First Report of Anuran Trypanosoma DNA in Flat-Tailed House Geckos (Reptilia: Gekkonidae) Collected from Southern Thailand: No Evidence as a Reservoir for Human Trypanosomatids
Abstract
:1. Introduction
2. Results
2.1. Molecular Detection of Trypanosoma and Leishmania DNA from Dissected Organ Specimens
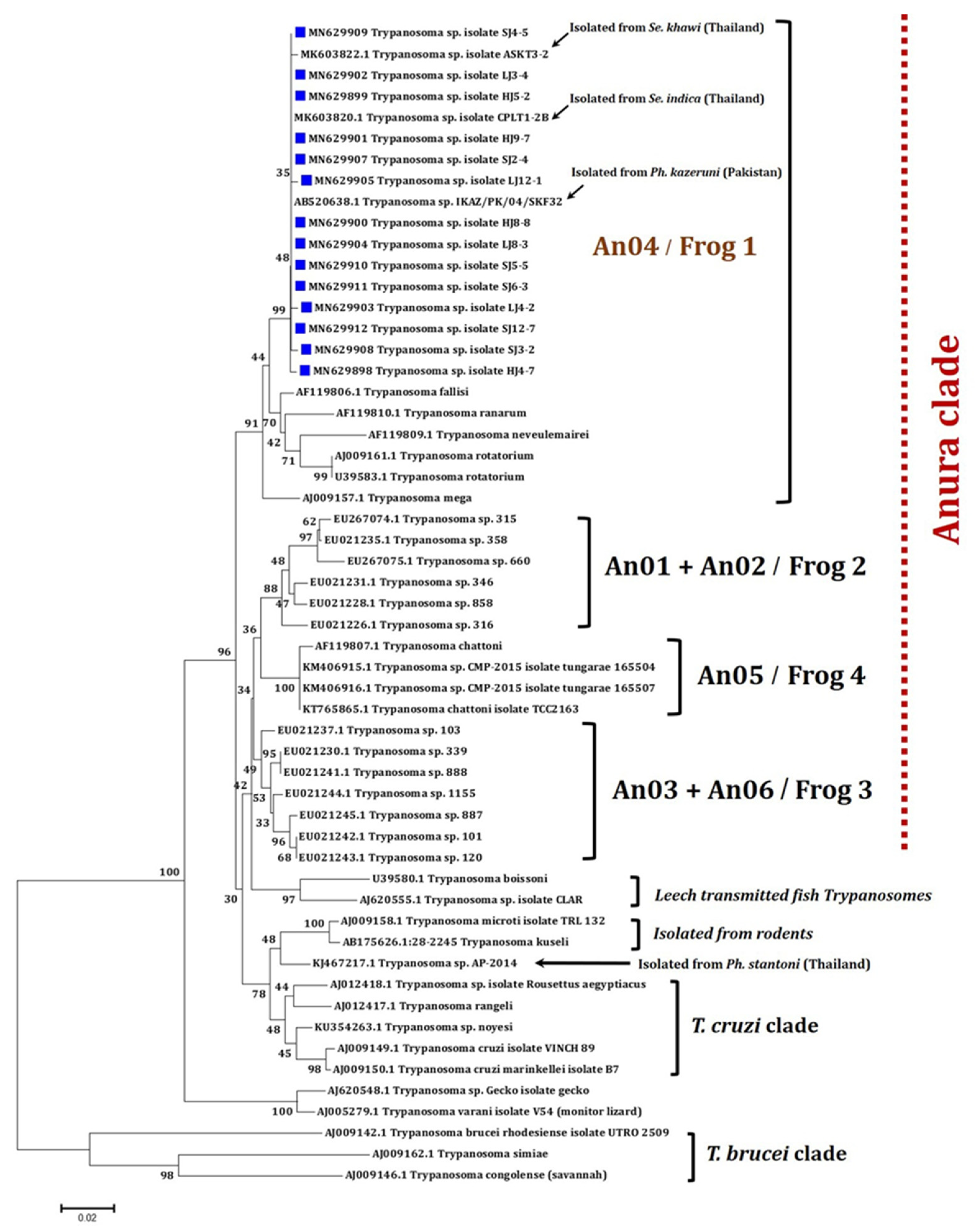
2.2. Nucleotide BLAST Analysis and Phylogenetic Tree Construction
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Gecko Collection and Visceral Organ Dissection
4.3. Genomic DNA Extraction
4.4. Detection of Trypanosoma and Leishmania DNA in Flat-Tailed House Geckos
4.5. Molecular Identification of Flat-Tailed House Geckos
4.6. TA Cloning and Sanger Sequencing
4.7. Phylogenetic Tree Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nussbaum, K.; Honek, J.; Cadmus, C.M.; Efferth, T. Trypanosomatid parasites causing neglected diseases. Curr. Med. Chem. 2010, 17, 1594–1617. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.G.; Stevens, J.R.; Lukes, J. The evolution and diversity of kinetoplastid flagellates. Trends Parasitol. 2006, 22, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Magri, A.; Galuppi, R.; Fioravanti, M. Autochthonous Trypanosoma spp. in European Mammals: A Brief Journey amongst the Neglected Trypanosomes. Pathogens 2021, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Lukeš, J.; Butenko, A.; Hashimi, H.; Maslov, D.A.; Votýpka, J.; Yurchenko, V. Trypanosomatids Are Much More than Just Trypanosomes: Clues from the Expanded Family Tree. Trends Parasitol. 2018, 34, 466–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunantaraporn, S.; Thepparat, A.; Phumee, A.; Sor-Suwan, S.; Boonserm, R.; Bellis, G.; Siriyasatien, P. Culicoides Latreille (Diptera: Ceratopogonidae) as potential vectors for Leishmania martiniquensis and Trypanosoma sp. in northern Thailand. PLoS Negl. Trop. Dis. 2021, 15, e0010014. [Google Scholar] [CrossRef]
- Chiewchanvit, S.; Tovanabutra, N.; Jariyapan, N.; Bates, M.D.; Mahanupab, P.; Chuamanochan, M.; Tantiworawit, A.; Bates, P.A. Chronic generalized fibrotic skin lesions from disseminated leishmaniasis caused by Leishmania martiniquensis in two patients from northern Thailand infected with HIV. Br. J. Dermatol. 2015, 173, 663–670. [Google Scholar] [CrossRef]
- Jariyapan, N.; Daroontum, T.; Jaiwong, K.; Chanmol, W.; Intakhan, N.; Sor-Suwan, S.; Siriyasatien, P.; Somboon, P.; Bates, M.D.; Bates, P.A. Leishmania (Mundinia) orientalis n. sp. (Trypanosomatidae), a parasite from Thailand responsible for localised cutaneous leishmaniasis. Parasites Vectors 2018, 11, 351. [Google Scholar] [CrossRef]
- Leelayoova, S.; Siripattanapipong, S.; Manomat, J.; Piyaraj, P.; Tan-Ariya, P.; Bualert, L.; Mungthin, M. Leishmaniasis in Thailand: A Review of Causative Agents and Situations. Am. J. Trop. Med. Hyg. 2017, 96, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Ugochukwu, E.I. Animal trypanosomiasis in Africa: Aetiology and Epidemiology. Anim. Res. Int. 2008, 5, 811–815. [Google Scholar] [CrossRef]
- Truc, P.; Büscher, P.; Cuny, G.; Gonzatti, M.I.; Jannin, J.; Joshi, P.; Juyal, P.; Lun, Z.R.; Mattioli, R.; Pays, E.; et al. Atypical human infections by animal trypanosomes. PLoS Negl. Trop. Dis. 2013, 7, e2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deborggraeve, S.; Koffi, M.; Jamonneau, V.; Bonsu, F.A.; Queyson, R.; Simarro, P.P.; Herdewijn, P.; Büscher, P. Molecular analysis of archived blood slides reveals an atypical human Trypanosoma infection. Diagn. Microbiol. Infect. Dis. 2008, 61, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Sarataphan, N.; Vongpakorn, M.; Nuansrichay, B.; Autarkool, N.; Keowkarnkah, T.; Rodtian, P.; Stich, R.W.; Jittapalapong, S. Diagnosis of a Trypanosoma lewisi-like (Herpetosoma) infection in a sick infant from Thailand. J. Med. Microbiol. 2007, 56, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roldan, J.A.; Modry, D.; Otranto, D. Zoonotic Parasites of Reptiles: A Crawling Threat. Trends Parasitol. 2020, 36, 677–687. [Google Scholar] [CrossRef]
- Njagu, Z.; Mihok, S.; Kokwaro, E.; Verloo, D. Isolation of Trypanosoma brucei from the monitor lizard (Varanus niloticus) in an endemic focus of rhodesian sleeping sickness in Kenya. Acta Trop. 1999, 72, 137–148. [Google Scholar] [CrossRef]
- Waiswa, C.; Picozzi, K.; Olaho-Mukani, W.; Katunguka-Rwakishaya, E. Monitor lizard (Varanus niloticus, Linnaeus, 1766) as a host for tsetse (Glossina fuscipes fuscipes, Newstead, 1910) in the sleeping sickness endemic foci of Uganda. Afr. J. Ecol. 2003, 41, 349–351. [Google Scholar] [CrossRef]
- Zhang, J.R.; Guo, X.G.; Liu, J.L.; Zhou, T.H.; Gong, X.; Chen, D.L.; Chen, J.P. Molecular detection, identification and phylogenetic inference of Leishmania spp. in some desert lizards from Northwest China by using internal transcribed spacer 1 (ITS1) sequences. Acta Trop. 2016, 162, 83–94. [Google Scholar] [CrossRef]
- Zhang, J.R.; Guo, X.G.; Chen, H.; Liu, J.L.; Gong, X.; Chen, D.L.; Chen, J.P. Pathogenic Leishmania spp. detected in lizards from Northwest China using molecular methods. BMC Vet. Res. 2019, 15, 446. [Google Scholar] [CrossRef] [Green Version]
- Golding, N. Review of “Trypanosomes and Trypanosomiasis” by Stefan Magez and Magdalena Radwanska (Editors). Parasites Vectors 2013, 6, 365. [Google Scholar] [CrossRef] [Green Version]
- Pumhom, P.; Pongon, D.; Yanhtara, S.; Thaprathorn, N.; Milocco, C.; Douangboupha, B. Molecular prevalence of Trypanosoma spp. in wild rodents of Southeast Asia: Influence of human settlement habitat. Epidemiol. Infect. 2013, 142, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Njiokou, F.; Nimpaye, H.; Simo, G.; Njitchouang, G.R.; Asonganyi, T.; Cuny, G.; Herder, S. Domestic animals as potential reservoir hosts of Trypanosoma brucei gambiense in sleeping sickness foci in Cameroon. Parasite 2010, 17, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vourchakbé, J.; Tiofack, Z.; Kante, T.S.; Mpoame, M.; Simo, G. Molecular identification of Trypanosoma brucei gambiense in naturally infected pigs, dogs and small ruminants confirms domestic animals as potential reservoirs for sleeping sickness in Chad. Parasite 2020, 27, 63. [Google Scholar] [CrossRef] [PubMed]
- Rosal, G.G.; Nogueda-Torres, B.; Villagrán, M.E.; de Diego-Cabrera, J.A.; Montañez-Valdez, O.D.; Martínez-Ibarra, J.A. Chagas disease: Importance of rats as reservoir hosts of Trypanosoma cruzi (Chagas, 1909) in western Mexico. J. Infect. Public Health 2018, 11, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, J.; Zhang, J.; Guo, X.; Liu, J.; He, J.; Song, Q.; Zhang, J.; Chen, M.; Zheng, Z.; et al. Multi-locus characterization and phylogenetic inference of Leishmania spp. in snakes from Northwest China. PLoS ONE 2019, 14, e0210681. [Google Scholar] [CrossRef] [Green Version]
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Health Sci. J. 2017, 4, 519–524. [Google Scholar] [CrossRef]
- Abbate, J.M.; Maia, C.; Pereira, A.; Arfuso, F.; Gaglio, G.; Rizzo, M.; Caracappa, G.; Marino, G.; Pollmeier, M.; Giannetto, S.; et al. Identification of trypanosomatids and blood feeding preferences of phlebotomine sand fly species common in Sicily, Southern Italy. PLoS ONE 2020, 15, e0229536. [Google Scholar] [CrossRef] [Green Version]
- Chusri, S.; Hortiwakul, T.; Silpapojakul, K.; Siriyasatien, P. Case Report: Consecutive cutaneous and visceral leishmaniasis manifestations involving a novel Leishmania species in two HIV patients in Thailand. Am. J. Trop. Med. Hyg. 2012, 87, 76–80. [Google Scholar] [CrossRef]
- Phumee, A.; Chusri, S.; Kraivichian, K.; Wititsuwannakul, J.; Hortiwakul, T.; Thavara, U.; Silpapojakul, K.; Siriyasatien, P. Multiple Cutaneous Nodules in an HIV-Infected Patient. PLoS Negl. Trop. Dis. 2014, 8, e3291. [Google Scholar] [CrossRef] [Green Version]
- Phumee, A.; Tawatsin, A.; Thavara, U.; Pengsakul, T.; Thammapalo, S.; Depaquit, J.; Gay, F.; Siriyasatien, P. Detection of an Unknown Trypanosoma DNA in a Phlebotomus stantoni (Diptera: Psychodidae) Collected from Southern Thailand and Records of New Sand Flies With Reinstatement of Sergentomyia hivernus Raynal & Gaschen, 1935 (Diptera: Psychodidae). J. Med. Entomol. 2017, 54, 429–434. [Google Scholar] [CrossRef]
- Srisuton, P.; Phumee, A.; Sunantaraporn, S.; Boonserm, R.; Sor-Suwan, S.; Brownell, N.; Pengsakul, T.; Siriyasatien, P. Detection of Leishmania and Trypanosoma DNA in Field-Caught Sand Flies from Endemic and Non-Endemic Areas of Leishmaniasis in Southern Thailand. Insects 2019, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Silva Pereira, S.; Trindade, S.; De Niz, M.; Figueiredo, L.M. Tissue tropism in parasitic diseases. Open Biol. 2019, 9, 190036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuntasuvan, D.; Sarataphan, N.; Nishikawa, H. Cerebral trypanosomiasis in native cattle. Vet. Parasitol. 1997, 73, 357–363. [Google Scholar] [CrossRef]
- Sudarto, M.W.; Tabel, H.; Haines, D.M. Immunohistochemical demonstration of Trypanosoma evansi in tissues of experimentally infected rats and a naturally infected water buffalo (Bubalus bubalis). J. Parasitol. 1990, 76, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Tuntasuvan, D.; Mimapan, S.; Sarataphan, N.; Trongwongsa, L.; Intraraksa, R.; Luckins, A.G. Detection of Trypanosoma evansi in brains of the naturally infected hog deer by streptavidine-biotin immunohistochemistry. Vet. Parasitol. 2000, 87, 223–230. [Google Scholar] [CrossRef]
- Kato, H.; Uezato, H.; Sato, H.; Bhutto, A.M.; Soomro, F.R.; Baloch, J.H.; Iwata, H.; Hashiguchi, Y. Natural infection of the sand fly Phlebotomus kazeruni by Trypanosoma species in Pakistan. Parasites Vectors 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da S Ferreira, J.I.G.; da Costa, A.P.; Ramirez, D.; Roldan, J.A.M.; Saraiva, D.; Founier, G.F.R.D.S.; Sue, A.; Zambelli, E.R.; Minervino, A.; Verdade, V.K.; et al. Anuran trypanosomes: Phylogenetic evidence for new clades in Brazil. Syst. Parasitol. 2015, 91, 63–70. [Google Scholar] [CrossRef]
- Chusri, S.; Thammapalo, S.; Chusri, S.; Thammapalo, S.; Silpapojakul, K.; Siriyasatien, P. Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. Southeast Asian J. Trop. Med. Public Health 2014, 45, 13–19. [Google Scholar]
- Müller, N.; Welle, M.; Lobsiger, L.; Stoffel, M.H.; Boghenbor, K.K.; Hilbe, M.; Gottstein, B.; Frey, C.F.; Geyer, C.; von Bomhard, W. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet. Parasitol. 2009, 166, 346–351. [Google Scholar] [CrossRef]
- Reuss, S.M.; Dunbar, M.D.; Calderwood Mays, M.B.; Owen, J.L.; Mallicote, M.F.; Archer, L.L.; Wellehan, J.F., Jr. Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg. Infect. Dis. 2012, 18, 1545–1547. [Google Scholar] [CrossRef]
- Lobsiger, L.; Müller, N.; Schweizer, T.; Frey, C.F.; Wiederkehr, D.; Zumkehr, B.; Gottstein, B. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet. Parasitol. 2010, 169, 408–414. [Google Scholar] [CrossRef]
- Sriwongpan, P.; Nedsuwan, S.; Manomat, J.; Charoensakulchai, S.; Lacharojana, K.; Sankwan, J.; Kobpungton, N.; Sriwongpun, T.; Leelayoova, S.; Mungthin, M.; et al. Prevalence and associated risk factors of Leishmania infection among immunocompetent hosts, a community-based study in Chiang Rai, Thailand. PLoS Negl. Trop. Dis. 2021, 15, e0009545. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Clode, P.L.; Peacock, C.; Thompson, R.C. Host-Parasite Relationships and Life Histories of Trypanosomes in Australia. Adv. Parasitol. 2017, 97, 47–109. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; De Souza, A.A.; Freitas, R.A.; Campaner, M.; Takata, C.S.; Barrett, T.V.; Shaw, J.J.; Teixeira, M.M. A phylogenetic lineage of closely related trypanosomes (Trypanosomatidae, Kinetoplastida) of anurans and sand flies (Psychodidae, Diptera) sharing the same ecotopes in brazilian amazonia. J. Eukaryot. Microbiol. 2008, 55, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; Morais, D.H.; Carvalho, V.T.; D’Agosto, M. First record of Trypanosoma chattoni in Brazil and occurrence of other Trypanosoma species in Brazilian frogs (Anura, Leptodactylidae). J. Parasitol. 2008, 94, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Viola, L.B.; Campaner, M.; Takata, C.S.; Ferreira, R.C.; Rodrigues, A.C.; Freitas, R.A.; Duarte, M.R.; Grego, K.F.; Barrett, T.V.; Camargo, E.P.; et al. Phylogeny of snake trypanosomes inferred by SSU rDNA sequences, their possible transmission by phlebotomines, and taxonomic appraisal by molecular, cross-infection and morphological analysis. Parasitology 2008, 135, 595–605. [Google Scholar] [CrossRef]
- Kanjanopas, K.; Siripattanapipong, S.; Ninsaeng, U.; Hitakarun, A.; Jitkaew, S.; Kaewtaphaya, P.; Tan-Ariya, P.; Mungthin, M.; Charoenwong, C.; Leelayoova, S. Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infect. Dis. 2013, 13, 333. [Google Scholar] [CrossRef] [Green Version]
- Siripattanapipong, S.; Leelayoova, S.; Ninsaeng, U.; Mungthin, M. Detection of DNA of Leishmania siamensis in Sergentomyia (Neophlebotomus) iyengari (Diptera: Psychodidae) and Molecular Identification of Blood Meals of Sand Flies in an Affected Area, Southern Thailand. J. Med. Entomol. 2018, 55, 1277–1283. [Google Scholar] [CrossRef]
- Hysek, J.; Zizka, Z. Transmission of Trypanosoma rotatorium from frogs to white mice. Nature 1976, 260, 608–609. [Google Scholar] [CrossRef]
- Noyes, H.A.; Stevens, J.R.; Teixeira, M.; Phelan, J.; Holz, P. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999, 29, 331–339. [Google Scholar] [CrossRef]
- Spanakos, G.; Piperaki, E.T.; Menounos, P.G.; Tegos, N.; Flemetakis, A.; Vakalis, N.C. Detection and species identification of Old World Leishmania in clinical samples using a PCR-based method. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 46–53. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Pääbo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carranza, S.; Arnold, E.N. Systematics, biogeography, and evolution of Hemidactylus geckos (Reptilia: Gekkonidae) elucidated using mitochondrial DNA sequences. Mol. Phylogenetics Evol. 2006, 38, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]


| Isolate Code | SSU rRNA-PCR | Gecko Species Identification | ||
|---|---|---|---|---|
| Heart (H) | Liver (L) | Spleen (S) | ||
| J1 | − | − | − | Hemidactylus platyurus |
| J2 | − | − | + | Hemidactylus platyurus |
| J3 | − | + | + | Hemidactylus platyurus |
| J4 | + | + | + | Hemidactylus platyurus |
| J5 | + | − | + | Hemidactylus platyurus |
| J6 | − | − | + | Hemidactylus platyurus |
| J7 | − | − | − | Hemidactylus platyurus |
| J8 | + | + | − | Hemidactylus platyurus |
| J9 | + | − | − | Hemidactylus platyurus |
| J10 | − | − | − | Hemidactylus platyurus |
| J11 | − | − | − | Hemidactylus platyurus |
| J12 | − | + | + | Hemidactylus platyurus |
| J13 | − | − | − | Hemidactylus platyurus |
| J14 | − | − | − | Hemidactylus platyurus |
| J15 | − | − | − | Hemidactylus platyurus |
| J16 | − | − | − | Hemidactylus platyurus |
| J17 | − | − | − | Hemidactylus platyurus |
| J18 | − | − | − | Hemidactylus platyurus |
| J19 | − | − | − | Hemidactylus platyurus |
| Total | 4 | 4 | 6 | |
| 14 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toontong, P.; Sunantaraporn, S.; Tiawsirisup, S.; Pengsakul, T.; Boonserm, R.; Phumee, A.; Siriyasatien, P.; Preativatanyou, K. First Report of Anuran Trypanosoma DNA in Flat-Tailed House Geckos (Reptilia: Gekkonidae) Collected from Southern Thailand: No Evidence as a Reservoir for Human Trypanosomatids. Pathogens 2022, 11, 247. https://doi.org/10.3390/pathogens11020247
Toontong P, Sunantaraporn S, Tiawsirisup S, Pengsakul T, Boonserm R, Phumee A, Siriyasatien P, Preativatanyou K. First Report of Anuran Trypanosoma DNA in Flat-Tailed House Geckos (Reptilia: Gekkonidae) Collected from Southern Thailand: No Evidence as a Reservoir for Human Trypanosomatids. Pathogens. 2022; 11(2):247. https://doi.org/10.3390/pathogens11020247
Chicago/Turabian StyleToontong, Prapimporn, Sakone Sunantaraporn, Sonthaya Tiawsirisup, Theerakamol Pengsakul, Rungfar Boonserm, Atchara Phumee, Padet Siriyasatien, and Kanok Preativatanyou. 2022. "First Report of Anuran Trypanosoma DNA in Flat-Tailed House Geckos (Reptilia: Gekkonidae) Collected from Southern Thailand: No Evidence as a Reservoir for Human Trypanosomatids" Pathogens 11, no. 2: 247. https://doi.org/10.3390/pathogens11020247










