rCsHscB Derived from Clonorchis sinensis: A Carcinogenic Liver Fluke Ameliorates LPS-Induced Acute Hepatic Injury by Repression of Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experiment Protocol and Tissue Collection
2.3. Preparation of rCsHscB
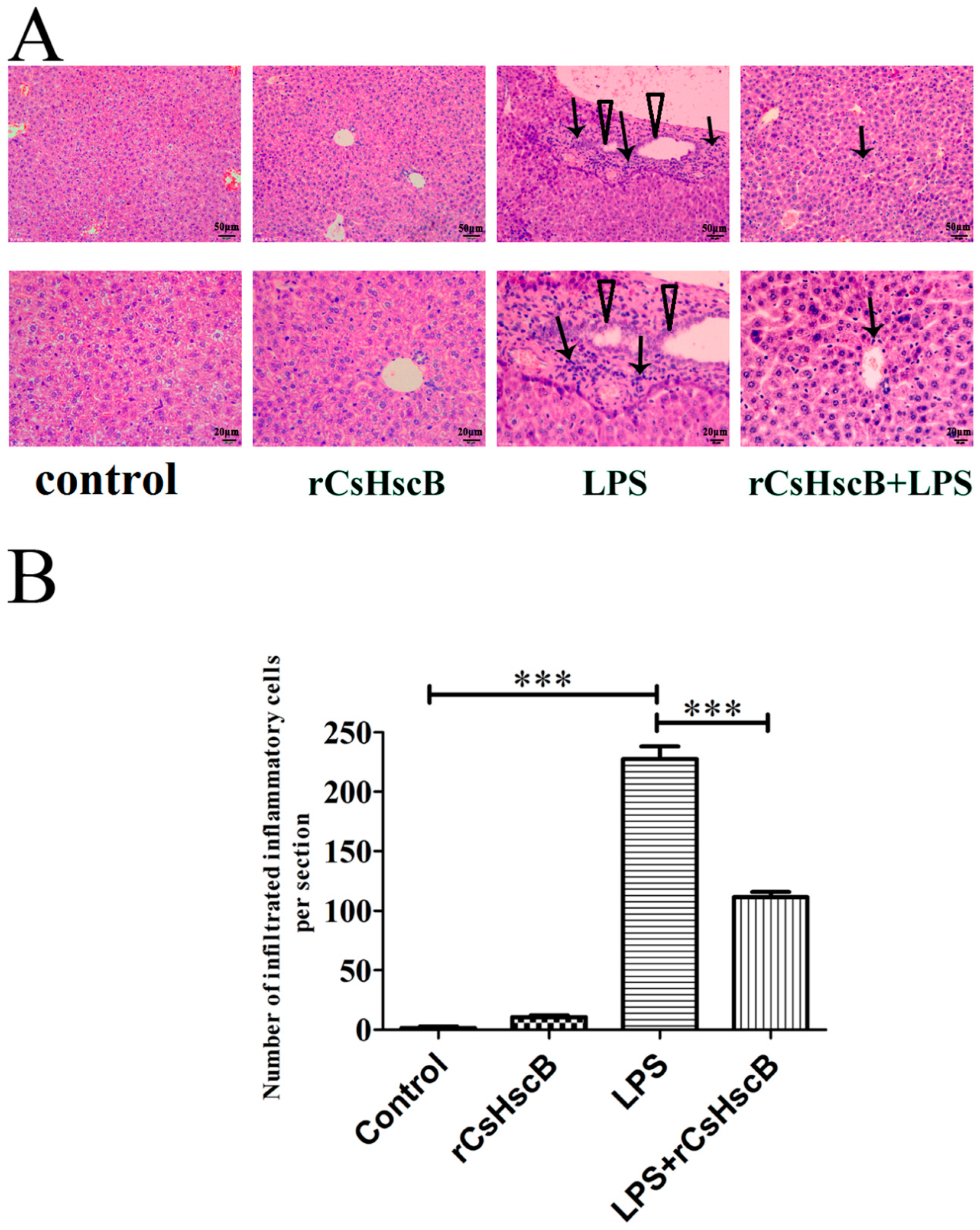
2.4. H&E Staining
2.5. Determination of Serum ALT and AST Activity
2.6. Cell Culture
2.7. ELISA
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. rCsHscB Protects against Pathological Changes in Mice with LPS-Induced Liver Injury
3.2. rCsHscB Improves the Liver Damage Caused by LPS
3.3. rCsHscB Significantly Reverses LPS-Induced High Levels of IL-6 and MCP-1 in the Sera of Mice
3.4. rCsHscB Suppresses LPS-Induced Macrophage Inflammatory Response
3.5. rCsHscB Protects against LPS-Induced Liver Injury by Anti-Inflammation Associated with MAPK Signaling Pathway
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver—guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, Y.; Yao, Y. Advances in sepsis-associated liver dysfunction. Burn. Trauma 2014, 2, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Hsing, C.H.; Wang, J.J. Clinical implication of perioperative inflammatory cytokine alteration. Acta Anaesthesiol. Taiwanica Off. J. Taiwan Soc. Anesthesiol. 2015, 53, 23–28. [Google Scholar] [CrossRef]
- Hsia, C.H.; Velusamy, M.; Jayakumar, T.; Chen, Y.J.; Hsia, C.W.; Tsai, J.H.; Teng, R.D.; Sheu, J.R. Mechanisms of TQ-6, a Novel Ruthenium-Derivative Compound, against Lipopolysaccharide-Induced In Vitro Macrophage Activation and Liver Injury in Experimental Mice: The Crucial Role of p38 MAPK and NF-kappaB Signaling. Cells 2018, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ge, R.; Zhao, C.; Tang, L.; Li, J.; Li, Q. Farrerol regulates occludin expression in hydrogen peroxide-induced EA.hy926 cells by modulating ERK1/2 activity. Eur. J. Pharmacol. 2014, 734, 9–14. [Google Scholar] [CrossRef]
- Yoshinari, O.; Shiojima, Y.; Igarashi, K. Hepatoprotective effect of germanium-containing Spirulina in rats with (D)-galactosamine- and lipopolysaccharide-induced hepatitis. Br. J. Nutr. 2014, 111, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Ariyadi, B.; Isobe, N.; Yoshimura, Y. Toll-like receptor signaling for the induction of mucin expression by lipopolysaccharide in the hen vagina. Poult. Sci. 2014, 93, 673–679. [Google Scholar] [CrossRef]
- Zhang, X.; Vallabhaneni, R.; Loughran, P.A.; Shapiro, R.; Yin, X.M.; Yuan, Y.; Billiar, T.R. Changes in FADD levels, distribution, and phosphorylation in TNFalpha-induced apoptosis in hepatocytes is caspase-3, caspase-8 and BID dependent. Apoptosis Int. J. Program. Cell Death 2008, 13, 983–992. [Google Scholar] [CrossRef]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, M.Y.; Mordvinov, V.A. The liver fluke Opisthorchis felineus: Biology, epidemiology and carcinogenic potential. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.S.; Pak, J.H.; Kim, J.B.; Bahk, Y.Y. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: A brief review. BMB Rep. 2016, 49, 590–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.M.; Kwak, Y.S.; Yi, M.H.; Kim, J.Y.; Sohn, W.M.; Yong, T.S. Clonorchis sinensis antigens alter hepatic macrophage polarization in vitro and in vivo. PLoS Negl. Trop. Dis. 2017, 11, e0005614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, S.M.; Oikonomou, K.D.; Zhou, W.L.; Acker, C.D.; Popovic, M.A.; Zecevic, D.; Antic, S.D. The stochastic nature of action potential backpropagation in apical tuft dendrites. J. Neurophysiol. 2017, 118, 1394–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, H.; Dong, X.; Zhang, Y.; Fang, F.; Zhang, B.; Li, X.; Yu, Q.; Zheng, K.; Yan, C. rCsHscB derived from Clonorchis sinensis has therapeutic effect on dextran sodium sulfate-induced chronic ulcerative colitis in mice. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 664–670. [Google Scholar] [CrossRef]
- Xiong, X.; Ren, Y.; Cui, Y.; Li, R.; Wang, C.; Zhang, Y. Obeticholic acid protects mice against lipopolysaccharide-induced liver injury and inflammation. Biomed. Pharmacother. 2017, 96, 1292–1298. [Google Scholar] [CrossRef]
- Yan, C.; Fang, F.; Zhang, Y.Z.; Dong, X.; Wu, J.; Liu, H.L.; Fan, C.Y.; Koda, S.; Zhang, B.B.; Yu, Q.; et al. Recombinant CsHscB of carcinogenic liver fluke Clonorchis sinensis induces IL-10 production by binding with TLR2. PLoS Negl. Trop. Dis. 2020, 14, e0008643. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.F. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nat. Rev. Immunol. 2018, 18, 105–120. [Google Scholar] [CrossRef] [PubMed]
- De Laval, B.; Sieweke, M.H. Trained macrophages support hygiene hypothesis. Nat. Immunol. 2017, 18, 1279–1280. [Google Scholar] [CrossRef]
- Harnett, M.M.; Harnett, W. Can Parasitic Worms Cure the Modern World’s Ills? Trends Parasitol. 2017, 33, 694–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maizels, R.M. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnett, M.M.; Kean, D.E.; Boitelle, A.; McGuiness, S.; Thalhamer, T.; Steiger, C.N.; Egan, C.; Al-Riyami, L.; Alcocer, M.J.; Houston, K.M.; et al. The phosphorycholine moiety of the filarial nematode immunomodulator ES-62 is responsible for its anti-inflammatory action in arthritis. Ann. Rheum. Dis. 2008, 67, 518–523. [Google Scholar] [CrossRef]
- Pineda, M.A.; Al-Riyami, L.; Harnett, W.; Harnett, M.M. Lessons from helminth infections: ES-62 highlights new interventional approaches in rheumatoid arthritis. Clin. Exp. Immunol. 2014, 177, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coltherd, J.C.; Rodgers, D.T.; Lawrie, R.E.; Al-Riyami, L.; Suckling, C.J.; Harnett, W.; Harnett, M.M. The parasitic worm-derived immunomodulator, ES-62 and its drug-like small molecule analogues exhibit therapeutic potential in a model of chronic asthma. Sci. Rep. 2016, 6, 19224. [Google Scholar] [CrossRef] [Green Version]
- Aprahamian, T.R.; Zhong, X.; Amir, S.; Binder, C.J.; Chiang, L.K.; Al-Riyami, L.; Gharakhanian, R.; Harnett, M.M.; Harnett, W.; Rifkin, I.R. The immunomodulatory parasitic worm product ES-62 reduces lupus-associated accelerated atherosclerosis in a mouse model. Int. J. Parasitol. 2015, 45, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xie, H.; Xu, L.; Liao, Q.; Wan, S.; Yu, Z.; Lin, D.; Zhang, B.; Lv, Z.; Wu, Z.; et al. rSj16 Protects against DSS-Induced Colitis by Inhibiting the PPAR-alpha Signaling Pathway. Theranostics 2017, 7, 3446–3460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Han, Q.; Lu, N.; Xu, D.; Tian, Z.; Zhang, J. HMBOX1 in hepatocytes attenuates LPS/D-GalN-induced liver injury by inhibiting macrophage infiltration and activation. Mol. Immunol. 2018, 101, 303–311. [Google Scholar] [CrossRef]
- Liu, D.; Cao, S.; Zhou, Y.; Xiong, Y. Recent advances in endotoxin tolerance. J. Cell. Biochem. 2019, 120, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, H.; Nishiguchi, S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int. J. Mol. Sci. 2014, 15, 7711–7730. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- He, H.Q.; Wu, Y.X.; Nie, Y.J.; Wang, J.; Ge, M.; Qian, F. LYRM03, an ubenimex derivative, attenuates LPS-induced acute lung injury in mice by suppressing the TLR4 signaling pathway. Acta Pharmacol. Sin. 2017, 38, 342–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Zhang, P.; Wan, R.; Zhang, B. Hepatoprotective effect of quercetin against LPS/d-GalN induced acute liver injury in mice by inhibiting the IKK/NF-kappaB and MAPK signal pathways. Int. Immunopharmacol. 2017, 52, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Ci, X.; He, J.; Wei, M.; Yang, X.; Cao, Q.; Li, H.; Guan, S.; Deng, Y.; Pang, D.; et al. A novel anti-inflammatory role for ginkgolide B in asthma via inhibition of the ERK/MAPK signaling pathway. Molecules 2011, 16, 7634–7648. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.L.; Lian, L.H.; Wan, Y.; Nan, J.X. Baicalein inhibits nuclear factor-kappaB and apoptosis via c-FLIP and MAPK in D-GalN/LPS induced acute liver failure in murine models. Chem. Biol. Interact. 2010, 188, 526–534. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Z.; Shen, B.; Wu, L.; Han, L.; Zhang, Q.; Feng, H. The protective effects of Shikonin on lipopolysaccharide/d-galactosamine-induced acute liver injury via inhibiting MAPK and NF-κB and activating Nrf2/HO-1 signaling pathways. RSC Adv. 2017, 7, 34846–34856. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yu, Y.H.; Wang, S.T.; Ren, J.; Camer, D.; Hua, Y.Z.; Zhang, Q.; Huang, J.; Xue, D.L.; Zhang, X.F.; et al. Chlorogenic acid protects D-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Fan, C.; Tan, Q.; Zhang, Y.; Jiang, Q.; Yu, Q.; Zhang, B.; Zheng, K.; Yan, C. rCsHscB Derived from Clonorchis sinensis: A Carcinogenic Liver Fluke Ameliorates LPS-Induced Acute Hepatic Injury by Repression of Inflammation. Pathogens 2022, 11, 1548. https://doi.org/10.3390/pathogens11121548
Zhang B, Fan C, Tan Q, Zhang Y, Jiang Q, Yu Q, Zhang B, Zheng K, Yan C. rCsHscB Derived from Clonorchis sinensis: A Carcinogenic Liver Fluke Ameliorates LPS-Induced Acute Hepatic Injury by Repression of Inflammation. Pathogens. 2022; 11(12):1548. https://doi.org/10.3390/pathogens11121548
Chicago/Turabian StyleZhang, Bo, Chunyang Fan, Qi Tan, Yuzhao Zhang, Qing Jiang, Qian Yu, Beibei Zhang, Kuiyang Zheng, and Chao Yan. 2022. "rCsHscB Derived from Clonorchis sinensis: A Carcinogenic Liver Fluke Ameliorates LPS-Induced Acute Hepatic Injury by Repression of Inflammation" Pathogens 11, no. 12: 1548. https://doi.org/10.3390/pathogens11121548
APA StyleZhang, B., Fan, C., Tan, Q., Zhang, Y., Jiang, Q., Yu, Q., Zhang, B., Zheng, K., & Yan, C. (2022). rCsHscB Derived from Clonorchis sinensis: A Carcinogenic Liver Fluke Ameliorates LPS-Induced Acute Hepatic Injury by Repression of Inflammation. Pathogens, 11(12), 1548. https://doi.org/10.3390/pathogens11121548








