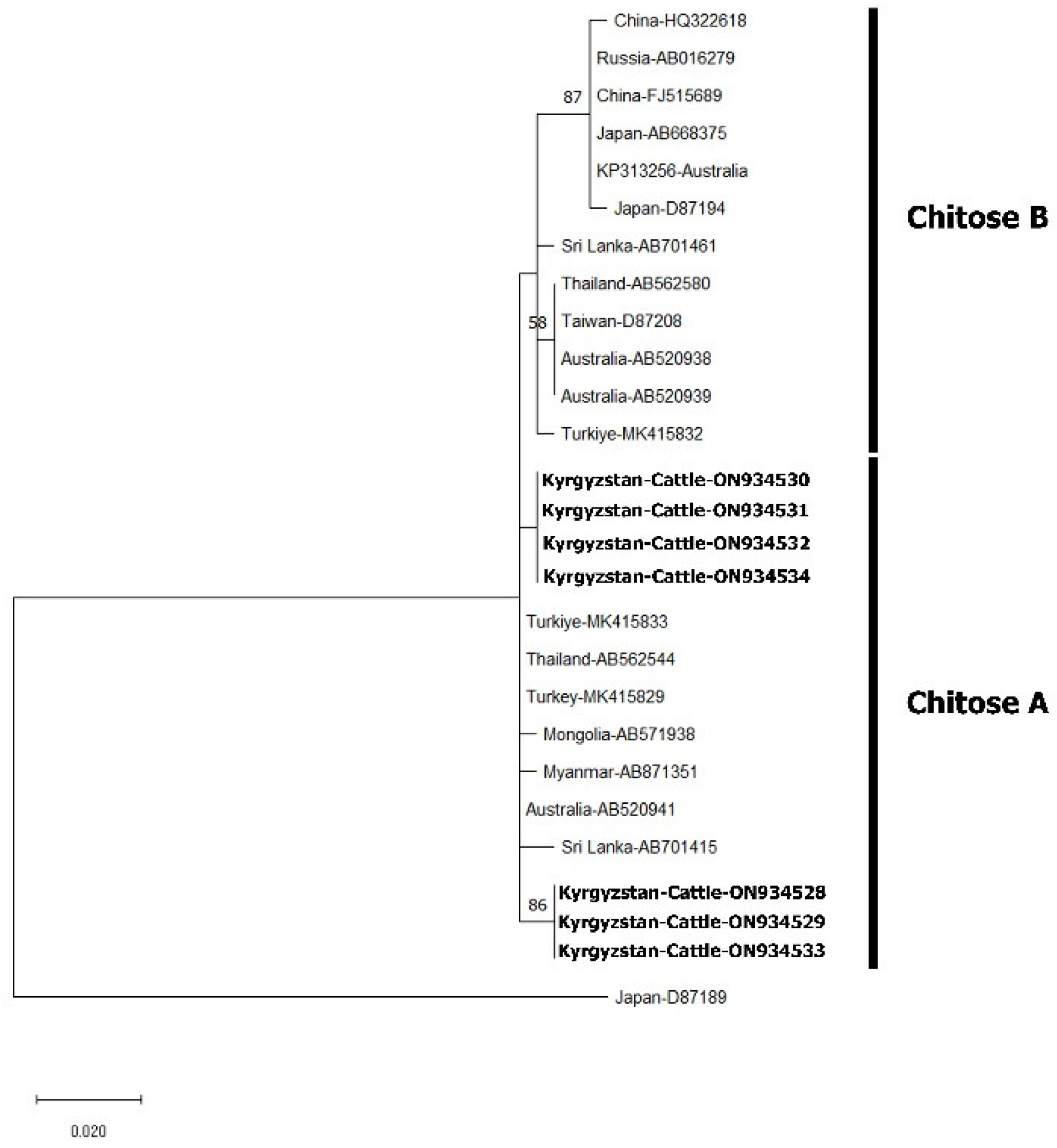
PCR Amplification, Single-Strand Conformation Polymorphism (SSCP) and Phylogenetic Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aktas, M.; Altay, K.; Dumanli, N. A molecular survey of bovine Theileria parasites among apparently healthy cattle and with a note on the distribution of ticks in eastern Turkey. Vet. Parasitol. 2006, 138, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Hayashida, K.; Sugimoto, C.; Yokoyama, N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014, 27, 250–263. [Google Scholar] [PubMed] [Green Version]
- Uilenberg, G.; Perie, N.; Spanjer, A.; Franssen, F. Theileria orientalis, a cosmopolitan blood parasite of cattle: Demonstration of the schizont stage. Vet. Sci. Res. J. 1985, 38, 352–360. [Google Scholar] [CrossRef]
- Uilenberg, G. Theileria sergenti . Vet. Parasitol. 2011, 175, 386. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.; Perry, C.; Brühlmann, F.; Olias, P. Theileria’s strategies and effector mechanisms for host cell transformation: From invasion to immortalization. Front. Cell Dev. Biol. 2021, 9, 662805. [Google Scholar] [CrossRef] [PubMed]
- Eamens, G.; Bailey, G.; Gonsalves, J.; Jenkins, C. Distribution and temporal prevalence of Theileria orientalis major piroplasm surface protein types in eastern Australian cattle herds. Aust. Vet. J. 2013, 91, 332–340. [Google Scholar] [CrossRef]
- Riek, R. Epidemiology and transmission of Theileria sp. of cattle in Australia. Aust. Vet. J. 1982, 59, 89–92. [Google Scholar] [CrossRef]
- Stewart, N.; De Vos, A.; Shiels, I.; McGregor, W. The experimental transmission of Theileria buffeli of cattle in Australia by Haemaphysalis humerosa. Aust. Vet. J. 1987, 64, 81–83. [Google Scholar] [CrossRef]
- Lakew, B.T.; Kheravii, S.K.; Wu, S.-B.; Eastwood, S.; Andrew, N.R.; Nicholas, A.H.; Walkden-Brown, S.W. Detection and distribution of haematophagous flies and lice on cattle farms and potential role in the transmission of Theileria orientalis. Vet. Parasitol. 2021, 298, 109516. [Google Scholar] [CrossRef]
- Lawrence, K.; Gedye, K.; Hickson, R.; Wang, B.; Carvalho, L.; Zhao, Y.; Pomroy, W. The role of sheep (Ovis aries) in maintaining Theileria orientalis Ikeda type infection. Vet. Parasitol. 2021, 291, 109391. [Google Scholar] [CrossRef]
- Aktas, M.; Kısadere, I.; Ozubek, S.; Cihan, H.; Salıkov, R.; Cirak, V.Y. First molecular survey of piroplasm species in cattle from Kyrgyzstan. Parasitol. Res. 2019, 118, 2431–2435. [Google Scholar] [CrossRef]
- Ota, N.; Mizuno, D.; Kuboki, N.; Igarashi, I.; Nakamura, Y.; Yamashina, H.; Hanzaike, T.; Fujii, K.; Onoe, S.; Hata, H. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan. J. Vet. Med. Sci. 2009, 71, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Cufos, N.; Jabbar, A.; de Carvalho, L.M.; Gasser, R.B. Mutation scanning-based analysis of Theileria orientalis populations in cattle following an outbreak. Electrophoresis 2012, 33, 2036–2040. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Kubota, S.; Sugimoto, C.; Kakuda, T.; Onuma, M. Analysis of immunodominant piroplasm surface antigen alleles in mixed populations of Theileria sergenti and T. buffeli. Int. J. Parasitol. 1996, 26, 741–747. [Google Scholar] [CrossRef]
- Matsuba, T.; Kubota, H.; Tanaka, M.; Hattori, M.; Murata, M.; Sugimoto, C.; Onuma, M. Analysis of mixed parasite populations of Theileria sergenti using cDNA probes encoding a major piroplasm surface protein. Parasitology 1993, 107, 369–377. [Google Scholar] [CrossRef]
- Kawazu, S.-i.; Kamio, T.; Kakuda, T.; Terada, Y.; Sugimoto, C.; Fujisaki, K. Phylogenetic relationships of the benign Theileria species in cattle and Asian buffalo based on the major piroplasm surface protein (p33/34) gene sequences. Int. J. Parasitol. 1999, 29, 613–618. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+ C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Zhumanova, M.; Maharjan, K.L. Trends in livestock population and composition through derived productivity in Kyrgyzstan: A case study in Ala-Buka District. J. Int. Dev. Coop. 2012, 18, 159–176. [Google Scholar]
- Karadag, H. Bağımsızlık Sonrası Kırgızıstan’da Hayvancılıkta Gelişmeler. YYUSBED 2019, 45, 151–171. [Google Scholar]
- Wikel, S.K. Ticks and tick-borne infections: Complex ecology, agents, and host interactions. Vet. Sci. 2018, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Schnittger, L.; Ganzinelli, S.; Bhoora, R.; Omondi, D.; Nijhof, A.M.; Florin-Christensen, M. The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 2022, 121, 1207–1245. [Google Scholar] [PubMed]
- Altay, K.; Erol, U.; Sahin, O.F.; Aytmirzakizi, A. First molecular detection of Anaplasma species in cattle from Kyrgyzstan; molecular identification of human pathogenic novel genotype Anaplasma capra and Anaplasma phagocytophilum related strain. Ticks Tick-Borne Dis. 2022, 13, 101861. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Z.; Liu, A.; Wang, J.; Guan, G.; Yin, H.; Luo, J. Identification and isolation of pathogenic Theileria orientalis Ikeda genotype from confined dairy cattle, in Hebei, China. Parasitol. Res. 2022, 121, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Gralén, B. Tick-Borne Diseases in Tajikistan; SLU Publication Database: St. Louis, MI, USA, 2009. [Google Scholar]
- Shkap, V.; Rasulov, I.; Abdurasulov, S.; Fish, L.; Leibovitz, B.; Krigel, Y.; Molad, T.; Mazuz, M.; Savitsky, I. Babesia bigemina: Attenuation of an Uzbek isolate for immunization of cattle with live calf-or culture-derived parasites. Vet. Parasitol. 2007, 146, 221–226. [Google Scholar] [CrossRef]
- Rasulov, I.; Fish, L.; Shkap, V. Vaccination of cattle against tropical theileriosis in Uzbekistan using autochthonous live vaccine. Vaccine 2008, 26, G14–G16. [Google Scholar] [CrossRef] [PubMed]
- Kamau, J.; de Vos, A.J.; Playford, M.; Salim, B.; Kinyanjui, P.; Sugimoto, C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasites Vectors 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFadden, A.; Rawdon, T.; Meyer, J.; Makin, J.; Morley, C.; Clough, R.; Tham, K.; Müllner, P.; Geysen, D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N. Z. Vet. J. 2011, 59, 79–85. [Google Scholar] [CrossRef]
- Oakes, V.J.; Yabsley, M.J.; Schwartz, D.; LeRoith, T.; Bissett, C.; Broaddus, C.; Schlater, J.L.; Todd, S.M.; Boes, K.M.; Brookhart, M. Theileria orientalis Ikeda genotype in cattle, Virginia, USA. Emerg. Infect. Dis. 2019, 25, 1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, J.; Playford, M.; Hickey, K. Theileria orientalis: A review. N. Z. Vet. J. 2016, 64, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.; de Burgh, S.; Dinh, T.H.H.H.; Rolls, P.; Carter, P. Merozoites of Theileria orientalis buffeli reduce the parasitaemia of T. orientalis ikeda following tick challenge. Vet. Parasitol. 2021, 298, 109532. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Micallef, M.; Alex, S.; Collins, D.; Djordjevic, S.; Bogema, D. Temporal dynamics and subpopulation analysis of Theileria orientalis genotypes in cattle. Infect. Genet. Evol. 2015, 32, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.L. Approaches to Integrated Parasite Management (IPM) for Theileria orientalis with an Emphasis on Immunity. Pathogens 2021, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Gedye, K.; McFadden, A.; Pulford, D.; Heath, A.; Pomroy, W. Re-view of the New Zealand Theileria orientalis Ikeda Type Epidemic and Epidemiologi-cal Research since 2012. Pathogens 2021, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, S.Z. Ixodidae ticks in Bishkek. Meditsinskaia Parazitol. Parazit. Bolezn. 2005, 4, 34–37. [Google Scholar]
| Primer Name | Primer Sequence 5′-3′ | PCR Condition | References |
|---|---|---|---|
| MPSP-F | CTTTGCCTAGGATACTTCCT | 95 °C, 3 min; 95 °C, 30 s; 58 °C, 30 s; and 72 °C, 30 s (35 cycles); final extension of 72 °C, 5 min. | [12] |
| MPSP-R | ACGGCAAGTGGTGAGAACT | ||
| MPSP-AJ-F | TTCACTCCAACAGTCGCCCACA | 95 °C, 3 min; 95 °C, 30 s; 60 °C, 30 s; and 72 °C, 30 s (35 cycles); final extension of 72 °C, 5 min. | [13] |
| MPSP-AJ-R1 | ACGTAAACTTTGACTGCGGTG | ||
| TSB | CACCTTCCTCATCGTCTCTGCAACT | 95 °C, 3 min; 95 °C, 30 s; 55 °C, 30 s; and 72 °C, 30 s (35 cycles); final extension of 72 °C, 5 min. | [15] |
| TSR | CACCTGCTCTGCAACCGCAGAG | ||
| TSC | CACCTTCCTCATCGTCTCTGCAACT | 95 °C, 3 min; 95 °C, 30 s; 55 °C, 30 s; and 72 °C, 30 s (35 cycles); final extension of 72 °C, 5 min. | [16] |
| TSR | CACCTGCTCTGCAACCGCAGAG | ||
| TSI | CACCATCGTCTGCTACCGCCGC | 95 °C, 3 min; 95 °C, 30 s; 55 °C, 30 s; and 72 °C, 30 s (35 cycles); final extension of 72 °C, 5 min. | [17] |
| TSR | CACCTGCTCTGCAACCGCAGAG |
| Number of T. orientalis Genotypes | |||||
|---|---|---|---|---|---|
| Province | No. of Positive Samples | Type 1 (Chitose) | Type2 (Ikeda) | Type 3 (Buffeli) | Type 1 + Type 3 |
| Tokmok | 13 | 1 | - | 2 | 10 |
| Sokuluk | 22 | 1 | - | 7 | 14 |
| Karashar | 3 | - | - | - | 3 |
| Kyzyl-Töbö | 3 | - | - | 1 | 2 |
| Kopuro Bazar | 19 | - | - | 5 | 14 |
| Tamga | 2 | - | - | - | 2 |
| Kayyngdy | 27 | - | - | 8 | 19 |
| Maldovanovka | 60 | 1 | - | 16 | 43 |
| Total | 149 | 3 (2%) | - | 39 (26.2%) | 107 (71.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozubek, S.; Ulucesme, M.C.; Cirak, V.Y.; Aktas, M. Detection of Theileria orientalis Genotypes from Cattle in Kyrgyzstan. Pathogens 2022, 11, 1185. https://doi.org/10.3390/pathogens11101185
Ozubek S, Ulucesme MC, Cirak VY, Aktas M. Detection of Theileria orientalis Genotypes from Cattle in Kyrgyzstan. Pathogens. 2022; 11(10):1185. https://doi.org/10.3390/pathogens11101185
Chicago/Turabian StyleOzubek, Sezayi, Mehmet Can Ulucesme, Veli Yılgor Cirak, and Munir Aktas. 2022. "Detection of Theileria orientalis Genotypes from Cattle in Kyrgyzstan" Pathogens 11, no. 10: 1185. https://doi.org/10.3390/pathogens11101185






