Epidemiological, Clinical, and Microbiological Characteristics in a Large Series of Patients Affected by Dermacentor-Borne-Necrosis-Erythema-Lymphadenopathy from a Unique Centre from Spain
Abstract
:1. Introduction
2. Results
2.1. Microbiological Tests
2.1.1. Serological Assays
2.1.2. Molecular Methods (PCR)
2.2. Epidemiological and Clinical Comparison of ‘Ca. R. rioja’, R. slovaca and R. raoultii Infections
2.2.1. ‘Ca. R. rioja’ Infection
2.2.2. R. slovaca Infection
2.2.3. R. raoultii Infection
3. Discussion
4. Materials and Methods
4.1. Case Definition
4.2. Patients and Samples
4.3. Microbiological Tests
4.3.1. Serological Assays
4.3.2. Molecular Methods (PCR)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Lakos, A. Tick-borne lymphadenopathy—A new rickettsial disease? Lancet 1997, 350, 1006. [Google Scholar] [CrossRef]
- Raoult, D.; Lakos, A.; Fenollar, F.; Beytout, J.; Brouqui, P.; Fournier, P.E. Spotless rickettsiosis caused by Rickettsia slovaca and associated with Dermacentor ticks Clin. Infect. Dis. 2002, 34, 1331–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oteo, J.A.; Ibarra, V. DEBONEL (Dermacentor-borne-necrosis-erythema-lymphadenopathy). ¿Una nueva enfermedad transmitida por garrapatas? Enferm. Infecc. Microbiol. Clin. 2002, 20, 51–52. [Google Scholar] [CrossRef]
- Cazorla, C.; Enea, M.; Lucht, F.; Raoult, D. First isolation of Rickettsia slovaca from a patient, France. Emerg. Infect. Dis. 2003, 9, 135. [Google Scholar] [CrossRef]
- Oteo, J.A.; Ibarra, V.; Blanco, J.R.; Martínez de Artola, V.; Márquez, F.J.; Portillo, A.; Raoult, D.; Anda, P. Dermacentor-borne necrosis erythema and lymphadenopathy: Clinical and epidemiological features of a new tick-borne disease. Clin. Microbiol. Infect. 2004, 10, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, V.; Oteo, J.A.; Portillo, A.; Santibáñez, S.; Blanco, J.R.; Metola, L.; Eiros, J.M.; Pérez-Martínez, L.; Sanz, M. Rickettsia slovaca Infection: DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 2006, 1078, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Komitova, R.; Lakos, A.; Aleksandrov, A.; Christova, I.; Murdjeva, M. A case of tick-transmitted lymphadenopathy in Bulgaria associated with Rickettsia slovaca. Scand. J. Infect. Dis. 2003, 35, 213. [Google Scholar] [CrossRef]
- Selmi, M.; Bertolotti, L.; Tomassone, L.; Mannelli, A. Rickettsia slovaca in Dermacentor marginatus and tick-borne lymphadenopathy, Tuscany, Italy. Emerg. Infect. Dis. 2008, 14, 817–820. [Google Scholar] [CrossRef]
- Porta, F.S.; Nieto, E.A.; Creus, B.F.; Espín, T.M.; Casanova, F.J.; Sala, I.S.; García, S.L.; Aguilar, J.L.; Vilaseca, M.Q. Tick-borne lymphadenopathy: A new infectious disease in children. Pediatr. Infect. Dis. J. 2008, 27, 618–622. [Google Scholar] [CrossRef]
- Parola, P.; Rovery, C.; Rolain, J.M.; Brouqui, P.; Davoust, B.; Raoult, D. Rickettsia slovaca and R. raoultii in tick-borne Rickettsioses. Emerg. Infect. Dis. 2009, 15, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Rieg, S.; Schmoldt, S.; Theilacker, C.; de With, K.; Wölfel, S.; Kern, W.V.; Dobler, G. Tick-borne lymphadenopathy (TIBOLA) acquired in Southwestern Germany. BMC Infect. Dis. 2011, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielewski, T.; Rudzka, D.; Fiecek, B.; Maczka, I.; Tylewska-Wierzbanowska, S. Case of TIBOLA/DEBONEL (tick-borne lymphadenopathy/Dermacentor spp.-borne necrosis-erythema-lymphadenopathy) in Poland. Przegl. Epidemiol. 2011, 65, 583–586. [Google Scholar]
- Gaston, J.; Durox, H.; Sparsa, A.; Bonnetblanc, J.M.; Doffoel-Hantz, V. Dermohypodermitis on the face revealing TIBOLA. Arch. Pediatr. 2011, 18, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.A.; Portillo, A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012, 3, 271–278. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.; Pereira, B.I.; Nazareth, C.; Cabral, S.; Ventura, C.; Crespo, P.; Marques, N.; da Cunha, S. Rickettsia slovaca infection in humans, Portugal. Emerg. Infect. Dis. 2013, 19, 1627–1629. [Google Scholar] [CrossRef] [Green Version]
- Rigal, E.; Dorcier, D.; Lesens, O.; Texier, C.; D’Incan, M. TIBOLA: An emerging clinically polymorphous rickettsiosis. Ann. Dermatol. Venereol. 2014, 141, 186–191. [Google Scholar] [CrossRef]
- Pietzsch, M.E.; Hansford, K.M.; Cull, B.; Jahfari, S.; Sprong, H.; Medlock, J.M. Detection of Dermacentor marginatus and a possible Rickettsia slovaca case in the United Kingdom-the risk of the visiting traveller. Travel Med. Infect. Dis. 2015, 13, 200–201. [Google Scholar] [CrossRef]
- Silva, J.T.; López-Medrano, F.; Fernández-Ruiz, M.; Foz, E.R.; Portillo, A.; Oteo, J.A.; Aguado, J.M. Tickborne Lymphadenopathy Complicated by Acute Myopericarditis, Spain. Emerg. Infect. Dis. 2015, 21, 2240–2242. [Google Scholar] [CrossRef] [Green Version]
- Barlozzari, G.; Romiti, F.; Zini, M.; Magliano, A.; De Liberato, C.; Corrias, F.; Capponi, G.; Galli, L.; Scarpulla, M.; Montagnani, C. Scalp eschar and neck lymphadenopathy by Rickettsia slovaca after Dermacentor marginatus tick bite case report: Multidisciplinary approach to a tick-borne disease. BMC Infect. Dis. 2021, 21, 103. [Google Scholar] [CrossRef]
- Raoult, D.; Berbis, P.; Roux, V.; Xu, W.; Maurin, M. A new tick-transmitted disease due to Rickettsia slovaca. Lancet 1997, 350, 112–113. [Google Scholar] [CrossRef]
- Oteo, J.A.; Ibarra, V.; Blanco, J.R. Eritema, Necrosis y Linfedenopatía. Una nueva enfermedad (DEBONEL) transmitida por Dermacentor marginatus Sulzer, 1776. In Zubía Monográfico; Instituto de Estudios Riojanos: Logroño, Spain, 2000; Volume 12, pp. 49–58. [Google Scholar]
- Ibarra, V.; Portillo, A.; Santibanez, S.; Blanco, J.R.; Pérez-Martínez, L.; Márquez, F.J.; Oteo, J.A. DEBONEL/TIBOLA: Is Rickettsia slovaca the only etiological agent? Ann. N. Y. Acad. Sci. 2005, 1063, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Ibarra, V.; Santibáñez, S.; Pérez-Martínez, L.; Blanco, J.R.; Oteo, J.A. Genetic characterisation of ompA, ompB and gltA genes from Candidatus Rickettsia rioja. Clin. Microbiol. Infect. 2009, 15, 307–308. [Google Scholar] [CrossRef] [Green Version]
- Mediannikov, O.; Matsumoto, K.; Samoylenko, I.; Drancourt, M.; Roux, V.; Rydkina, E.; Davoust, B.; Tarasevich, I.; Brouqui, P.; Fournier, P.E. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Syst. Evol. Microbiol. 2008, 58, 1635–1639. [Google Scholar] [CrossRef] [Green Version]
- Rydkina, E.; Roux, V.; Rudakov, N.; Gafarova, M.; Tarasevich, I.; Raoult, D. New Rickettsiae in ticks collected in territories of the former soviet union. Emerg. Infect. Dis. 1999, 5, 811–814. [Google Scholar] [CrossRef]
- Shpynov, S.; Parola, P.; Rudakov, N.; Samoilenko, I.; Tankibaev, M.; Tarasevich, I.; Raoult, D. Detection and identification of spotted fever group rickettsiae in Dermacentor ticks from Russia and central Kazakhstan. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 903–905. [Google Scholar] [CrossRef]
- Angelakis, E.; Pulcini, C.; Waton, J.; Imbert, P.; Socolovschi, C.; Edouard, S.; Dellamonica, P.; Raoult, D. Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after Tick Bite. Clin. Infect. Dis. 2010, 50, 549–551. [Google Scholar] [CrossRef] [Green Version]
- Edouard, S.; Gonin, K.; Turc, Y.; Angelakis, E.; Socolovschi, C.; Raoult, D. Eschar and neck lymphadenopathy caused by Francisella tularensis after a tick bite: A case report. J. Med. Case Rep. 2011, 5, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascio, A.; Torina, A.; Valenzise, M.; Blanda, V.; Camarda, N.; Bombaci, S.; Iaria, C.; De Luca, F.; Wasniewska, M. Scalp eschar and neck lymphadenopathy caused by Rickettsia massiliae. Emerg. Infect. Dis. 2013, 19, 836–837. [Google Scholar] [CrossRef] [Green Version]
- Foissac, M.; Socolovschi, C.; Raoult, D. Update on SENLAT syndrome: Scalp eschar and neck lymph adenopathy after a tick bite. Ann. Dermatol. Venereol. 2013, 140, 598–609. [Google Scholar] [CrossRef]
- Dubourg, G.; Socolovschi, C.; Del Giudice, P.; Fournier, P.E.; Raoult, D. Scalp eschar and neck lymphadenopathy after tick bite: An emerging syndrome with multiple causes. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, M.; Popescu, C.P.; Florescu, S.A.; Ceausu, E.; Raoult, D.; Parola, P.; Socolovschi, C. Rickettsia massiliae infection and SENLAT syndrome in Romania. Ticks Tick Borne Dis. 2016, 7, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Kim, C.M.; Yun, N.R.; Kim, D.M.; Kim, S.S.; Choi, S.; Chu, H. Scalp eschar and neck lymphadenopathy after tick bite (SENLAT) caused by Bartonella henselae in Korea: A case report. BMC Infect. Dis. 2020, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, V.; Blanco, J.R.; Portillo, A.; Santibáñez, S.; Metola, L.; A Oteo, J. Effect of antibiotic treatment in patients with DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 2005, 1063, 257–258. [Google Scholar] [CrossRef]
- Faccini-Martínez, Á.A.; García-Álvarez, L.; Hidalgo, M.; Oteo, J.A. Syndromic classification of rickettsioses: An approach for clinical practice. Int. J. Infect. Dis. 2014, 28, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Oteo, J.A.; Martínez de Artola, V.; Casas, J.M. Tick-borne diseases in Spain. In Proceedings of the 6th International Congress for Infectious Diseases, Prague, Czech Republic, 26–30 April 1994. [Google Scholar]
- Oteo Revuelta, J.A.; Blanco Ramos, J.R.; Martínez de Artola, V.; Grandival García, R.; Ibarra Cucalón, V.; Dopereiro Gómez, R. Eritema migratorio (borreliosis de Lyme). Características clinicoepidemiológicas de 50 pacientes [Migratory erythema (Lyme borreliosis). Clinicoepidemiologic features of 50 patients]. Rev. Clin. Esp. 2000, 200, 60–63. [Google Scholar] [CrossRef]
- Hornok, S. Dermacentor marginatus (Sulzer, 1776). In Ticks of Europe and North Africa. A Guide to Species Identification; Estrada-Peña, A., Mihalca, A.D., Petney, T.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 281–286. [Google Scholar]
- Silva-Pinto, A.; Santos Mde, L.; Sarmento, A. Tick-borne lymphadenopathy, an emerging disease. Ticks Tick Borne Dis. 2014, 5, 656–659. [Google Scholar] [CrossRef]
- Sanogo, Y.O.; Davoust, B.; Parola, P.; Camicas, J.L.; Brouqui, P.; Raoult, D. Prevalence of Rickettsia spp. in Dermacentor marginatus ticks removed from game pigs (Sus scrofa) in southern France. Ann. N. Y. Acad. Sci. 2003, 990, 191–195. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Pérez-Sánchez, R.; Alamo-Sanz, R.; Encinas-Grandes, A. Spotted fever group rickettsiae in ticks feeding on humans in northwestern Spain: Is Rickettsia conorii vanishing? Ann. N. Y. Acad. Sci. 2006, 1078, 331–333. [Google Scholar] [CrossRef]
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.J.; Bjoërsdorff, A.; Blanco, J.R.; Caruso, G.; Cinco, M.; Fournier, P.E.; Francavilla, E.; et al. ESCMID Study Group on Coxiella, Anaplasma, Rickettsia and Bartonella; European Network for Surveillance of Tick-Borne Diseases. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132. [Google Scholar] [CrossRef]
- Santibanez, S.; Ibarra, V.; Portillo, A.; Blanco, J.R.; Martínez de Artola, V.; Guerrero, A.; Oteo, J.A. Evaluation of IgG antibody response against Rickettsia conorii and Rickettsia slovaca in patients with DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 2006, 1078, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, S.; Portillo, A.; Santibáñez, P.; Palomar, A.M.; Oteo, J.A. Usefulness of rickettsial PCR assays for the molecular diagnosis of human rickettsioses. Enferm. Infecc. Microbiol. Clin. 2013, 31, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic iden.ntification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.J.; Jang, W.J.; Kim, J.H.; Kim, J.H.; Ryu, J.S.; Lee, S.H.; Park, K.H.; Paik, H.S.; Koh, Y.S.; Choi, M.S.; et al. Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg. Infect. Dis. 2005, 11, 237–244. [Google Scholar] [CrossRef]
- Roux, V.; Fournier, P.E.; Raoult, D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 1996, 34, 2058–2065. [Google Scholar] [CrossRef] [Green Version]
- Renesto, P.; Gouvernet, J.; Drancourt, M.; Roux, V.; Raoult, D. Use of rpoB gene analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 2001, 39, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Karhukorpi, E.K.; Karhukorpi, J. Rapid laboratory diagnosis of ulceroglandular tularemia with polymerase chain reaction. Scand. J. Infect. Dis. 2001, 33, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Hendricks, A.; Burge, D. Molecular Identification and Analysis of Borrelia burgdorferi Sensu Lato in Lizards in the Southeastern United States. Appl. Environ. Microbiol. 2005, 71, 2616–2625. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.J.B.; Happ, C.M.; Mayer, L.W.; Piesman, J. Detection of Borrelia burgdorferi in ticks by species-specific amplification of the flagellin gene. Am. J. Trop. Med. Hyg. 1992, 47, 730–741. [Google Scholar] [CrossRef]
- Willems, H.; Thiele, D.; Frölich-Ritter, R.; Krauss, H. Detection of Coxiella burnetii in cow’s milk using the polymerase chain reaction (PCR). J. Vet. Med. 1994, 41, 580–587. [Google Scholar] [CrossRef]
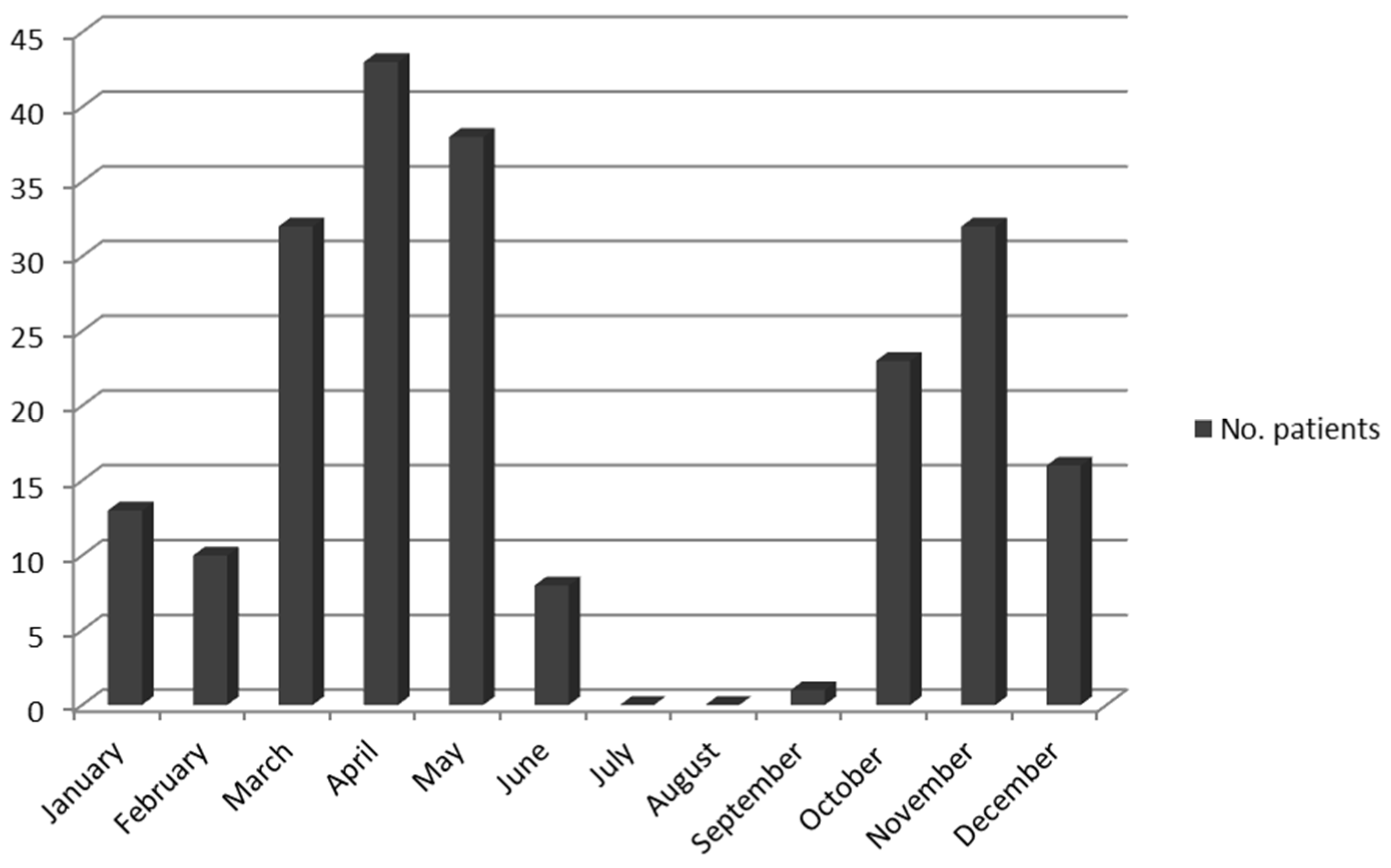
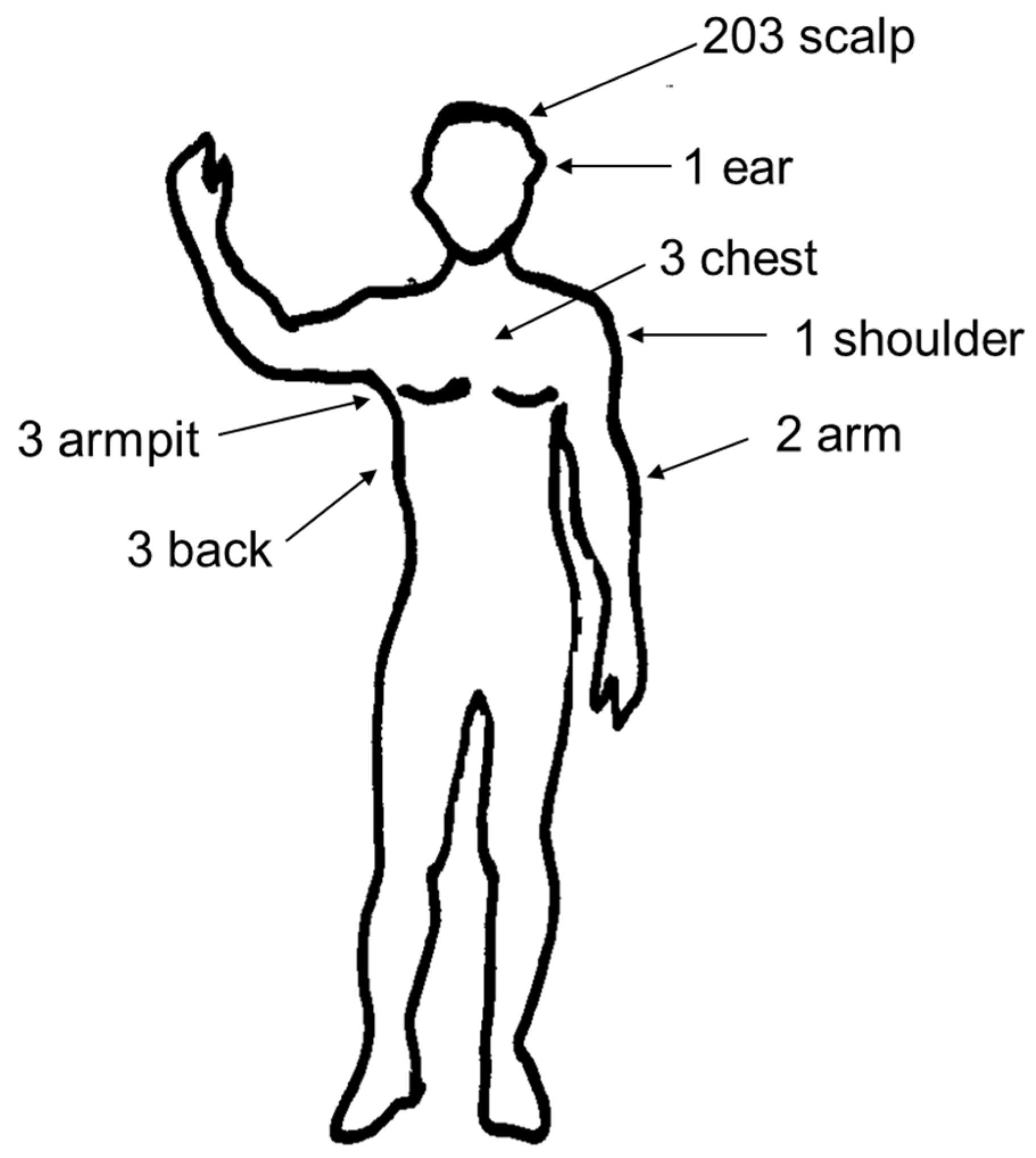

| Clinical and Epidemiological Data | ‘Ca. R. rioja’ Infection (n:91) | R. slovaca Infection (n:66) | R. raoultii Infection (n:4) | Patients with DEBONEL by Unknown Agent (n:55) | p Value | Total Patients (n:216) |
|---|---|---|---|---|---|---|
| Sex (female) | 49/91 (53.85%) | 45/66 (68.18%) | 3/4 (75.00%) | 44/55 (80.00%) ** | 0.008 | 141 |
| Mean age (years) | 32.98 ± 2.42 | 37.62 ± 3.09 | 27.00 ± 12.27 | 39.13 ± 2.80 | 0.325 | 37.7 |
| IP (days) | 5.43 ± 0.31 | 4.68 ± 0.28 | 5.00 ± 0.41 | 5.78 ± 0.40 | 0.161 | 5.61 |
| Low grade fever 1 | 34/91 (37.36%) | 19/66 (28.79%) | 2/4 (50.00%) | 16/55 (29.09%) | 0.505 | 71 (32.87%) |
| Fever 2 | 2/91 (2.20%) | 3/66 (4.55%) | 0/4 (0.00%) | 3/55 (5.45%) | 0.653 | 8 (3.70%) |
| Persistent alopecia 3 | 26/89 (29.21%) | 40/64 (62.50%) *** | 1/3 (33.33%) | 9/47 (19.15%) ### | <0.001 | 76/203 (37.44%) |
| Evidence of recent infection by IFA (seroconversion or fourfold rise in titer) | 39/48 (81.25%) | 28/34 (82.35%) | 1/1 (100.00%) | 23/26 (88.46%) | 0.798 | 91/109 (83.49%) |
| Gene | Primers | Primer Sequence (5′→3′) | Fragment Size (bp) | Tm (°C) | Reference |
|---|---|---|---|---|---|
| gltA (nested) | RpCS.877p | GGGGGCCTGCTCACGGCGG | 381 | 48 | [46,47] |
| RpCS.1258n | ATTGCAAAAAGTACAGTGAACA | ||||
| RpCS.896p | GGCTAATGAAGCAGTGATAA | 337 | 54 | ||
| RpCS.1233n | ATTGCAAAAAGTACAGTGAACA | ||||
| ompA (semi nested) | Rr190.70p | ATGGCGAATATTTCTCCAAAA | 631 | 46 | [46,48] |
| Rr190.701n | GTTCCGTTAATGGCAGCATCT | ||||
| Rr190.70p | ATGGCGAATATTTCTCCAAAA | 532 | 48 | ||
| Rr190.602n | AGTGCAGCATTCGCTCCCCCT | ||||
| ompB (nested) | rompB OF | GTAACCGGAAGTAATCGTTTCGTAA | 511 | 54 | [47] |
| rompB OR | GCTTTATAACCAGCTAAACCACC | ||||
| rompB SFG IF | GTTTAATACGTGCTGCTAACCAA | 420 | 56 | ||
| rompB SFG/TG IR | GGTTTGGCCCATATACCATAAG |
| Bacteria | Target Gene | Primer Sequence (5′→3′) | Fragment Size (bp) | Tm (°C) | Reference |
|---|---|---|---|---|---|
| Bartonella spp. | rpoB | CGCATTGGCTTACTTCGTATG GTAGACTGATTAGAACGCTG | 825 | 53 | [49] |
| Francisella tularensis | 17 KDa lipoprotein | ATGGCGAGTGATACTGCTTG GCATCATCAGAGCCACCTAA | 250 | 56 | [50] |
| Borrelia burgdorferi sensu lato | Flagellin (nested) | AARGAATTGGCAGTTCAATC GCATTTTCWATTTTAGCAAGTGATG | 497 | 52 | [51] |
| ACATATTCAGATGCAGACAGAGGTTCTA GAAGGTGCTGTAGCAGGTGCTGGCTGT | 389 | 55 | [51,52] | ||
| Coxiella burnetii | IS1111 | TATGTATCCACCGTAGCCAGTC CCCAACAACACCTCCTTATTC | 685 | 48 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santibáñez, S.; Portillo, A.; Ibarra, V.; Santibáñez, P.; Metola, L.; García-García, C.; Palomar, A.M.; Cervera-Acedo, C.; Alba, J.; Blanco, J.R.; et al. Epidemiological, Clinical, and Microbiological Characteristics in a Large Series of Patients Affected by Dermacentor-Borne-Necrosis-Erythema-Lymphadenopathy from a Unique Centre from Spain. Pathogens 2022, 11, 528. https://doi.org/10.3390/pathogens11050528
Santibáñez S, Portillo A, Ibarra V, Santibáñez P, Metola L, García-García C, Palomar AM, Cervera-Acedo C, Alba J, Blanco JR, et al. Epidemiological, Clinical, and Microbiological Characteristics in a Large Series of Patients Affected by Dermacentor-Borne-Necrosis-Erythema-Lymphadenopathy from a Unique Centre from Spain. Pathogens. 2022; 11(5):528. https://doi.org/10.3390/pathogens11050528
Chicago/Turabian StyleSantibáñez, Sonia, Aránzazu Portillo, Valvanera Ibarra, Paula Santibáñez, Luís Metola, Concepción García-García, Ana M. Palomar, Cristina Cervera-Acedo, Jorge Alba, José R. Blanco, and et al. 2022. "Epidemiological, Clinical, and Microbiological Characteristics in a Large Series of Patients Affected by Dermacentor-Borne-Necrosis-Erythema-Lymphadenopathy from a Unique Centre from Spain" Pathogens 11, no. 5: 528. https://doi.org/10.3390/pathogens11050528
APA StyleSantibáñez, S., Portillo, A., Ibarra, V., Santibáñez, P., Metola, L., García-García, C., Palomar, A. M., Cervera-Acedo, C., Alba, J., Blanco, J. R., & Oteo, J. A. (2022). Epidemiological, Clinical, and Microbiological Characteristics in a Large Series of Patients Affected by Dermacentor-Borne-Necrosis-Erythema-Lymphadenopathy from a Unique Centre from Spain. Pathogens, 11(5), 528. https://doi.org/10.3390/pathogens11050528








