Concentrations of PGE2 and TXB2 in the Eyes of Mice with Disseminated Acanthamoebiasis
Abstract
:1. Introduction
2. Results
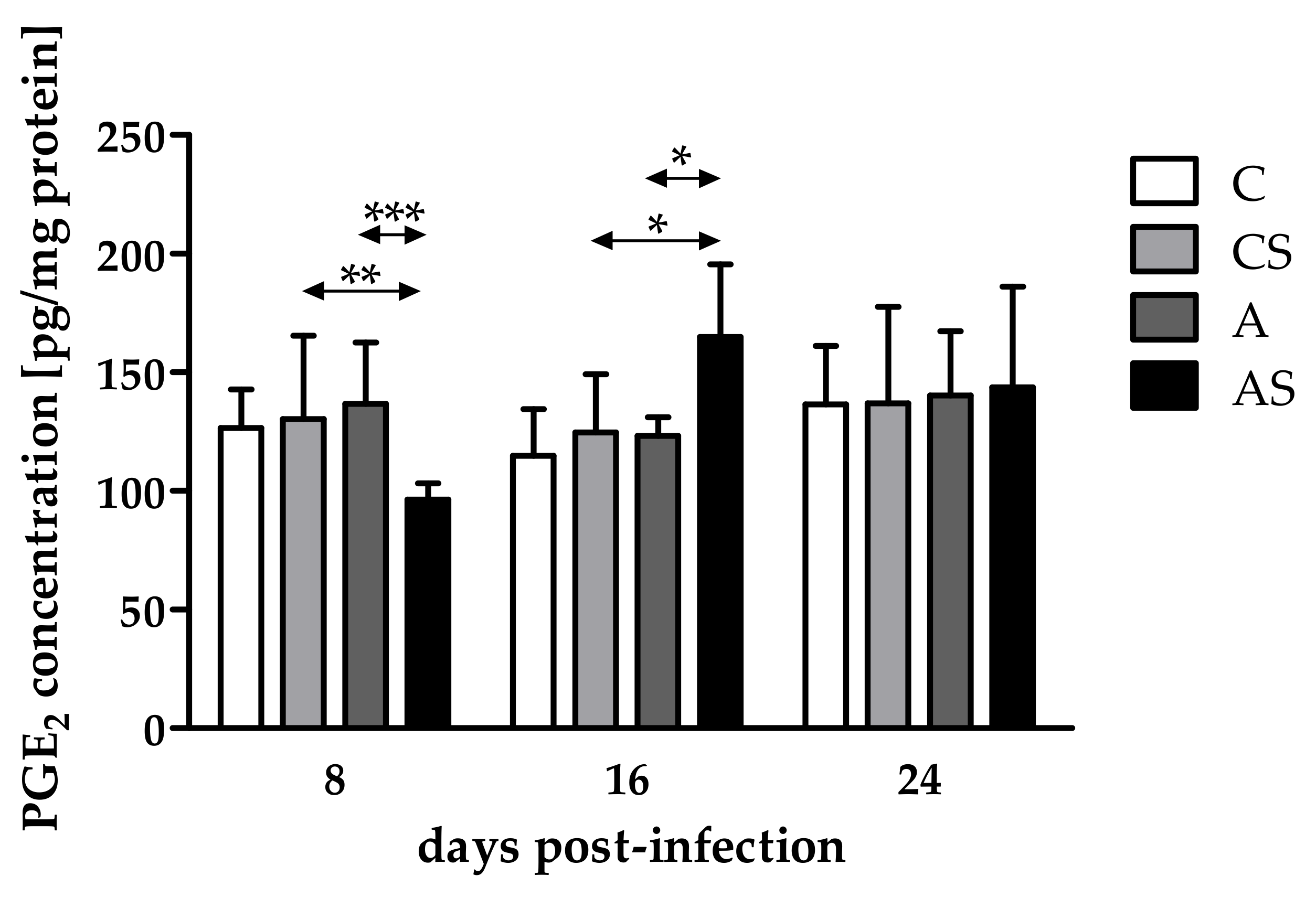
2.1. Concentration of PGE2
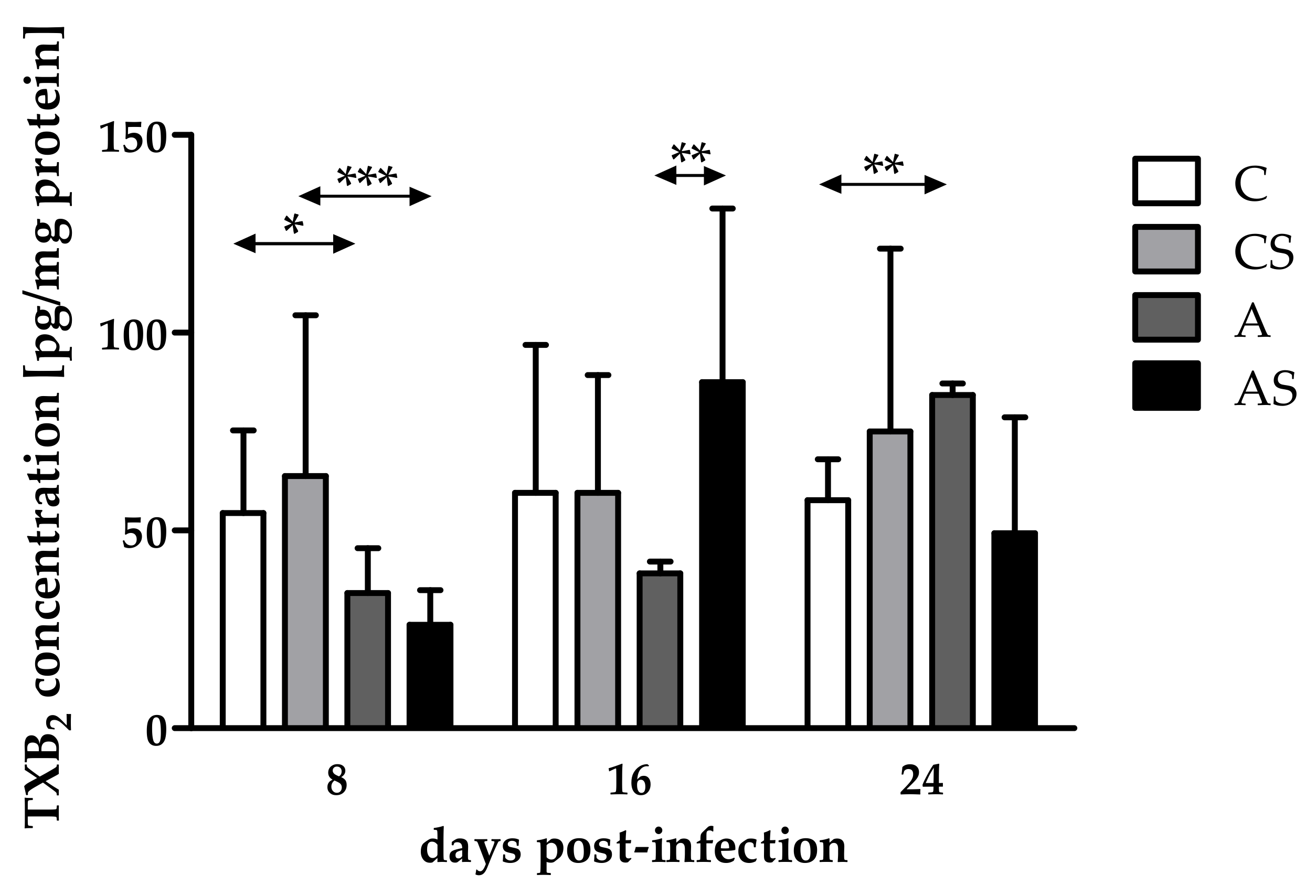
2.2. Concentration of TXB2
3. Discussion
4. Materials and Methods
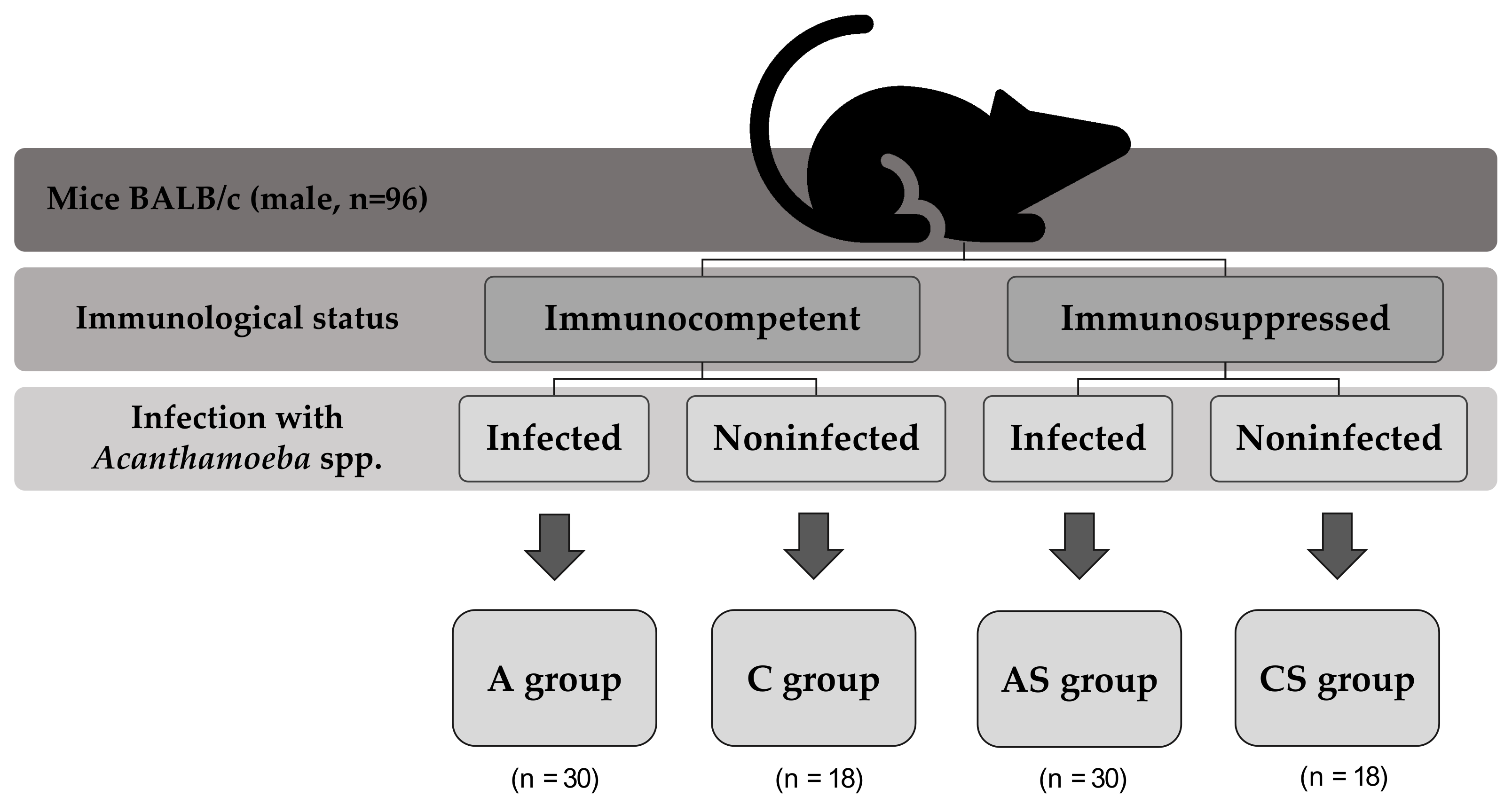
4.1. Animal Model
4.2. Homogenization
4.3. Total Protein Concentration
4.4. PGE2 and TXB2 Concentrations
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Król-Turmińska, K.; Olender, A. Human infections caused by free-living amoebae. Ann. Agric. Environ. Med. 2017, 24, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.R.; Adwani, S.; Mahadevan, A. Acanthamoeba meningoencephalitis. Ann. Indian Acad. Neurol. 2014, 17, 108–112. [Google Scholar] [CrossRef]
- Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Wojtkowiak-Giera, A.; Kolasa-Wołosiuk, A. Expression of toll-like receptors (TLR2 and TLR4) in the eyes of mice with disseminated acanthamoebiasis. Biomed. Res. Int. 2019, 2019, 1401894. [Google Scholar] [CrossRef] [Green Version]
- Kot, K.; Kosik-Bogacka, D.; Kupnicka, P.; Łanocha-Arendarczyk, N. Antioxidant defense in the eyes of immunocompetent and immunosuppressed mice infected with Acanthamoeba spp. Parasites Vectors 2020, 13, 123. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Cheng, C.K.; Zhang, C.L.; Huang, Y. Interplay between oxidative stress, cyclooxygenases, and prostanoids in cardiovascular diseases. Antioxid. Redox Signal. 2021, 34, 784–799. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Cyclooxygenase pathways. Acta Biochim. Pol. 2014, 61, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Liclican, E.L.; Nguyen, V.; Sullivan, A.B.; Gronert, K. Selective activation of the prostaglandin E2 circuit in chronic injury-induced pathologic angiogenesis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6311–6320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doucette, L.P.; Walter, M.A. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Lucotti, S.; Cerutti, C.; Soyer, M.; Bernabé, A.M.J.; Gomes, A.L.; Allen, P.D.; Smart, S.; Markelc, B.; Watson, K.; Armstrong, P.C.; et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A2. J. Clin. Investig. 2019, 129, 1845–1862. [Google Scholar] [CrossRef] [Green Version]
- Cartier, A.; Parent, A.; Labrecque, P.; Laroche, G.; Parent, J.L. WDR36 acts as a scaffold protein tethering a G-protein-coupled receptor, Gαq and phospholipase Cβ in a signalling complex. J. Cell Sci. 2011, 124, 3292–3304. [Google Scholar] [CrossRef]
- Lonardoni, M.V.; Russo, M.; Jancar, S. Essential role of platelet-activating factor in control of Leishmania (Leishmania) amazonensis infection. Infect. Immun. 2000, 68, 6355–6361. [Google Scholar] [CrossRef]
- Lejeune, M.; Moreau, F.; Chadee, K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am. J. Pathol. 2011, 179, 807–818. [Google Scholar] [CrossRef]
- Abdalla, G.K.; Faria, G.E.; Silva, K.T.; Castro, E.C.; Reis, M.A.; Michelin, M.A. Trypanosoma cruzi: The role of PGE2 in immune response during the acute phase of experimental infection. Exp. Parasitol. 2008, 118, 514–521. [Google Scholar] [CrossRef]
- Anyona, S.B.; Kempaiah, P.; Davenport, G.C.; Vulule, J.M.; Hittner, J.B.; Ong’echa, J.M.; Perkins, D.J. Suppressed circulating bicyclo-PGE2 levels and leukocyte COX-2 transcripts in children co-infected with P. falciparum malaria and HIV-1 or bacteremia. Biochem. Biophys. Res. Commun. 2013, 436, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Łanocha-Arendarczyk, N.; Baranowska-Bosiacka, I.; Kot, K.; Gutowska, I.; Kolasa-Wołosiuk, A.; Chlubek, D.; Kosik-Bogacka, D. Expression and activity of COX-1 and COX-2 in Acanthamoeba sp.-infected lungs according to the host immunological status. Int. J. Mol. Sci. 2018, 19, 121. [Google Scholar] [CrossRef] [Green Version]
- Fu, N.X.; Song, J.; Huang, X.; Lin, G.H. Granulomatous amoebic encephalitis presenting as a solitary mass lesion. Radiol. Infect. Dis. 2020, 7, 204–207. [Google Scholar] [CrossRef]
- Paudel, A.C.; Patel, N.; Quang, J.; Casella, C.; Sigal, A.; Parajuli, P.; Oladunjove, O.; Oke, I.O.; Khanal, S.; Bhattari, K. Rapidly progressive granulomatous amoebic encephalitis in a diabetic individual. Cureus 2021, 13, e19336. [Google Scholar] [CrossRef]
- Akpek, G.; Uslu, A.; Huebner, T.; Taner, A.; Rapoport, A.P.; Gojo, I.; Akpolat, Y.T.; Loffe, O.; Kleinberg, M.; Baer, M.R. Granulomatous amebic encephalitis: An under-recognized cause of infectious mortality after hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2011, 13, 366–373. [Google Scholar] [CrossRef]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp. Parasitol. 2020, 208, 107788. [Google Scholar] [CrossRef]
- Schoenberger, S.D.; Kim, S.J.; Sheng, J.; Rezaei, K.A.; Lalezary, M.; Cherney, E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest. Ophthalmol. Vis. Sci. 2012, 53, 5906–5911. [Google Scholar] [CrossRef] [Green Version]
- Yamanishi, R.; Okada, N.; Shimizu, E.; Fujishima, H. Elevated levels of prostaglandin E2 in the tears of patients with severe allergic conjunctivitis and primary cultured conjunctival cells are suppressed by ketotifen and dexamethasone. BMJ Open Ophthalmol. 2021, 6, e000571. [Google Scholar] [CrossRef]
- Toris, C.B.; Gulati, V. The biology, pathology and therapeutic use of prostaglandins in the eye. Clin. Lipidol. 2011, 6, 577–591. [Google Scholar] [CrossRef]
- Kim, S.J.; Flach, A.J.; Jampol, L.M. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv. Ophthalmol. 2010, 55, 108–133. [Google Scholar] [CrossRef]
- Ambaw, Y.A.; Chao, C.; Ji, S.; Raida, M.; Torta, F.; Wenk, M.R.; Tong, L. Tear eicosanoids in healthy people and ocular surface disease. Sci. Rep. 2018, 8, 11296. [Google Scholar] [CrossRef]
- Kallinikos, P.; Efron, N. On the etiology of keratocyte loss during contact lens wear. Invest. Ophthalmol. Vis. Sci. 2004, 45, 3011–3020. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; Fitz-Gerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Johnson, E.I.; Dunlop, M.E.; Larkins, R.G. Increased vasodilatory prostaglandin production in the diabetic rat retinal vasculature. Curr. Eye Res. 1999, 18, 79–82. [Google Scholar] [CrossRef]
- Amrite, A.C.; Ayalasomayajula, S.P.; Cheruvu, N.P.; Kompella, U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekhanont, K.; Sathianvichitr, K.; Pisitpayat, P.; Anothaisintawee, T.; Soontrapa, K.; Udomsubpayakul, U. Association between the levels of prostaglandin E2 in tears and severity of dry eye. Int. J. Ophthalmol. 2019, 12, 1127–1133. [Google Scholar] [CrossRef]
- Greigert, V.; Bittich-Fahmi, F.; Pfaff, A.W. Pathophysiology of ocular toxoplasmosis: Facts and open questions. PLoS Negl. Trop. Dis. 2020, 14, e0008905. [Google Scholar] [CrossRef] [PubMed]
- Sander, W.J.; O’Neill, H.G.; Pohl, C.H. Prostaglandin E2 As a Modulator of Viral Infections. Front Physiol. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degraaf, A.J.; Zasłona, Z.; Bourdonnay, E.; Peters-Golden, M. Prostaglandin E2 reduces Toll-like receptor 4 expression in alveolar macrophages by inhibition of translation. Am. J. Respir. Cell Mol. Biol. 2014, 51, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, J.; Yang, X.; Han, X. Several transcription factors regulate COX-2 gene expression in pancreatic beta-cells. Mol. Biol. Rep. 2007, 34, 199–206. [Google Scholar] [CrossRef]
- Hu, Y.P.; Peng, Y.B.; Zhang, Y.F.; Wang, Y.; Yu, W.R.; Yao, M.; Fu, X.J. Reactive Oxygen Species Mediated Prostaglandin E2 Contributes to Acute Response of Epithelial Injury. Oxid. Med. Cell Longev. 2017, 2017, 4123854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, V.E.; Mackrell, P.J.; Concannon, E.M.; Naama, H.A.; Schaefer, P.A.; Shaftan, G.W.; Stapleton, P.P.; Daly, J.M. Blocking prostgalndin E2 after trauma attenuates pro-inflammatory cytokines and improves survivial. Shock 2000, 14, 374–379. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Xie, T.; Zhan, P.; Zou, J.; Nie, X.; Shao, J.; Zhuang, M.; Tan, C.; Tan, J.; et al. Prostaglandin E2/EP2 receptor signalling pathway promotes diabetic retinopathy in a rat model of diabetes. Diabetologia 2019, 62, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Fuller, R.W.; Keisey, C.R.; Cole, P.J.; Dollery, C.T.; MacDermot, J. Dexamethasone inhibits the production of thromboxane B2 and leukotriene B4 by human alveolar and peritoneal macrophages in culture. Clin. Sci. 1984, 67, 653–656. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, P.; Li, F.; Kijlstra, A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS ONE 2011, 6, e18139. [Google Scholar] [CrossRef] [Green Version]
- Sauer, A.; Pfaff, A.W.; Villard, O.; Creuzot-Garcher, C.; Dalle, F.; Chiquet, C.; Pelloux, H.; Speeg-Schatz, C.; Gaucher, D.; Prevost, G.; et al. Interleukin 17A as an effective target for anti-inflammatory and antiparasitic treatment of toxoplasmic uveitis. J. Infect. Dis. 2012, 206, 1319–1329. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, A.; Cao, Z.; Sampson, J.F.; Panjwani, N. IL-17A-mediated protection against Acanthamoeba keratitis. J. Immunol. 2015, 194, 650–663. [Google Scholar] [CrossRef] [Green Version]
- Łanocha-Arendarczyk, N.; Kolasa-Wołosiuk, A.; Wojciechowska-Koszko, I.; Kot, K.; Roszkowska, P.; Krasnodębska-Szponder, B.; Paczkowska, E.; Machaliński, B.; Łuczkowska, K.; Wiszniewska, B.; et al. Changes in the immune system in experimental acanthamoebiasis in immunocompetent and immunosuppressed hosts. Parasites Vectors 2018, 11, 517. [Google Scholar] [CrossRef]
- Östling, J.; van Geest, M.; Schofield, J.P.R.; Jevnikar, Z.; Wilson, S.; Ward, J.; Lutter, R.; Shaw, D.E.; Bakke, P.S.; Caruso, M.; et al. U-BIOPRED Study Group. IL-17-high asthma with features of a psoriasis immunophenotype. J. Allergy Clin. Immunol. 2019, 144, 1198–1213. [Google Scholar] [CrossRef] [Green Version]
- Boniface, K.; Bak-Jensen, K.S.; Li, Y.; Blumenschein, W.M.; McGeachy, M.J.; McClanahan, T.K.; McKenzie, B.S.; Kastelein, R.A.; Cua, D.J.; de Waal Malefyt, R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 2009, 206, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Dejani, N.N.; Orlando, A.B.; Niño, V.E.; Penteado, L.A.; Verdan, F.F.; Bazzano, J.M.R.; Codo, A.C.; Salina, A.C.G.; Saraiva, A.C.; Avelar, M.R.; et al. Intestinal host defense outcome is dictated by PGE2 production during efferocytosis of infected cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8469–E8478. [Google Scholar] [CrossRef] [Green Version]
- Górnik, K.; Kuźna-Grygiel, W. Histological studies of selected organs of mice experimentally infected with Acanthamoeba spp. Folia Morphol. 2005, 64, 161–167. [Google Scholar]
- Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Ptak, M.; Roszkowska, P.; Kram, A. Histological changes in the kidneys and heart in experimental acanthamoebiasis in immunocompetent and immunosuppressed hosts. Folia Biol. 2021, 4, 167–178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kot, K.; Kołodziej, D.; Kupnicka, P.; Kosik-Bogacka, D.I.; Łanocha-Arendarczyk, N. Concentrations of PGE2 and TXB2 in the Eyes of Mice with Disseminated Acanthamoebiasis. Pathogens 2022, 11, 438. https://doi.org/10.3390/pathogens11040438
Kot K, Kołodziej D, Kupnicka P, Kosik-Bogacka DI, Łanocha-Arendarczyk N. Concentrations of PGE2 and TXB2 in the Eyes of Mice with Disseminated Acanthamoebiasis. Pathogens. 2022; 11(4):438. https://doi.org/10.3390/pathogens11040438
Chicago/Turabian StyleKot, Karolina, Daria Kołodziej, Patrycja Kupnicka, Danuta Izabela Kosik-Bogacka, and Natalia Łanocha-Arendarczyk. 2022. "Concentrations of PGE2 and TXB2 in the Eyes of Mice with Disseminated Acanthamoebiasis" Pathogens 11, no. 4: 438. https://doi.org/10.3390/pathogens11040438
APA StyleKot, K., Kołodziej, D., Kupnicka, P., Kosik-Bogacka, D. I., & Łanocha-Arendarczyk, N. (2022). Concentrations of PGE2 and TXB2 in the Eyes of Mice with Disseminated Acanthamoebiasis. Pathogens, 11(4), 438. https://doi.org/10.3390/pathogens11040438






