Development of a Real-Time Recombinase Polymerase Amplification Assay for the Rapid Detection of African Swine Fever Virus Genotype I and II
Abstract
:1. Introduction
2. Results
2.1. Analytical Sensitivity of Real-Time RPA Assay
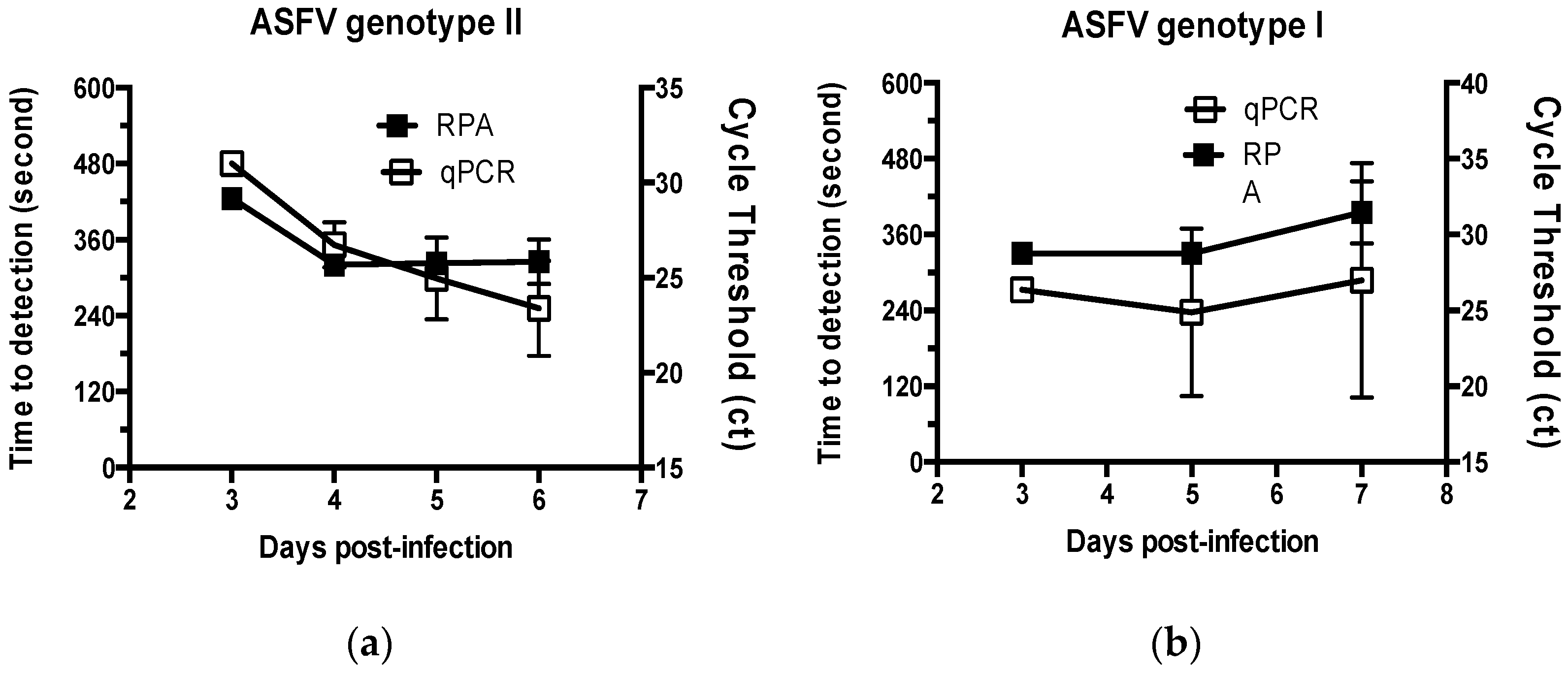
2.2. Performance of the Real-Time RPA Assay on ASFV Specimens
3. Discussion
4. Materials and Methods
4.1. ASFV DNA Specimens
4.2. Synthesis of Standard DNA Samples
4.3. Primers and Exo-Probes
4.4. Real-Time RPA Assay
4.5. qPCR
4.6. Performance Assessment of the Real-Time RPA Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Ar-bovirus. Front. Vet. Sci. 2020, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Sauter-Louis, C.; Conraths, F.J.; Probst, C.; Blohm, U.; Schulz, K.; Sehl, J.; Fischer, M.; Forth, J.H.; Zani, L.; Depner, K.; et al. African Swine Fever in Wild Boar in Europe—A Review. Viruses 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Li, J.; Fan, X.; Liu, F.; Li, L.; Wang, Q.; Ren, W.; Bao, J.; Liu, C.; Wang, H.; et al. Molecular Characterization of African Swine Fever Virus, China, 2018. Emerg. Infect. Dis. 2018, 24, 2131–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Yu, E.Y.W.; Wang, S.; Sun, C. African swine fever epidemic in China. Veter. Rec. 2019, 184, 713. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, H.; Lee, J.Y.; Yoo, H.S. African swine fever: Etiology, epidemiological status in Korea, and perspective on control. J. Veter. Sci. 2020, 21, e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Kim, J.; Son, K.; Choi, Y.; Jeong, H.-S.; Kim, Y.-K.; Park, J.-E.; Hong, Y.-J.; Lee, S.-I.; Wang, S.-J.; et al. Wild boar harbouring African swine fever virus in the demilitarized zone in South Korea, 2019. Emerg. Microbes Infect. 2020, 9, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Fernández-Pinero, J.; Arias, M. African swine fever (ASF) diagnosis, an essential tool in the epidemiological in-vestigation. Virus Res. 2019, 271, 197676. [Google Scholar] [CrossRef] [PubMed]
- Piepenburg, O.; Williams, C.H.; Stemple, D.; Armes, N.A. DNA Detection Using Recombination Proteins. PLOS Biol. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Macdonald, J. Recombinase polymerase amplification: Emergence as a critical molecular technology for rapid, low-resource diagnostics. Expert Rev. Mol. Diagn. 2015, 15, 1475–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Sun, A.; Wan, B.; Du, Y.; Wu, Y.; Zhang, A.; Jiang, D.; Ji, P.; Wei, Z.; Zhuang, G.; et al. Development of a Directly Visualized Recombinase Polymerase Amplification–SYBR Green I Method for the Rapid Detection of African Swine Fever Virus. Front. Microbiol. 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Zhang, J.; Li, N.; Chen, T.; Wang, L.; Zhang, F.; Mi, L.; Zhang, J.; Wang, S.; Wang, Y.; et al. Rapid and Sensitive Recombinase Polymerase Amplification Combined with Lateral Flow Strip for Detecting African Swine Fever Virus. Front. Microbiol. 2019, 15, 1004. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, L.; Zhao, Y.; Liu, Y.; Liu, C.; Wang, Q.; Dong, Y.; Wang, S.; Chi, T.; Song, F.; et al. Clinical Validation of Two Recombinase-Based Isothermal Amplification Assays (RPA/RAA) for the Rapid Detection of African Swine Fever Virus. Front. Microbiol. 2020, 21, 1696. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Ma, P.; Fu, X.; Zhang, L.; Cui, P.; Li, H.; Yan, W.; Wang, H.; Yang, X. A recombinase polymerase amplification combined with lateral flow dipstick for rapid and specific detection of African swine fever virus. J. Virol. Methods 2020, 285, 113885. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Geng, Y.; Yuan, W. A recombinase polymerase amplification-based assay for rapid detection of African swine fever virus. Can. J. Vet. Res. 2017, 81, 308–312. [Google Scholar] [PubMed]
- Afonso, C.L.; Alcaraz, C.; Brun, A.; Sussman, M.D.; Onisk, D.V.; Escribano, J.M.; Rock, D.L. Characterization of P30, a highly antigenic membrane and secreted protein of African Swine Fever Virus. Virology 1992, 189, 368–373. [Google Scholar] [CrossRef]
- Pornthummawat, A.; Truong, Q.L.; Hoa, N.T.; Lan, N.T.; Izzati, U.Z.; Suwanruengsri, M.; Nnueangphuet, P.; Hirai, T.; Ymaguchi, R. Pathological lesions and presence of viral antigens in four surviving pigs in African swine fever outbreak farms in Vietnam. J. Vet. Med. Sci. 2021, 83, 1653–1660. [Google Scholar] [CrossRef]
- Petrovan, V.; Yuan, F.; Li, Y.; Shang, P.; Murgia, M.V.; Misra, S.; Rowland, R.R.; Fang, Y. Development and characterization of monoclonal antibodies against p30 protein of African swine fever virus. Virus Res. 2019, 269, 197632. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhou, J.; Zheng, Y.; Gamson, A.S.; Roembke, B.T.; Nakayama, S.; Sintim, H.O. Isothermal amplified detection of DNA and RNA. Mol. BioSyst. 2014, 10, 970–1003. [Google Scholar] [CrossRef] [Green Version]
- Davi, S.D.; Kissenkötter, J.; Faye, M.; Böhlken-Fascher, S.; Stahl-Hennig, C.; Faye, O.; Faye, O.; Sall, A.A.; Weidmann, M.; Ademowo, O.G.; et al. Recombinase polymerase amplification assay for rapid de-tection of Monkeypox virus. Diagn. Microbiol. Infect. Dis. 2019, 95, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; An, D.; Fan, Y.; Cheng, Z.; Tang, Y.; Diao, Y. A novel recombinase polymerase amplification assay for rapid detection of epidemic fowl adenovirus. Poult. Sci. 2020, 99, 6446–6453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zeng, F.; Sun, J.; Liu, X.; Wu, M.; Huang, B.; Lian, Y.; Xiao, L.; Ma, L.; Zhang, S.; et al. Application of recombinase polymerase amplification method for rapid detection of infectious laryngotracheitis virus. Mol. Cell. Probes 2020, 54, 101646. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Branavan, M.; Naveenathayalan, A.; Balachandran, W. Recombinase polymerase amplification–nucleic acid lateral flow immunoassays for Newcastle disease virus and infectious bronchitis virus detection. Mol. Biol. Rep. 2019, 46, 6391–6397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Li, R.; Liu, L.; Yuan, W. Rapid and sensitive detection of canine distemper virus by real-time reverse tran-scription recombinase polymerase amplification. BMC Vet. Res. 2017, 15, 241. [Google Scholar] [CrossRef]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Koltsova, G.; Koltsov, A.; Krutko, S.; Kholod, N.; Tulman, E.; Kolbasov, D. Growth Kinetics and Protective Efficacy of Attenuated ASFV Strain Congo with Deletion of the EP402 Gene. Viruses 2021, 13, 1259. [Google Scholar] [CrossRef] [PubMed]
- Andrew, S. Calculation for Determining the Number of Copies of a Template. URI Genomics & Sequencing. Available online: http://cels.uri.edu/gsc/cdna.html (accessed on 5 August 2021).


| Name | Nucleotide Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| 2RPAP30F | AGATCATCTTCACAAGTTGTGTTTCATGCGGGTAG | 141 |
| 2RPAP30R | CGAGCAGATTTCACAATATCATACTTAACAGTACT | |
| 2P30Exo v1 | TAGCGGTCGTAACAATTCTACCGCTATTGA(Fam dT)A (THF)TC(BHQ1-dT)CAACAGAAAACCAAT-C3spacer |
| Virus/Specimens | Real-Time RPA | qPCR | ||
|---|---|---|---|---|
| No. of Positives | No. of Negatives | No. of Positives | No. of Negatives | |
| ASFV GII isolates (n = 11) | 11 | 0 | 11 | 0 |
| ASFV GII animal specimens (n = 14) | 13 | 1 | 13 | 1 |
| ASFV GI animal specimens (n = 6) | 6 | 0 | 6 | 0 |
| Other viruses (n = 11) | 0 | 11 | 0 | 11 |
| Sensitivity | 96.8% (95% CI: 83.3–99.9) | 96.8% (95% CI: 83.3–99.9) | ||
| Specificity | 100% (95% CI: 71.5–100.0) | 100% (95% CI: 71.5–100.0) | ||
| Positive predictive value | 100% (95% CI: 88.4–100.0) | 100% (95% CI: 88.4–100.0) | ||
| Negative predictive value | 91.7% (95% CI: 61.5–99.8) | 91.7% (95% CI: 61.5–99.8) | ||
| qPCR | ||||
|---|---|---|---|---|
| No. of Positives | No. of Negatives | Total | ||
| Real-time RPA | No. of positive | 30 | 0 | 30 |
| No. of negative | 0 | 1 | 1 | |
| Total | 30 | 1 | 31 | |
| Name | Isolates/Origin | Genotype/Serotype | Source |
|---|---|---|---|
| D 1 | ASFV Stavropol 01/8 | II | FRCVM |
| D 2 | ASFV Irkutsk 2017 | II | FRCVM |
| D 3 | ASFV Stavropol | II | FRCVM |
| D 4 | ASFV Omsk #1 | II | FRCVM |
| D 5 | ASFV Omsk #2 | II | FRCVM |
| D 6 | ASFV Nizhny Novgorod #1 | II | FRCVM |
| D 7 | ASFV Nizhny Novgorod #2 | II | FRCVM |
| D 8 | ASFV Nizhny Novgorod #3 | II | FRCVM |
| D 9 | ASFV Nizhny Novgorod #4 | II | FRCVM |
| D 10 | ASFV Krasnodar | II | FRCVM |
| D 11 | ASFV Saratov | II | FRCVM |
| D 12 | ASFV DNA from pig # 1-Volgograd-v (3 dpi) | II | FRCVM |
| D 13 | ASFV DNA from pig # 1-Volgograd-v (5 dpi) | II | FRCVM |
| D 14 | ASFV DNA from pig #2-Volgograd-v (3 dpi) | II | FRCVM |
| D 15 | ASFV DNA from pig #2-Volgograd-v (5 dpi) | II | FRCVM |
| D 16 | ASFV DNA from pig #3-Volgograd-v (3 dpi) | II | FRCVM |
| D 17 | ASFV DNA from pig #3-Volgograd-v (4 dpi) | II | FRCVM |
| D 18 | ASFV DNA from pig #3-Volgograd-v (5 dpi) | II | FRCVM |
| D 19 | ASFV DNA from pig #3-Volgograd-v (6 dpi) | II | FRCVM |
| D 20 | ASFV DNA from pig #4-Volgograd-v (4 dpi) | II | FRCVM |
| D 21 | ASFV DNA from pig #4-Volgograd-v (5 dpi) | II | FRCVM |
| D 22 | ASFV DNA from pig #4-Volgograd-v (6 dpi) | II | FRCVM |
| D 23 | ASFV DNA from pig #5-Volgograd-v (3 dpi) | II | FRCVM |
| D 24 | ASFV DNA from pig #5-Volgograd-v (4 dpi) | II | FRCVM |
| D 25 | ASFV DNA from pig #5-Volgograd-v (5 dpi) | II | FRCVM |
| D 26 | ASFV DNA from pig #1-Congo-v (3 dpi) | I | FRCVM |
| D 27 | ASFV DNA from pig #1-Congo-v (5 dpi) | I | FRCVM |
| D 28 | ASFV DNA from pig #1-Congo-v (7 dpi) | I | FRCVM |
| D 29 | ASFV DNA from pig #2- Congo-v (3 dpi) | I | FRCVM |
| D 30 | ASFV DNA from pig #2-Congo-v (5 dpi) | I | FRCVM |
| D 31 | ASFV DNA from pig #2-Congo-v (7 dpi) | I | FRCVM |
| V 1 | Influenza A virus | H1N1 | KBPV |
| V 2 | Influenza A virus | H3N2 | KBPV |
| V 3 | Parainfluenza virus 2 | KBPV | |
| V 4 | Parainfluenza virus 4 | KBPV | |
| V 5 | Adenovirus | Serotype 1 | KBPV |
| V 6 | Adenovirus | Serotype 3 | KBPV |
| V 7 | Adenovirus | Serotype 7 | KBPV |
| V 8 | Enterovirus A | Enterovirus 71 | KBPV |
| V 9 | Rota virus A | Type 1 | KBPV |
| V 10 | Rota virus A | Type 3 | KBPV |
| V 11 | Cytomegalovirus | KBPV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilya, T.; Monoldorova, S.; Kang, S.-S.; Yun, S.; Byeon, H.-S.; Mariia, N.; Jeon, B.-Y. Development of a Real-Time Recombinase Polymerase Amplification Assay for the Rapid Detection of African Swine Fever Virus Genotype I and II. Pathogens 2022, 11, 439. https://doi.org/10.3390/pathogens11040439
Ilya T, Monoldorova S, Kang S-S, Yun S, Byeon H-S, Mariia N, Jeon B-Y. Development of a Real-Time Recombinase Polymerase Amplification Assay for the Rapid Detection of African Swine Fever Virus Genotype I and II. Pathogens. 2022; 11(4):439. https://doi.org/10.3390/pathogens11040439
Chicago/Turabian StyleIlya, Titov, Sezim Monoldorova, Shin-Seok Kang, Seungri Yun, Hyeon-Seop Byeon, Nefedeva Mariia, and Bo-Young Jeon. 2022. "Development of a Real-Time Recombinase Polymerase Amplification Assay for the Rapid Detection of African Swine Fever Virus Genotype I and II" Pathogens 11, no. 4: 439. https://doi.org/10.3390/pathogens11040439
APA StyleIlya, T., Monoldorova, S., Kang, S.-S., Yun, S., Byeon, H.-S., Mariia, N., & Jeon, B.-Y. (2022). Development of a Real-Time Recombinase Polymerase Amplification Assay for the Rapid Detection of African Swine Fever Virus Genotype I and II. Pathogens, 11(4), 439. https://doi.org/10.3390/pathogens11040439






