The Promotional Effect of GW4869 on C. albicans Invasion and Cellular Damage in a Murine Model of Oral Candidiasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Fungal Cells
2.2. Animals
2.3. Oral Candidiasis Model and GW4869 Treatment
2.4. Macroscopic Scoring of Candidiasis Lesions
2.5. Histopathologic Examination and Periodic Acid Solution and Schiff Staining
2.6. Scanning Electron Microscopy
2.7. Transmission Electron Microscopy
2.8. Fluorescence
2.9. Statistics
3. Results
3.1. GW4869 Promoted Oral Candidiasis and Biofilm Formation
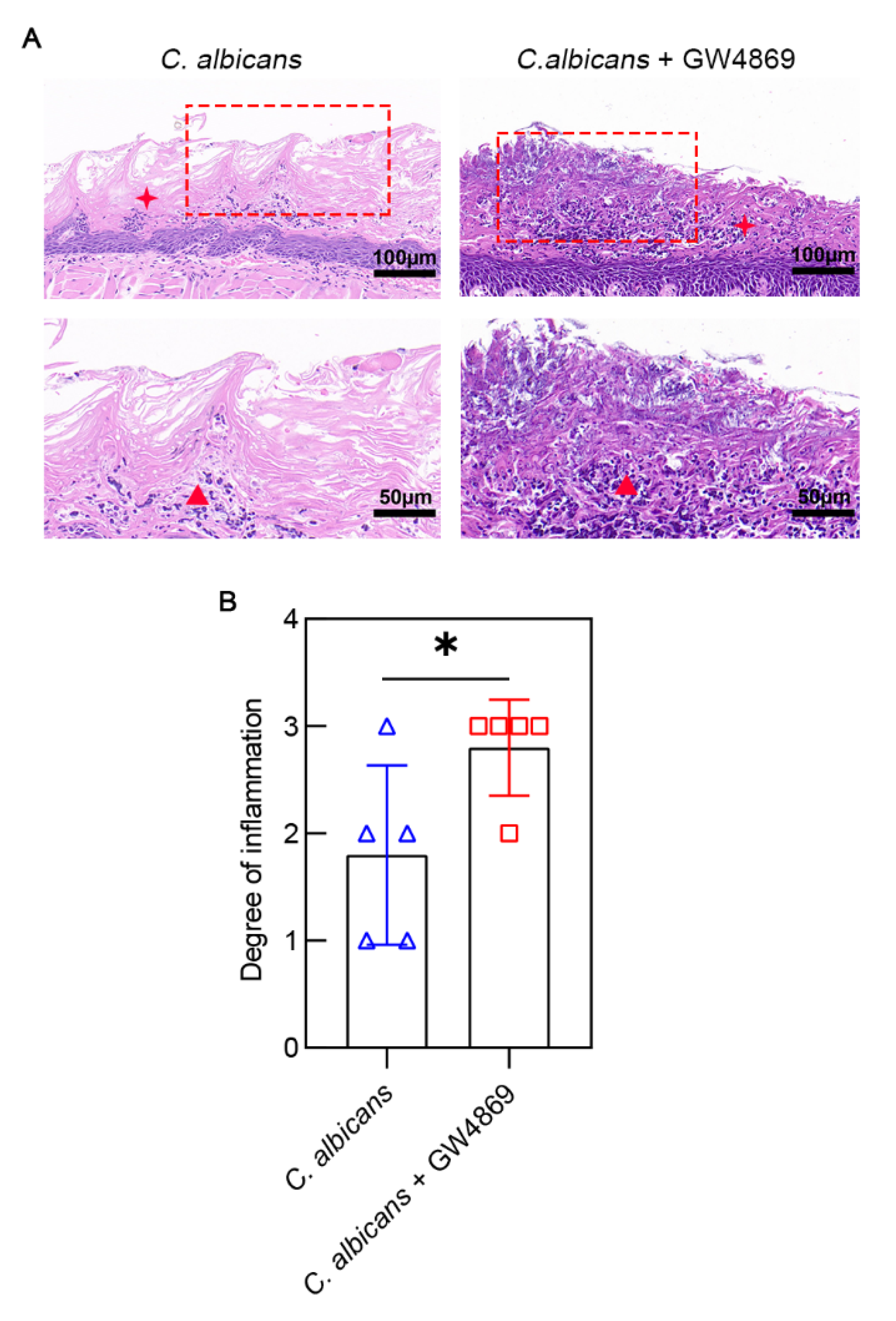
3.2. GW4869 Aggravated C. albicans Infection-Induced Mucosal Inflammation
3.3. GW4869 Increased the Invasion of C. albicans into Mucosal Epithelium
3.4. GW4869 Facilitated C. albicans Growth and Bacterial Biofilm Formation
3.5. GW4869 Exacerbated C. albicans Infection-Induced Ultrastructural Damages of Oral Mucosal Epithelial Cells
3.6. GW4869 Stabilized the Cell Wall Structure of C. albicans
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iliev, I.D.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef]
- Gunsalus, K.T.; Tornberg-Belanger, S.N.; Matthan, N.R.; Lichtenstein, A.H.; Kumamoto, C.A. Manipulation of Host Diet To Reduce Gastrointestinal Colonization by the Opportunistic Pathogen Candida albicans. mSphere 2016, 1, e00020-15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Hu, Y.; Li, R.; Li, T. Single-cell atlas of murine adrenal glands reveals immune-adrenal crosstalk during systemic Candida albicans infection. Front. Immunol. 2022, 13, 966814. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sun, L.; Lu, C.; Gong, Y.; Li, M.; Sun, S. Promising Antifungal Targets Against Candida albicans Based on Ion Homeostasis. Front. Cell. Infect. Microbiol. 2018, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [Green Version]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef]
- Sitrin, R.G.; Sassanella, T.M.; Petty, H.R. An obligate role for membrane-associated neutral sphingomyelinase activity in orienting chemotactic migration of human neutrophils. Am. J. Respir. Cell Mol. Biol. 2011, 44, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Gon, Y.; Maruoka, S.; Inoue, T.; Kuroda, K.; Yamagishi, K.; Kozu, Y.; Shikano, S.; Soda, K.; Lötvall, J.; Hashimoto, S. Selective release of miRNAs via extracellular vesicles is associated with house-dust mite allergen-induced airway inflammation. Clin. Exp. Allergy 2017, 47, 1586–1598. [Google Scholar] [CrossRef]
- Yang, S.W.; Lee, Y.S.; Chang, L.C.; Yang, C.H.; Luo, C.M.; Wu, P.W. Oral tongue leukoplakia: Analysis of clinicopathological characteristics, treatment outcomes, and factors related to recurrence and malignant transformation. Clin. Oral Investig. 2021, 25, 4045–4058. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wang, X.; Ge, S.; Chen, W.; Wang, W.; Han, X. Long-term cigarette smoking suppresses NLRP3 inflammasome activation in oral mucosal epithelium and attenuates host defense against Candida albicans in a rat model. Biomed. Pharmacother. 2019, 113, 108597. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xiong, T.X.; Zhang, K.; Zhou, F.C.; Wang, H.Y.; Li, B.; Dong, Y.J.; He, X.; Li, L.Z.; Yu, Q.X.; et al. Benefit Effect ofDendrobium officinaleUltrafine Powder on DSS-Induced Ulcerative Colitis Rats by Improving Colon Mucosal Barrier. Evid.-Based Complement. Alternat. Med. 2021, 2021, 9658638. [Google Scholar] [CrossRef]
- Rollenhagen, C.; Agyeman, H.; Eszterhas, S.; Lee, S.A. Candida albicans END3Mediates Endocytosis and Has Subsequent Roles in Cell Wall Integrity, Morphological Switching, and Tissue Invasion. Microbiol. Spectr. 2022, 10, e0188021. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Lynch, N.; McCullough, M.J. Oral fungal infections: An update for the general practitioner. Aust. Dent. J. 2010, 55 (Suppl. S1), 48–54. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef]
- Valand, N.; Girija, U.V. Candida Pathogenicity and Interplay with the Immune System. Adv. Exp. Med. Biol. 2021, 1313, 241–272. [Google Scholar] [CrossRef]
- Cho, E.; Park, Y.; Kim, K.Y.; Han, D.; Kim, H.S.; Kwon, J.S.; Ahn, H.J. Clinical Characteristics and Relevance of Oral Candida Biofilm in Tongue Smears. J. Fungi 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Bielska, E.; Sisquella, M.A.; Aldeieg, M.; Birch, C.; O’Donoghue, E.J.; May, R.C. Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii. Nat. Commun. 2018, 9, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, G.; Rocha, J.D.; Oliveira, D.L.; Albuquerque, P.C.; Frases, S.; Santos, S.S.; Nosanchuk, J.D.; Gomes, A.M.; Medeiros, L.C.; Miranda, K.; et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol. 2015, 17, 389–407. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Freire-de-Lima, C.G.; Nosanchuk, J.D.; Casadevall, A.; Rodrigues, M.L.; Nimrichter, L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 2010, 78, 1601–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratitong, B.; Marshall, M.; Pearlman, E. β-Glucan-stimulated neutrophil secretion of IL-1α is independent of GSDMD and mediated through extracellular vesicles. Cell Rep. 2021, 35, 109139. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, M.; Xu, K.; Wu, K.; Xie, R.; Li, R.; Wang, Q.; Liu, W.; Wang, W.; Wang, X. Antimicrobial Effect of Extracellular Vesicles Derived From Human Oral Mucosal Epithelial Cells on Candida albicans. Front. Immunol. 2022, 13, 777613. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef] [Green Version]
- West, A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 2017, 391, 54–63. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.C.; Everts, B.; Ivanova, Y.; O’Sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’Neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef]
- D’Souza, A.; Burch, A.; Dave, K.M.; Sreeram, A.; Reynolds, M.J.; Dobbins, D.X.; Kamte, Y.S.; Zhao, W.; Sabatelle, C.; Joy, G.M.; et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J. Control. Release 2021, 338, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.E.; Esher, S.K.; Alspaugh, J.A. Chitin: A “Hidden Figure” in the Fungal Cell Wall. Curr. Top. Microbiol. Immunol. 2020, 425, 83–111. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Gottfried, L.; Lehrer, N. Candidal vaginitis in hormone-treated mice: Prevention by a chitin extract. Mycopathologia 1988, 102, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.; Altboum, Z.; Savage, D.C.; Segal, E. Adhesion of Candida albicans to epithelial cells effect of polyoxin D. Mycopathologia 1991, 115, 197–205. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Alvarez, M.; Fonseca, F.L.; Casadevall, A. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot. Cell 2008, 7, 602–609. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, R.; Zhou, Y.; Xie, R.; Ma, J.; Liu, H.; Qin, Y.; Zhao, M.; Duan, N.; Ye, P.; et al. The Promotional Effect of GW4869 on C. albicans Invasion and Cellular Damage in a Murine Model of Oral Candidiasis. Pathogens 2022, 11, 1522. https://doi.org/10.3390/pathogens11121522
Zhang M, Li R, Zhou Y, Xie R, Ma J, Liu H, Qin Y, Zhao M, Duan N, Ye P, et al. The Promotional Effect of GW4869 on C. albicans Invasion and Cellular Damage in a Murine Model of Oral Candidiasis. Pathogens. 2022; 11(12):1522. https://doi.org/10.3390/pathogens11121522
Chicago/Turabian StyleZhang, Miaomiao, Ruowei Li, Yifan Zhou, Ruiqi Xie, Jingjing Ma, Hong Liu, Yao Qin, Maomao Zhao, Ning Duan, Pei Ye, and et al. 2022. "The Promotional Effect of GW4869 on C. albicans Invasion and Cellular Damage in a Murine Model of Oral Candidiasis" Pathogens 11, no. 12: 1522. https://doi.org/10.3390/pathogens11121522
APA StyleZhang, M., Li, R., Zhou, Y., Xie, R., Ma, J., Liu, H., Qin, Y., Zhao, M., Duan, N., Ye, P., Wang, W., & Wang, X. (2022). The Promotional Effect of GW4869 on C. albicans Invasion and Cellular Damage in a Murine Model of Oral Candidiasis. Pathogens, 11(12), 1522. https://doi.org/10.3390/pathogens11121522







