Linguatula serrata (Fröhlich, 1789) in Gray Wolf (Canis lupus) from Italy: A Neglected Zoonotic Parasite
Abstract
1. Introduction
2. Materials and Methods
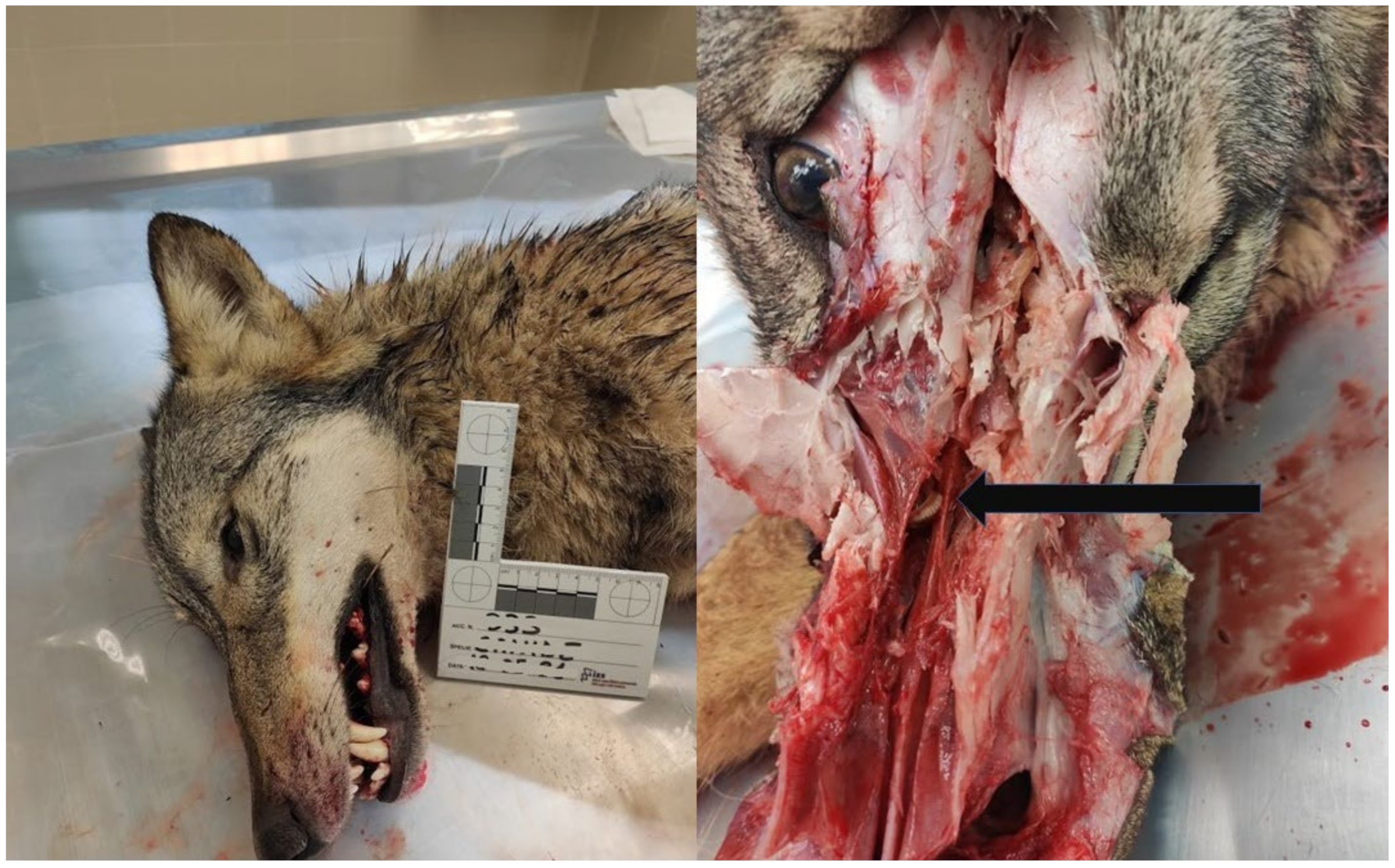
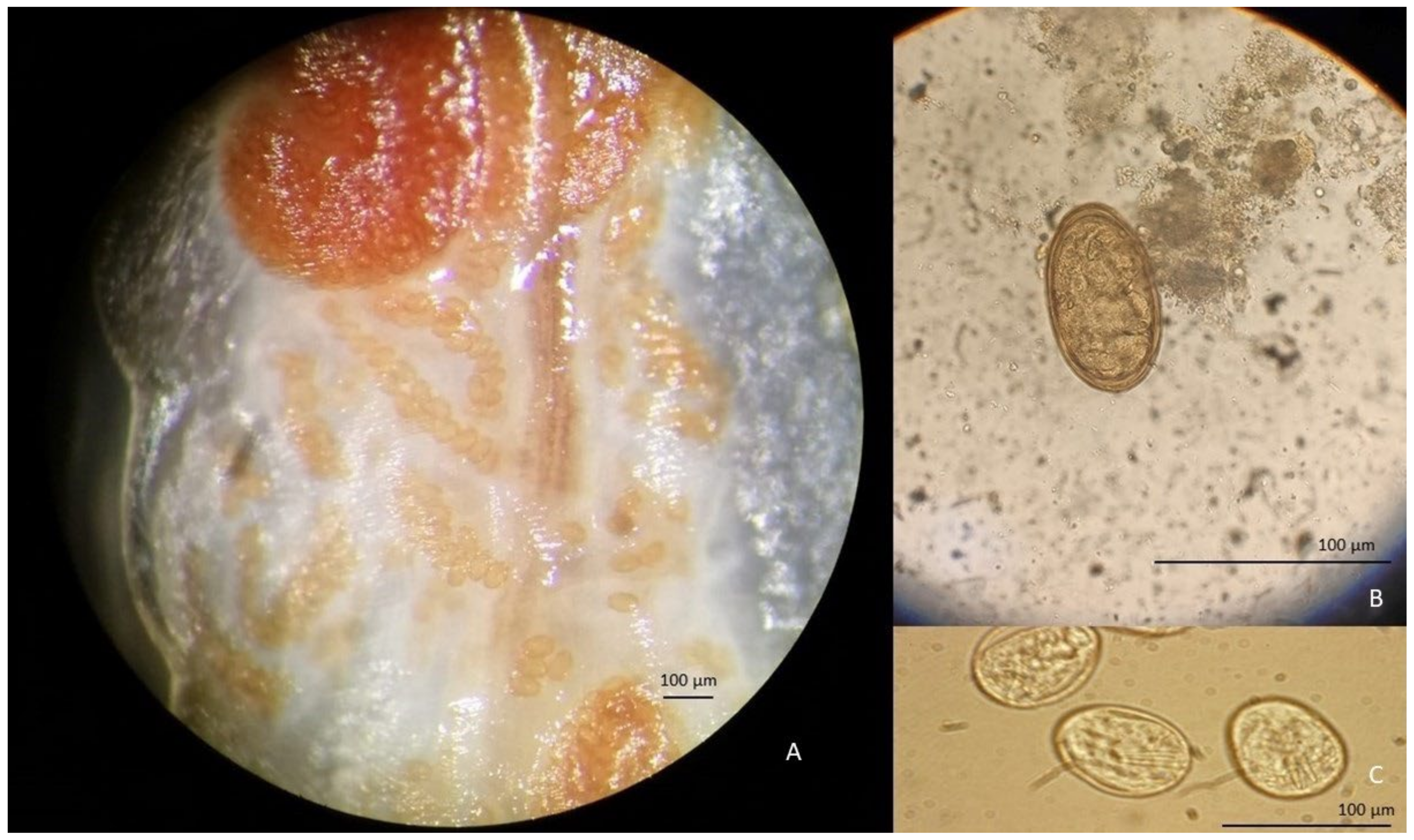
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riley, J. The Biology of Pentastomids. In Advances in Parasitology; Baker, J.R., Muller, R., Eds.; Academic Press: London, UK, 1986; Volume 25, pp. 45–128. [Google Scholar]
- Christoffersen, M.L.; De Assis, J.E. A systematic monograph of the recent Pentastomida, with a compilation of their hosts. Zool. Meded. 2013, 87, 1–206. [Google Scholar]
- Tabaripour, R.; Shokri, A.; Hosseini Teshnizi, S.; Fakhar, M.; Keighobadi, M. Status of Linguatula serrata infection in livestock: A systematic review with meta-analysis in Iran. Paras. Epid. Cont. 2019, 7, e00111. [Google Scholar] [CrossRef] [PubMed]
- Musharrafıeh, U.; Hamadeh, G.; Touma, A.; Fares, J. Nasopharyngeal linguatulosis or halzoun syndrome: Clinical diagnosis and treatment. Rev. Assoc. Médica Bras. 2018, 64, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Tabaripour, R.; Keighobadi, M.; Sharifpour, A.; Azadeh, H.; Shokri, A.; Banimostafavi, E.S.; Fakhar, M.; Abedi, S. Global status of neglected human Linguatula infection: A systematic review of published case reports. Parasitol. Res. 2021, 120, 3045–3050. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Ashrafihelan, J.; Nematollahi, A.; Mostafavi, E. The prevalence of Linguatula serrata nymphs in goats slaughtered in Tabriz, Iran. J. Parasit Dis. 2012, 36, 200–202. [Google Scholar] [CrossRef][Green Version]
- Drabick, J.J. Pentastomiasis. Rev. Infect. Dis. 1987, 6, 1087–1094. [Google Scholar] [CrossRef]
- Dryden, M.W.; Payne, P.A.; Ridley, R.; Smith, V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet. Ther. 2005, 6, 15–28. [Google Scholar]
- Neveu-Lemaire, M. Traité D’Entomologie Médicale et Vétérinaire; Vigot Frères, Editeurs: Paris, France, 1938; Volume 28, p. 1339. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 5, 294–299. [Google Scholar]
- Shamsi, S.; Zhu, X.; Halajian, A.; Barton, D.P. 28S rRNA sequences for Linguatula spp. Parasitol. Res. 2022, 6, 1799–1804. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Masatoshi, G.S.; Kumar, N.S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Bogdaschew, V.N. Der Zusammenhang der anatomischenformen der metacarpal und metatarsalknochen der haustieremit dem histologischenbau und der chemisch-physikalischeneigenschafternderselben. Anat. Anz. 1930, 70, 113–154. [Google Scholar]
- Tasan, E. Distribution of Linguatula serrata, Frölich, 1789 in dogs from rural districts of Elaziğ. Doğa. Vet. Hay Derg. 1987, 11, 86–89. [Google Scholar]
- Jones, D.A.; Riley, J. An ELISA for the detection of pentatomid infections in the rat. Parasitology 1991, 3, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.A.; Bamorovat, M.; Sharifi, I.; Mostafavi, M.; Zarandi, M.B.; Kheirandish, R.; Karamoozian, A.; Khatami, M.; Zadeh, S.H. Linguatula serrata in cattle in southeastern Iran: Epidemiological, histopathological and phylogenetic profile and its zoonotic importance. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100465. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Sharifi, I.; Bamorovat, M.; Mohammadi, M.A.M.A. Human Linguatulosis caused by Linguatula serrata in the City of Kerman, South-eastern Iran- case report. Iran. J. Parasitol. 2014, 9, 282–285. [Google Scholar] [PubMed]
- Islam, R.; Hossain, M.S.; Alam, Z.; Islam, A.; Khan, A.H.N.A.; Kabir, M.E.; Hatta, T.; Alim, A.; Tsuji, N. Linguatula serrata, a food-borne zoonotic parasite, in livestock in Bangladesh: Some pathologic and epidemiologic aspects. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Sudan, V.; Jaiswal, A.K.; Shanker, D. Infection rates of Linguatula serrata nymphs in mesenteric lymph nodes from water buffaloes in North India. Vet. Parasitol. 2014, 205, 408–411. [Google Scholar] [CrossRef]
- Hajipour, N.; Tavassoli, M. Prevalence and associated risk factors of Linguatula serrata infection in definitive and intermediate hosts in Iran and other countries: A systematic review. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100288. [Google Scholar] [CrossRef]
- Gül, A.; Değer, S.; Denizhan, V. Van Ili Koyunlarinda Linguatula serrata (Fröhlich, 1789) NimflerininYayginliği [The prevalence of Linguatula serrata (Fröhlich, 1789) nymphs in sheep in the Van province]. Turk. Parazitol. Derg. 2009, 33, 25–27. [Google Scholar]
- Gothe, R.; Barutzki, D.; Schöl, H.; Heinen, H. Importierte Infestationen nasopharyngealer Parasiten beim Hund [Imported infestations of nasopharyngeal parasites in dogs]. Tierarztl. Prax. 1991, 19, 84–87. [Google Scholar]
- Thomas, M. Linguatula serrata in an imported Romanian street dog. Vet. Rec. 2018, 182, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Bell, S.; Wright, I.; Wall, R.; Jeckel, S.; Blake, D.; Marshall, P.; Andrews, C.; Lee, M.; Walsh, A. Tongue worm (Linguatula species) in stray dogs imported into the UK. Vet. Rec. 2016, 179, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Ioniță, M.; Mitrea, I.L. Linguatula serrata (Pentastomida: Linguatulidae) infection in dog, Romania: A case report. AgroLife Sci. J. 2016, 5, 85–89. [Google Scholar]
- Ferenc, C.; Pavlović, I.; Tamas, C.; Lengyel, B. Occurrence of Linguatula serrata in dogs in Nagybecskerek. A case study. KisallatPraxis 2014, 15, 64–67. [Google Scholar]
- Bordicchia, M.; Falcioni, D.; Scarpona, S.; Rossi, G. Nasal carcinoma in a dog with Linguatula serrata infection. Vet. Rec. Case Rep. 2014, 2, e000015. [Google Scholar] [CrossRef]
- Principato, M.; Polidori, G.A.; Dacomo, F.; Giannetto, S. Nasal pentastomiasis in dogs by Linguatula serrata Frohlich 1789: Notes on its very high biological potentiality. Parassitologia 1994, 36, 119. [Google Scholar]
- Cafiero, M.A.; Cavaliere, N.; Lia, R. Linguatula serrata, Frohlich, 1789 in a dog in Apulia region. In Proceedings of the 51th Congresso Nazionale S.I.S.Vet, Bologna, Italy, 17–20 September 1997; pp. 617–618. [Google Scholar]
- Pavlović, I.; Penezić, A.; Ćosić, N.; Burazerović, J.; Maletić, V.; Ćirović, D. The first report of Linguatula serrata in grey wolf (Canis lupus) from Central Balkans. J. Hellenic. Vet. Med. Soc. 2017, 68, 687–690. [Google Scholar] [CrossRef]
- Sinclair, K.B. The incidence and life cycle of Linguatula serrata (Frohlich 1789) in Great Britain. J. Comp. Pathol. 1954, 64, 371–383. [Google Scholar] [CrossRef]
- Dorchies, P.; De Lahitte, D.J.; Pangui, L.J.; Alzieu, J.P. Recherche de Fasciola hepatica, Dicrocoeliumlanceolatum et Linguatula denticulate dans les foies de bovinssaisis (a) l’abattoire de Pamiers. Revue Méd. Vét. 1988, 39, 307–309. [Google Scholar]
- Bertolini, G. Un caso di Pentastomatenioide in una pecora. Giorn. Vet. Mil. 1892, 5, 352–355. [Google Scholar]
- Tessé, G. Frequentissimi casi di Linguatula denticulata nei gangli mesenterici dei bovini sardi. Clin. Vet. 1913, 36, 147–157. [Google Scholar]
- Pampiglione, S.; Rivasi, F.; Villani, G. Linguatula serrata: Un caso umano con manifestazioni pseudoappendicolari, nel Molise (Italia Centrale). Parassitologia 1998, 40, 124. [Google Scholar]
- Pampiglione, S.; Gentile, A.; Maggi, P.; Scattone, A.; Sollitto, F. A nodularpulmonarylesion due to Linguatula serrata in an HIV-positive man. Parassitologia 2001, 43, 105–108. [Google Scholar] [PubMed]
- Parenzan, P.; Chieffi, G. Primo caso clinico di infestazione umana da Linguatula serrata in Italia [First clinical case of human infestation from Linguatula serrata in Italy]. Acta Med. Ital. 1951, 6, 67–69. [Google Scholar] [PubMed]
- Koehsler, M.; Walochnik, J.; Georgopoulos, M.; Pruente, C.; Boeckeler, W.; Auer, H.; Barisani-Asenbauer, T. Linguatula serrata Tongue Worm in Human Eye, Austria. Emerg. Infec. Dis. 2011, 17, 870–872. [Google Scholar] [CrossRef]
- Gjerde, B. Phylogenetic position of Linguatulaarctica and Linguatula serrata (Pentastomida) as inferred from the nuclear 18S rRNA gene and the mitochondrial cytochrome c oxidase subunit I gene. Parasitol. Res. 2013, 112, 3517–3525. [Google Scholar] [CrossRef]
- Ghorashi, S.A.; Tavassoli, M.; Peters, A.; Shamsi, S.; Hajipour, N. Phylogenetic relationships among Linguatula serrata isolates from Iran based on 18S rRNA and mitochondrial cox1 gene sequences. Acta Parasitol. 2016, 61, 195–200. [Google Scholar] [CrossRef]


| GenBank Accession Number/Species | Country | Host | % Homology |
|---|---|---|---|
| MW947492/L. serrata * | Italy | Wolf | 100% |
| MZ052082/L. serrata | Italy | Dog | 100% |
| LC150783/L. serrata | Bangladesh | Cattle | 99.83% |
| KF830141/L. serrata | Iran | Camel | 99.50% |
| LC150782/L. serrata | Bangladesh | Cattle | 99.83% |
| KY829107/L. serrata | Peru | Camel | 99.67% |
| KY829108/L. serrata | Peru | Camel | 99.67% |
| KY829109/L. serrata | Peru | Camel | 99.67% |
| KF830139/L. serrata | Iran | Dog | 99.00% |
| LC150781/L. serrata | Bangladesh | Cattle | 99.83% |
| KU234193/L. serrata | Iran | Sheep | 99.52% |
| KF029447/L. serrata | Norway | Dog | 100% |
| KF830138/L. serrata | Iran | Sheep | 99.50% |
| KF830137/L. serrata | Iran | Cattle | 98.00% |
| KF029443/L. arctica # | Norway | Reindeer | 89.95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raele, D.A.; Petrella, A.; Troiano, P.; Cafiero, M.A. Linguatula serrata (Fröhlich, 1789) in Gray Wolf (Canis lupus) from Italy: A Neglected Zoonotic Parasite. Pathogens 2022, 11, 1523. https://doi.org/10.3390/pathogens11121523
Raele DA, Petrella A, Troiano P, Cafiero MA. Linguatula serrata (Fröhlich, 1789) in Gray Wolf (Canis lupus) from Italy: A Neglected Zoonotic Parasite. Pathogens. 2022; 11(12):1523. https://doi.org/10.3390/pathogens11121523
Chicago/Turabian StyleRaele, Donato Antonio, Antonio Petrella, Pasquale Troiano, and Maria Assunta Cafiero. 2022. "Linguatula serrata (Fröhlich, 1789) in Gray Wolf (Canis lupus) from Italy: A Neglected Zoonotic Parasite" Pathogens 11, no. 12: 1523. https://doi.org/10.3390/pathogens11121523
APA StyleRaele, D. A., Petrella, A., Troiano, P., & Cafiero, M. A. (2022). Linguatula serrata (Fröhlich, 1789) in Gray Wolf (Canis lupus) from Italy: A Neglected Zoonotic Parasite. Pathogens, 11(12), 1523. https://doi.org/10.3390/pathogens11121523








