The Epidemiology and Control of Bovine Viral Diarrhoea Virus in Tropical Indonesian Cattle
Abstract
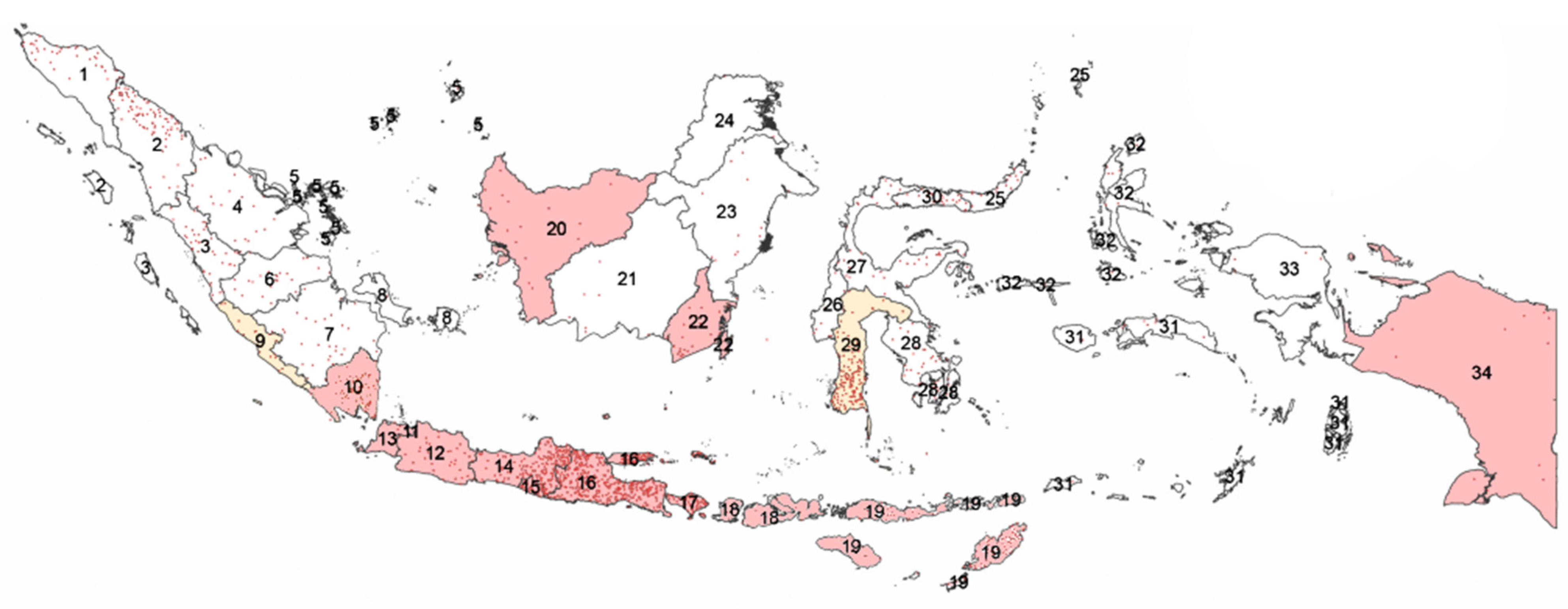
:1. Introduction
2. A brief Description of the Cattle Industries in Indonesia
2.1. Beef Cattle Industry
2.2. Dairy Industry
3. The Epidemiology of BVDV in Indonesian Cattle Populations
3.1. Isolation and Detection
3.2. Genetic Diversity
3.3. Prevalence
3.4. Impact of BVD on Cattle Production in Indonesia
3.5. Risk Factors of Infection with BVD in Indonesian Cattle
4. BVDV in Southeast Asia (SEA)
| Country | Period | Species | Test | Prevalence (n) | Risk Factors | Impact | Reference |
|---|---|---|---|---|---|---|---|
| Cambodia | 2016 | Buffalo | IDEXX BVDV Total Antibody Test Kit | 3.4% (29) | - | - | [74] |
| Cambodia | 2016 | Cattle | IDEXX BVDV Total Antibody Test Kit | 6.4% (471) | - | - | [74] |
| Malaysia, Selangor | 2014–2015 | Dairy cattle | PrioCHECK® BVDV Ab, Prionics AG, Switzerland | 33.2% (407) | - | - | [77] |
| Myanmar | 2016 | Dairy cattle (herd level) | Antibody detection, IDEXX | 2.1% (381) | - | - | [79] |
| Southern Vietnam | 2003 | Dairy cattle | ELISA-kit (SVANOVA Biotech AB, Uppsala, Sweden) | 81.9% (215) | - | - | [75] |
| Philippines | 2008 | Buffalo | RT-PCR: E2 | - b | - | - | [80] |
| Luzon, Philippines | 2007 | Brahman cows c | RT-PCR: 5′UTR, E2 | 47.1% (17) | - | Abortion, OR: 27.11 (p < 0.001) | [81] |
| Luzon, Philippines | 2007 | Brahman bulls | RT-PCR: 5′UTR, E2 | 12.5% (16) | - | - | [81] |
| Northeast Thailand | 2011 | Dairy cattle with a high level of BTM d seroprevalence | Indirect ELISA kit, SVANOVIR® BVDV-Ab (SVANOVA Biotech AB, Uppsala, Sweden) | 36.1% (1165 young stocks) | - | Longer calving interval (OR = 1.29; p = 0.02) Older age at first service (OR = 1.63; p = 0.02). | [76] |
| Northeast Thailand | 2011 | Dairy cattle with a high level of BTM seroprevalence | ELISA BVDV-Ag test kit (IDEXX Laboratories B.V. Switzerland) | 1.2% (1165 young stocks) | - | Longer calving interval (OR = 1.29; p = 0.02) Older age at first service (OR = 1.63; p = 0.02). | [76] |
| Lao PDR | 2013 | Buffalo | IDEXX BVDV Total Antibody Test Kit | 4.9% (61) | - | - | [78] |
| Lao PDR | 2013 | Cattle | IDEXX BVDV Total Antibody Test Kit | 10.0% (90) | - | - | [78] |
| Lao PDR | 2016–2018 | Cattle | IDEXX BVDV Total Antibody Test Kit | 7.7% (390) | Male (OR: 3.12, 1.22–7.99) Wet season (OR: 0.47, 0.24–0.93) N. caninum OD e (OR: 1.87, 1.01–3.45) FMD/HS f vaccinated (OR: 0.91, 0.85–0.97) | - | [82] |
| Timor-Leste | 1992 | Cattle | Agar Gel Precipitation Antigen was supplied by Elizabeth Macarthur Agricultural Institute (EMAI, NSW, Australia) | 9.3% (108) | - | - | [64] |
5. Control of BVD in Indonesian Cattle Population
5.1. Vaccination
5.2. Control of BVDV in the NAIC and LEC
5.3. Control of BVDV in Internationally Imported Cattle
5.4. Control of BVDV during Cattle Transport among Islands within Indonesia
5.5. Control of BVDV in Breeding Farms
5.6. Control of BVDV in Dairy Farms
5.7. Control of BVDV in Fattening Farms
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okumu, T.A.; John, N.M.; Wabacha, J.K.; Tsuma, V.; VanLeeuwen, J. Seroprevalence of antibodies for bovine viral diarrhoea virus, Brucella abortus and Neospora caninum, and their roles in the incidence of abortion/foetal loss in dairy cattle herds in Nakuru District, Kenya. BMC Vet. Res. 2019, 15, 95. [Google Scholar] [CrossRef] [Green Version]
- Gallina, L.; Koch, M.; Gentile, A.; Treglia, I.; Bombardi, C.; Mandrioli, L.; Bolcato, M.; Scagliarini, A.; Drögemüller, C.; Seuberlich, T. Bovine viral diarrhoea virus 1b infection associated with congenital tremor and hypomyelination in Holstein calves. Vet. Microbiol. 2021, 256, 109047. [Google Scholar] [CrossRef]
- Hasan, S.D.; Alsaad, K.M. Evaluation of clinical, hematological, blood coagulation and some biochemical parameter changes in clinically infected cattle with bovine viral diarrhea. IOSR J. Agric. Vet. Sci. 2018, 11, 64–70. [Google Scholar]
- Goto, Y.; Yaegashi, G.; Fukunari, K.; Suzuki, T. Clinical analysis for long-term sporadic bovine viral diarrhea transmitted by calves with an acute infection of bovine viral diarrhea virus 2. Viruses 2021, 13, 621. [Google Scholar] [CrossRef]
- Nonnecke, B.; McGill, J.; Ridpath, J.; Sacco, R.; Lippolis, J.; Reinhardt, T. Acute phase response elicited by experimental Bovine Diarrhea Virus (BVDV) infection is associated with decreased Vitamin D and E status of vitamin-replete preruminant calves. J. Dairy Sci. 2014, 97, 5566–5579. [Google Scholar] [CrossRef] [Green Version]
- Asmare, K.; Sibhat, B.; Ayelet, G.; Gebremedhin, E.Z.; Lidete, K.A.; Skjerve, E. Serological evidence of Bovine herpesvirus-1, Bovine Viral Diarrhea virus and Schmallenberg virus infections in relation to reproductive disorders in dairy cattle in Ethiopia. Acta Trop. 2018, 178, 236–241. [Google Scholar] [CrossRef]
- Waage, S. Influence of new infection with bovine virus diarrhoea virus on udder health in Norwegian dairy cows. Prev. Vet. Med. 2000, 43, 123–135. [Google Scholar] [CrossRef]
- Postel, A.; Smith, D.B.; Becher, P. Proposed update to the taxonomy of Pestiviruses: Eight additional species within the genus Pestivirus, family Flaviviridae. Viruses 2021, 13, 1542. [Google Scholar] [CrossRef]
- Smith, D.; Meyers, G.; Bukh, J.; Gould, E.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.; Simmonds, P. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef]
- Yeşilbağ, K.; Alpay, G.; Becher, P. Variability and global distribution of subgenotypes of bovine viral diarrhea virus. Viruses 2017, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Houe, H. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Vet. Microbiol. 1999, 64, 89–107. [Google Scholar] [CrossRef]
- Ridpath, J. Preventive strategy for BVDV infection in North America. Jpn. J. Vet. Res. 2012, 60, S41–S49. [Google Scholar] [PubMed]
- Voges, H.; Young, S.; Nash, M. Direct adverse effects of persistent BVDv infection in dairy heifers–a retrospective case control study. Vetscript 2006, 19, 22–25. [Google Scholar]
- Moennig, V.; Becher, P. Control of bovine viral diarrhea. Pathogens 2018, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, M.; Konradt, G.; De Souza, S.; Bassuino, D.; Silveira, S.; Mósena, A.; Canal, C.; Pavarini, S.; Driemeier, D. Natural outbreak of BVDV-1d–induced mucosal disease lacking intestinal lesions. Vet. Pathol. 2017, 54, 242–248. [Google Scholar] [CrossRef]
- Bianchi, M.V.; Silveira, S.; Mósena, A.C.S.; de Souza, S.O.; Konradt, G.; Canal, C.W.; Driemeier, D.; Pavarini, S.P. Pathological and virological features of skin lesions caused by BVDV in cattle. Braz. J. Microbiol. 2019, 50, 271–277. [Google Scholar] [CrossRef]
- Khodakaram-Tafti, A.; Farjanikish, G. Persistent bovine viral diarrhea virus (BVDV) infection in cattle herds. Iran. J. Vet. Res. 2017, 18, 154. [Google Scholar]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef]
- Fray, M.; Paton, D.; Alenius, S. The effects of bovine viral diarrhoea virus on cattle reproduction in relation to disease control. Anim. Reprod. Sci. 2000, 60, 615–627. [Google Scholar] [CrossRef]
- Newcomer, B.W.; Toohey-Kurth, K.; Zhang, Y.; Brodersen, B.W.; Marley, M.S.; Joiner, K.S.; Zhang, Y.; Galik, P.K.; Riddell, K.P.; Givens, M.D. Laboratory diagnosis and transmissibility of bovine viral diarrhea virus from a bull with a persistent testicular infection. Vet. Microbiol. 2014, 170, 246–257. [Google Scholar] [CrossRef]
- Evans, C.A.; Reichel, M.P. Non-bovine species and the risk to effective control of Bovine Viral Diarrhoea (BVD) in Cattle. Pathogens 2021, 10, 1263. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, S.; Du, R.; Wang, Q.; Sun, C.; Wang, N.; Zhang, P.; Zhang, L. Isolation and identification of a bovine viral diarrhea virus from sika deer in China. Virol. J. 2011, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huaman, J.L.; Pacioni, C.; Forsyth, D.M.; Pople, A.; Hampton, J.O.; Carvalho, T.G.; Helbig, K.J. Serosurveillance and molecular investigation of wild deer in Australia reveals seroprevalence of Pestivirus infection. Viruses 2020, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, F.S. Prevalence study of Bovine viral diarrhea virus by evaluation of antigen capture ELISA and RT-PCR assay in Bovine, Ovine, Caprine, Buffalo and Camel aborted fetuses in Iran. AMB Express 2011, 1, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, B.; Mitchell, S.; Carson, A.; Russell, G.; Hateley, G. BVD in sheep flocks. Vet. Rec. 2019, 185, 271. [Google Scholar] [CrossRef]
- Bachofen, C.; Vogt, H.-R.; Stalder, H.; Mathys, T.; Zanoni, R.; Hilbe, M.; Schweizer, M.; Peterhans, E. Persistent infections after natural transmission of bovine viral diarrhoea virus from cattle to goats and among goats. Vet. Res. 2013, 44, 32. [Google Scholar] [CrossRef] [Green Version]
- Carbrey, E.; Stewart, W.; Kresse, J.; Snyder, M. Natural infection of pigs with Bovine Viral Diarrhea Virus and its differential diagnosis from Hog Cholera. J. Am. Vet. Med. Assoc. 1976, 169, 1217–1219. [Google Scholar]
- Terpstra, C.; Wensvoort, G. A congenital persistent infection of bovine virus diarrhoea virus in pigs: Clinical, virological and immunological observations. Vet. Q. 1997, 19, 97–101. [Google Scholar] [CrossRef]
- Yarnall, M.J.; Thrusfield, M.V. Engaging veterinarians and farmers in eradicating bovine viral diarrhoea: A systematic review of economic impact. Vet. Rec. 2017, 181, 347. [Google Scholar] [CrossRef] [Green Version]
- Pinior, B.; Firth, C.L.; Richter, V.; Lebl, K.; Trauffler, M.; Dzieciol, M.; Hutter, S.E.; Burgstaller, J.; Obritzhauser, W.; Winter, P.; et al. A systematic review of financial and economic assessments of bovine viral diarrhea virus (BVDV) prevention and mitigation activities worldwide. Prev. Vet. Med. 2017, 137, 77–92. [Google Scholar] [CrossRef]
- Scharnböck, B.; Roch, F.-F.; Richter, V.; Funke, C.; Firth, C.L.; Obritzhauser, W.; Baumgartner, W.; Käsbohrer, A.; Pinior, B. A meta-analysis of bovine viral diarrhoea virus (BVDV) prevalences in the global cattle population. Sci. Rep. 2018, 8, 14420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soesilo, F. Epidemiology of Bovine Virus Diarrhea—Mucosal disease overview of an outbreak in five Provinces of Indonesia in 1988 and 1989. Hemera Zoa 1991, 74, 46–53. [Google Scholar]
- Wiyono, A.; Daniels, P.; Graydon, R. Serological studies of cattle affected by outbreaks of diarrhoeal disease in Kalimantan, Indonesia. In Proceedings of the 7th Congress of the Federation of Asian Veterinary Associations (FAVA), Chonburi, Thailand, 4–7 November 1990. [Google Scholar]
- Ministry of Agriculture. Keputusan Menteri Pertanian Nomor 4026/Kpts/OT.140/4/2013 Tentang Penetapan Jenis Penyakit Hewan Menular Strategis (Regulation of Minister of Agriculture Number 4026/Kpts/OT.140/4/2013 Concerning the Stipulation of Strategic Animal Diseases); Ministry of Agriculture: Jakarta, Indonesia, 2013; p. 4. [Google Scholar]
- BPS-Statistics Indonesia. Statistical Year Book of Indonesia; Sub-Directorate of Statistical Compilation and Publication, Ed.; BPS-Statistics Indonesia: Jakarta, Indonesia, 2020; p. xl+748. [Google Scholar]
- Asikin, Z.; Baker, D.; Villano, R.; Daryanto, A. Business models and innovation in the Indonesian smallholder beef value chain. Sustainability 2020, 12, 7020. [Google Scholar] [CrossRef]
- Agus, A.; Widi, T.S.M. Current situation and future prospects for beef cattle production in Indonesia–A review. Asian-Australas. J. Anim. Sci. 2018, 31, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyah Retno, P.; Dhiar Niken, L.; Ratu Fani, R. Analisis Rumah Tangga Usaha Peternakan di Indonesia Hasil Survei Rumah Tangga Usaha Peternakan 2014 (Analysis of Livestock Farmer Household in Indonesia, Survey of Livestock Farmer Households 2014); Marhaeni, H., Ed.; Badan Pusat Statistik: Jakarta, Indonesia, 2015; p. xvi+98. [Google Scholar]
- Primawidyawan, A.; Indrawati, A.; Lukman, D.W. Deteksi penyakit Bovine Viral Diarrhea pada sapi potong impor melalui pelabuhan tanjung priok (Detection of Bovine Viral Diarrhea in beef cattle imported through Tanjung Priok seaport). Acta Vet. Indones. 2016, 4, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Septiawaty, M.S. Kajian Serologis Bovine Viral Diarrhea (BVD) Pada Sapi Potong Impor Di Daerah Jawa Barat (Serological Study of Bovine Viral Diarrhea in Imported Beef Cattle in West Java). Bachelor’s Thesis, IPB University, Bogor, Indonesia, 2013. [Google Scholar]
- Agustiani, D. Kajian Serologis Bovine Viral Diarrhea (BVD) Pada Sapi Potong Impor Di Daerah Banten (Serological Study of Bovine Viral Diarrhea Virus in Imported Beef Cattle in Banten). Bachelor’s Thesis, IPB University, Bogor, Indonesia, 2013. [Google Scholar]
- Primatika, R.A.; Drastini, Y.; Widiasih, D.A. Kajian Epidemiologi Infeksi Bovine Viral Diarrhea (BVD) pada Sapi Perah di Kabupaten Sleman Yogyakarta (Epidemiological Study on the Infection with Bovine Viral Diarrhea (BVDV) in Dairy Cattle in Sleman, Yogyakarta). Acta Vet. Indones. 2020, 8, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Jannah, A.R. Profil Protein Seropositive Dan Seronegative BVDV Pada Sapi-Sapi Impor (Protein Profiles of BVDV Seropositive and Seronegative in Imported Cattle). Bachelor’s Thesis, Universitas Islam Negeri Syarif Hidayatullah, Jakarta, Indonesia, 2019. [Google Scholar]
- Hidayat, W.; Wuryastuty, H.; Wasito, R. Detection of Pestivirus in small ruminants in Central Java, Indonesia. Vet. World 2021, 14, 996. [Google Scholar] [CrossRef]
- Irianingsih, S.; Wuryastuty, H.; Wasito, R.; Wibawa, H.; Rasa, F.T.; Poermadjaja, B. Genetic analysis of NS5B gene from bovine viral diarrhea virus-infected cattle in Central and East Java, Indonesia. Vet. World 2019, 12, 1108. [Google Scholar] [CrossRef]
- Saepulloh, M.; Sendow, I. Identification and characterization of bovine viral diarrhea virus from Indonesian cattle. J. Vet. 2015, 16, 1–7. [Google Scholar]
- Suhaillah, L. Ragam Genetik Bovine Viral Diarrhea Virus Regio 5′-UTR Isolat Lapangan Pada Sapi Perah (Genetic Variation of Bovine Viral Diarrhea Virus Isolate Regio 5′UTR in Dairy Cattle). Ph.D. Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2016. [Google Scholar]
- Irianingsih, S.H.; Poermadjaja, B.; Wuryastuti, H.; Wasito, R. Genetic recombination of bovine viral diarrhea virus subgenotype-1a and-1c in persistently infected dairy cattle. Indones. J. Biotechnol. 2020, 25, 120–126. [Google Scholar] [CrossRef]
- Wuryastuti, H.; Wasito, R.; Sugiyono, S. Genotypes and biotypes variation of bovine viral diarrhea virus from persistently infected dairy cattle in Java, Indonesia. Integr. J. Vet. Biosci. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Wuryastuti, H.; Wasito, R. Generating a restriction map of the amplified DNA of non-cytopathic bovine viral diarrhea virus of Indonesia isolate. Indones. J. Biotechnol. 1998, 6, 193–198. [Google Scholar]
- Sutarno, S.; Setyawan, A.D. The diversity of local cattle in Indonesia and the efforts to develop superior indigenous cattle breeds. Biodiversitas J. Biol. Divers. 2016, 17, 275–295. [Google Scholar] [CrossRef]
- Mohamad, K.; Olsson, M.; van Tol, H.T.; Mikko, S.; Vlamings, B.H.; Andersson, G.; Rodríguez-Martínez, H.; Purwantara, B.; Paling, R.W.; Colenbrander, B. On the origin of Indonesian cattle. PLoS ONE 2009, 4, e5490. [Google Scholar] [CrossRef]
- Sutarno, S.; Setyawan, A.D. Genetic diversity of local and exotic cattle and their crossbreeding impact on the quality of Indonesian cattle. Biodiversitas J. Biol. Divers. 2015, 16, 327–354. [Google Scholar] [CrossRef]
- Nugroho, W.; Aditya, S.; Swastomo, R.; Aulanni’am, A.A. Productivity, absence of a bull and endoparasitic nematodiosis in beef cattle farms in an upland area of East Java, Indonesia. Vet. World 2020, 13, 1982–1987. [Google Scholar] [CrossRef]
- Priyanti, A.; Hanifah, V.W.; Mahendri, I.G.A.P.; Cahyadi, F.; Cramb, R.A. Small-Scale Beef Cattle Production in East Java, Indonesia. In Proceedings of the 56th AARES Annual Conference, Fremantle, Australia, 7–10 February 2012; pp. 1–22. [Google Scholar]
- IQFAST. Live Cattle Quarantine Inspection 2017–2020. Tanjung Priok Agricultural Quarantine Office, Agricultural Quarantine Agency, Ministry of Agriculture. 2021. Available online: https://intranet.karantina.pertanian.go.id/portalq/ (accessed on 17 August 2021).
- Jahroh, S.; Atmakusuma, J.; Fadillah, A. Comparative analysis of dairy farming management and business model between East Java and West Java, Indonesia. J. Manaj. Agribisnis 2020, 17, 96. [Google Scholar] [CrossRef]
- Untari, T.; Warsito, R.; Wuryastuti, H. Viabilitas Non-Cytopathic Bovine Viral Diarrhea Virus Isolat Baturaden (Viability of a Non-Cytopathic Bovine Viral Diarrhea Virus Isolate from Baturaden). Bul. Peternak. 1998, 22, 80–87. [Google Scholar]
- Edi, S.P.; Ibrahim, A.; Mahawan, T. Pewarnaan Immunoperoxidase (IPX) pada Biakan Sel Madin-Darby Bovine Kidney (MDBK) sebagai Salah Satu Upaya untuk Mendapatkan Isolat Lokal Virus Bovine Viral Diarrhea (BVD) (Immunoperoxidase staining on the Madin-Darby Bovine Kidney (MDBK) cell line as an effort to obtain local isolate of Bovine Viral Diarrhea Virus (BVDV)). In Proceedings of the Rapat Teknis dan Pertemuan Ilmiah (RATEKPIL), Penyidikan Penyakit Hewan dan Surveilans Kesehatan Hewan Tahun 2018 (Technical meeting and scientific conference, Chapter: Method development), Yogyakarta, Indonesia, 2–4 April 2018; pp. 151–160. [Google Scholar]
- Wuryastuti, H.; Wasito, R. Application of the immunoperoxidase monolayer assay to characterize non-cytopathic bovine viral diarrhea virus of Indonesian isolates. Indones. J. Biotechnol. 1997, 1, 154–158. [Google Scholar]
- Wuryastuty, H.; Irianingsih, S.H.; Wasito, R. Deteksi Kontaminasi Bovine Viral Diarrhea Virus pada Fetal Bovine Serum yang Tersedia Secara Komersial (The Detection of Bovine Viral Diarrhea Virus Contamination in Commercially Available of Fetal Bovine Serum). J. Vet. 2021, 22, 229–236. [Google Scholar]
- Nugroho, W.; Reichel, M.P.; Ruff, N.; Gazali, A.M.; Sakke, I.S. Infection with Bovine Viral Diarrhea Virus in cattle in Southern Papua, Indonesia. Acta Trop. 2020, 212, 105712. [Google Scholar] [CrossRef] [PubMed]
- Sudipa, P.H.; Sudimartini, L.M.; Wirata, I.W. Antibody survey of Bovine Viral Diarrhea in Bali cattle. J. Vet. Anim. Sci. 2020, 3, 14–19. [Google Scholar] [CrossRef]
- Ketut, S.A.; Dibia, N.; Purnatha, N.; Sutami, N.; Ardana, I.S. Survei Serologis antibodi bovine viral diarrhea pada ternak sapi di Propinsi Bali; NTB.; NTT; dan Timor Timur. Hemera Zoa 1993, 76, 10–17. [Google Scholar]
- Subekti, D.T.; Fatmawati, M.; Khoiriyah, A.; Pramesthi, A.; Fong, S.; Desem, M.I.; Azmi, Z.; Kusumaningtyas, E.; Endrawati, D.; Purwanto, E.S. Seroprevalence of seven reproductive diseases in beef and dairy cows from three Provinces in Indonesia. Vet. Med. Int. 2021, 2021, 6492289. [Google Scholar] [CrossRef]
- Murtimah, S. Seroprevalence, Seroincidence, and Risk Factors of Bovine Viral Diarrhea Virus Infection on Dairy Cattle in Sleman District. Ph.D. Thesis, Universitas Gadjah Mada, Depok, Indonesia, 2017. [Google Scholar]
- Sudarisman. Bovine Viral Diarrhea pada sapi di Indonesia dan Permasalahannya. Wartazoa 2011, 21, 18–24. [Google Scholar]
- Dradjat, A.S.; Imran, M.; Panjaitan, T.; Dahlanuddin, D. Diseases characteristic of pre-weaning Bali calf (Bos javanicus) in Central Lombok, Indonesia. J. Ilmu Dan Teknol. Peternak. Indones. Indones. J. Anim. Sci. Technol. 2019, 4, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Pratamasari, D.; Kusumawati, A. Review kejadian dan kebijakan pemerintah terhadap bovine viral diarrhea (BVD) di Indonesia. In Proceedings of the Prosiding Seminar Nasiona Peran Rumah Sakit Hewan dalam Penanggulangan Penyakit Zoonosis, Prof. Soeparwi Animal Hospital, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia, 23 November 2013; pp. 360–372. [Google Scholar]
- Adjid, R.A. Alternative strategies for controlling reproductive infectious diseases of beef cattle to increase reproduction eficiency. Indones. Bull. Anim. Vet. Sci. 2004, 14, 125–132. [Google Scholar]
- Aziz, N.; Maksudi, M.; Prakoso, Y.A. Correlation between hematological profile and theileriosis in Bali cattle from Muara Bulian, Jambi, Indonesia. Vet. World 2019, 12, 1358–1361. [Google Scholar] [CrossRef]
- Evans, C.; Cockcroft, P.; Reichel, M. Antibodies to bovine viral diarrhoea virus (BVDV) in water buffalo (Bubalus bubalis) and cattle from the Northern Territory of Australia. Aust. Vet. J. 2016, 94, 423–426. [Google Scholar] [CrossRef]
- Reichel, M.P.; Lanyon, S.R.; Hill, F.I. Perspectives on Current Challenges and Opportunities for Bovine Viral Diarrhoea Virus Eradication in Australia and New Zealand. Pathogens 2018, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Olmo, L.; Reichel, M.P.; Windsor, P.A.; Suon, S.; Wahl, L.C.; Thomson, P.C.; Bush, R.D. Are infectious reproductive pathogens of large ruminants a threat to improving food security? An investigation from Cambodia. Trop. Anim. Health Prod. 2021, 53, 480. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.C.; Alenius, S.; Huong, L.T.T.; Björkman, C. Prevalence of Neospora caninum and bovine viral diarrhoea virus in dairy cows in Southern Vietnam. Vet. J. 2008, 175, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Nilnont, T.; Aiumlamai, S.; Kanistanont, K.; Inchaisri, C.; Kampa, J. Bovine viral diarrhea virus (BVDV) infection in dairy cattle herds in northeast Thailand. Trop. Anim. Health Prod. 2016, 48, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Daves, L.; Yimer, N.; Arshad, S.; Sarsaifi, K.; Omar, M.; Yusoff, R.; Haron, A.; Abdullah, F. Seroprevalence of bovine viral diarrhea virus (BVDV) infection and associated risk factors in cattle in Selangor, Malaysia. Vet. Med. Open J. 2016, 1, 22–28. [Google Scholar] [CrossRef]
- Olmo, L.; Dye, M.T.; Reichel, M.P.; Young, J.R.; Nampanya, S.; Khounsy, S.; Thomson, P.C.; Windsor, P.A.; Bush, R.D. Investigation of infectious reproductive pathogens of large ruminants: Are neosporosis, brucellosis, leptospirosis and BVDV of relevance in Lao PDR? Acta Trop. 2018, 177, 118–126. [Google Scholar] [CrossRef]
- Aye, Y.; Aung, M.; Kyaw, W.; Naing, T.; Po, S. Prevalence and associated factors with bovine viral diarrhoea virus antibodies in the bulk tank milk of small scale dairy herds in central Myanmar. Adv. Anim. Vet. Sci. 2017, 5, 316–323. [Google Scholar]
- Mingala, C.N.; Konnai, S.; Tajima, M.; Onuma, M.; Ohashi, K. Classification of new BVDV isolates from Philippine water buffalo using the viral E2 region. J. Basic Microbiol. 2009, 49, 495–500. [Google Scholar] [CrossRef]
- Konnai, S.; Mingala, C.N.; Sato, M.; Abes, N.S.; Venturina, F.A.; Gutierrez, C.A.; Sano, T.; Omata, Y.; Cruz, L.C.; Onuma, M. A survey of abortifacient infectious agents in livestock in Luzon, the Philippines, with emphasis on the situation in a cattle herd with abortion problems. Acta Trop. 2008, 105, 269–273. [Google Scholar] [CrossRef]
- Olmo, L.; Reichel, M.P.; Nampanya, S.; Khounsy, S.; Wahl, L.C.; Clark, B.A.; Thomson, P.C.; Windsor, P.A.; Bush, R.D. Risk factors for Neospora caninum, bovine viral diarrhoea virus, and Leptospira interrogans serovar Hardjo infection in smallholder cattle and buffalo in Lao PDR. PLoS ONE 2019, 14, e0220335. [Google Scholar] [CrossRef] [Green Version]
- Wasito, R.; Wuryastuti, H. Development of DNA recombinant vaccine for type 1 bovine viral. Indones. J. Biotechnol. 1997, 168, 164–172. [Google Scholar]
- ASOHI. Indeks Obat Hewan Indonesia (Indonesian Veterinary Medicine Indexes), 12th ed.; Kurniawan, W., Uli, E., Prasetyo, A., Eds.; Ministry of Agriculture, Republic of Indonesia: Jakarta, Indonesia, 2019; p. 840. [Google Scholar]
- Anonymous. Target IB Upsus Siwab Tercapai 92,27% (The 92.27% of The Target of UPSUS SIWAB is Achieved). Trobos 2017. Available online: http://troboslivestock.com/detail-berita/2017/12/12/57/9623/target-ib-upsus-siwab-tercapai-9227 (accessed on 12 December 2017).
- LEC. Distribution of embryos and bulls. Available online: https://betcipelang.ditjenpkh.pertanian.go.id/newsite/page-detail.php?id=21 (accessed on 8 November 2021).
- Houe, H.; Lindberg, A.; Moennig, V. Test strategies in bovine viral diarrhea virus control and eradication campaigns in Europe. J. Vet. Diagn. Investig. 2006, 18, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mars, M.; Van Maanen, C. Diagnostic Assays Applied in BVDV Control in The Netherlands. Prev. Vet. Med. 2005, 72, 43–219. [Google Scholar] [CrossRef] [PubMed]
- Driskell, E.A.; Ridpath, J.F. A survey of bovine viral diarrhea virus testing in diagnostic laboratories in the United States from 2004 to 2005. J. Vet. Diagn. Investig. 2006, 18, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moennig, V.; Houe, H.; Lindberg, A. BVD control in Europe: Current status and perspectives. Anim. Health Res. Rev. 2005, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Rodning, S.P.; Marley, M.S.D.; Zhang, Y.; Eason, A.B.; Nunley, C.L.; Walz, P.H.; Riddell, K.P.; Galik, P.K.; Brodersen, B.W.; Givens, M.D. Comparison of three commercial vaccines for preventing persistent infection with bovine viral diarrhea virus. Theriogenology 2010, 73, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Dean, H.J.; Hunsaker, B.D.; Bailey, O.D.; Wasmoen, T. Prevention of persistent infection in calves by vaccination of dams with noncytopathic type-1 modified-live bovine viral diarrhea virus prior to breeding. Am. J. Vet. Res. 2003, 64, 530–537. [Google Scholar] [CrossRef]
- Raboisson, D.; Vosough Ahmadi, B.; Peyre, M.; Hogeveen, H.; Gunn, G.J.; Rushton, J. Editorial: Proceedings of the 2nd ISESSAH Conference 2018. Front. Vet. Sci. 2020, 7, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Stott, A.W.; Gunn, G.J. Use of a benefit function to assess the relative investment potential of alternative farm animal disease prevention strategies. Prev. Vet. Med. 2008, 84, 179–193. [Google Scholar] [CrossRef]

| Port | Number/Frequency | Feeder | Breeder Female | Breeder Bull | Total |
|---|---|---|---|---|---|
| Tanjung Priok | Number of cattle (n) | 1,127,220 | 6603 | 2101 | 1,135,924 |
| Shipments (n) | 796 | 24 | 4 | 824 | |
| Lampung | Number of cattle (n) | 843,328 | 3553 | - | 846,881 |
| Shipments (n) | 658 | 14 | - | 672 |
| No. | Province | Species | Region | Gene | Nucleotide Length | Result | Reference |
|---|---|---|---|---|---|---|---|
| 1 | East Java | Cattle | Pasuruan | NS5B | 360 | BVDV-1c | [45] |
| Malang | NS5B | 360 | BVDV-1a | [45] | |||
| Ngawi | 5′UTR | 288 | BVDV-2 | [47] | |||
| 2 | Central Java | Cattle | Boyolali | NS5B | 360 | BVDV-1a | [45] |
| Cilacap | NS5B | 360 | BVDV-1c | [45] | |||
| Semarang | NS5B | 360 | BVDV-1c | [45] | |||
| NS * | NS5B | 360 | BVDV-1 | [46] | |||
| Banyumas | NS5B | 360, 1038, 2157 | BVDV-1a, BVDV-1b, BVDV-1c | [45,48] | |||
| Banyumas | 5′UTR | 275 | BVDV-1a | [48] | |||
| Banyumas | NPro | 504 | BVDV-1a | [48] | |||
| Banyumas | NS3 | 2049 | BVDV-1a | [48] | |||
| Banyumas | E2 | 1093, 1122 | BVDV-1c | [48] | |||
| Goats | NS | 5′UTR | 288 | BVDV-1 | [44] | ||
| 3 | Jakarta | Cattle | NS | NS5B | 360 | BVDV-1 | [46] |
| 4 | West Java | Cattle | Pengalengan, Lembang, Bogor, Sumedang | NS5B | 360 | BVDV-1 | [46] |
| 5 | Java | Dairy Cattle | NS | 5′UTR | 288 | BVDV-1a, BVDV-1c | [49] |
| Province | Region | Period | Breed | Method of Diagnosis | n | Prevalence | Risk Factors | Impact | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Papua | Timika | 2017 | Bali cattle | ELISA Ab & Ag (IDEXX BVD Total Antibody Test kit, IDEXX HerdChek BVD Ag/Serum Plus Test kit) | 77 | 11.7% | Different village RR: 7.94 (1.01–62.27) | Pregnancy failure RR: 2.9 (1.02–8.14) | [62] |
| West Kalimantan | NS **** | 1989 | NS | Serum Neutralisation Test (SNT) | 161 | 34.8% | Possibly co-infection with IBR *** | Diarrhoea RR: 1.3 (1.2–1.5) | [33] |
| East Nusa Tenggara | Sikka, Kupang, Sumba Timur, Sumba Barat | 1992 | Agar Gel Precipitation Antigen was supplied by Elizabeth Macarthur Agricultural Institute (EMAI, NSW, Australia) | 320 | 0.3% | ND ** | ND | [64] | |
| West Nusa Tenggara | Lombok Tengah, Lombok Timur, Lombok Barat, Sumbawa Besar, Bima, Dompu. | 1992 | Agar Gel Precipitation Antigen was supplied by Elizabeth Macarthur Agricultural Institute (EMAI, NSW, Australia) | 642 | 14.2% | ND | ND | [64] | |
| Bali | Badung, Gianyar, Bangli, Klungkung, Karangasem, Buleleng, Jembrana, Tabanan. | 1992 | Agar Gel Precipitation Antigen was supplied by Elizabeth Macarthur Agricultural Institute (EMAI, NSW, Australia) | 682 | 13.5% | ND | ND | [64] | |
| Bali | Gianyar, Badung | 2020 | Bali cattle | ELISA Ab (NS) | 30 | 36.7% | ND ** | ND | [63] |
| East Java | Pasuruan, Batu | 2019-2020 | NS | ELISA Ab (IDEXX BVDV Total Ab Test, Switzerland) | 62 | 53.2% | ND | ND | [65] |
| Yogyakarta | Sleman | 2017 | FH * | (ID Screen® BVD p80 Antibody Competition, IDvet France), (IDEXX BVDV Ag/Serum Plus®, IDEXX, US) | 255 | 41.2% SS | Older age RR: 1.01 (CI: 1.02–1.04) Larger farm size RR:1.11 (CI: 1.01–1.22) | ND | [66] |
| Yogyakarta | Sleman | 2020 | FH | ELISA Ab (ID Screen® BVD p80 Antibody Competition, IDvet France) | 96 | 56.3% | Manure for Biog RR:1.4 (CI: 1.0–2.0) Clean pen RR: 1.5 (CI: 0.7–3.2) | ND | [42] |
| West Java | Cianjur, Bogor, Tangerang, Bandung, Subang. | 2016 | Brahman cross | ELISA Ab (IDEXX, NS) | 100 0 | 63.0% | Biosecurity 1 RR: 3.3 (CI: 1.4–8.0) Manure management 2 RR:2.7 (CI: 1.1–6.4) | ND | [39] |
| West Java | Cianjur, Bogor, Bandung, Subang | 2015 | Brahman cross | ELISA Ab (IDEXX-BGVV B233) | 474 | 67.5% | ND | ND | [40] |
| West Java | Livestock Embryo Center, Bogor | 2019 | FH, Ongole, Limousine, Simental, Angus, Wagyu | ELISA Ab (IDEXX, NS) | 43 3 | 48.8% | ND | ND | [43] |
| West Java | West Bandung, Bogor | 2019–2020 | NS | ELISA Ab (IDEXX BVDV Total Ab Test, Switzerland) | 47 | 46.8% | ND | ND | [65] |
| Banten | Legok, Tangerang | 2013 | Brahman cross | ELISA Ab A (IDEXX-BGVVB233) | 230 | 75.2% | ND | ND | [41] |
| Java | National Artificial Insemination Center | 2011 | NS | NS | 110 | 37.3% | ND | ND | [67] |
| Java | NS | 2018 | FH | RT-PCR (5′UTR) | 200 4 | 6% 5 | ND | ND | [49] |
| Lampung | NS | 2019–2020 | NS | ELISA Ab (IDEXX BVDV Total Ab Test, Switzerland) | 18 | 11.0% | ND | ND | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugroho, W.; Silitonga, R.J.P.; Reichel, M.P.; Irianingsih, S.H.; Wicaksono, M.S. The Epidemiology and Control of Bovine Viral Diarrhoea Virus in Tropical Indonesian Cattle. Pathogens 2022, 11, 215. https://doi.org/10.3390/pathogens11020215
Nugroho W, Silitonga RJP, Reichel MP, Irianingsih SH, Wicaksono MS. The Epidemiology and Control of Bovine Viral Diarrhoea Virus in Tropical Indonesian Cattle. Pathogens. 2022; 11(2):215. https://doi.org/10.3390/pathogens11020215
Chicago/Turabian StyleNugroho, Widi, Risma Juniarti Paulina Silitonga, Michael Philipp Reichel, Sri Handayani Irianingsih, and Muhammad Satryo Wicaksono. 2022. "The Epidemiology and Control of Bovine Viral Diarrhoea Virus in Tropical Indonesian Cattle" Pathogens 11, no. 2: 215. https://doi.org/10.3390/pathogens11020215
APA StyleNugroho, W., Silitonga, R. J. P., Reichel, M. P., Irianingsih, S. H., & Wicaksono, M. S. (2022). The Epidemiology and Control of Bovine Viral Diarrhoea Virus in Tropical Indonesian Cattle. Pathogens, 11(2), 215. https://doi.org/10.3390/pathogens11020215







