Biocontrol of Wheat Crown Rot Using Bacillus halotolerans QTH8
Abstract
:1. Introduction
2. Results
2.1. Antagonistic Effect of Bacterial Strains on Wheat Crown Rot
2.2. Determination of Antimicrobial Spectrum
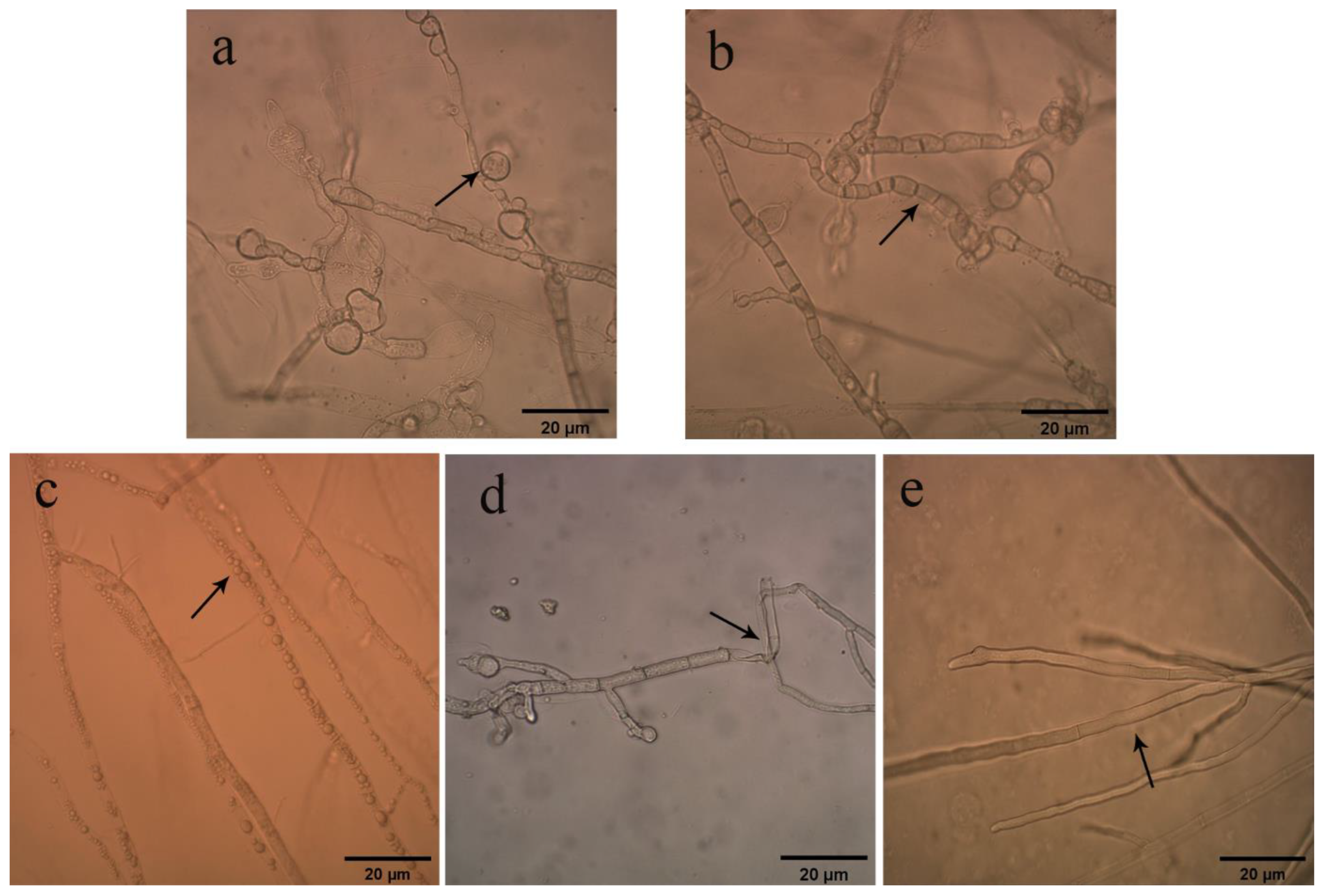
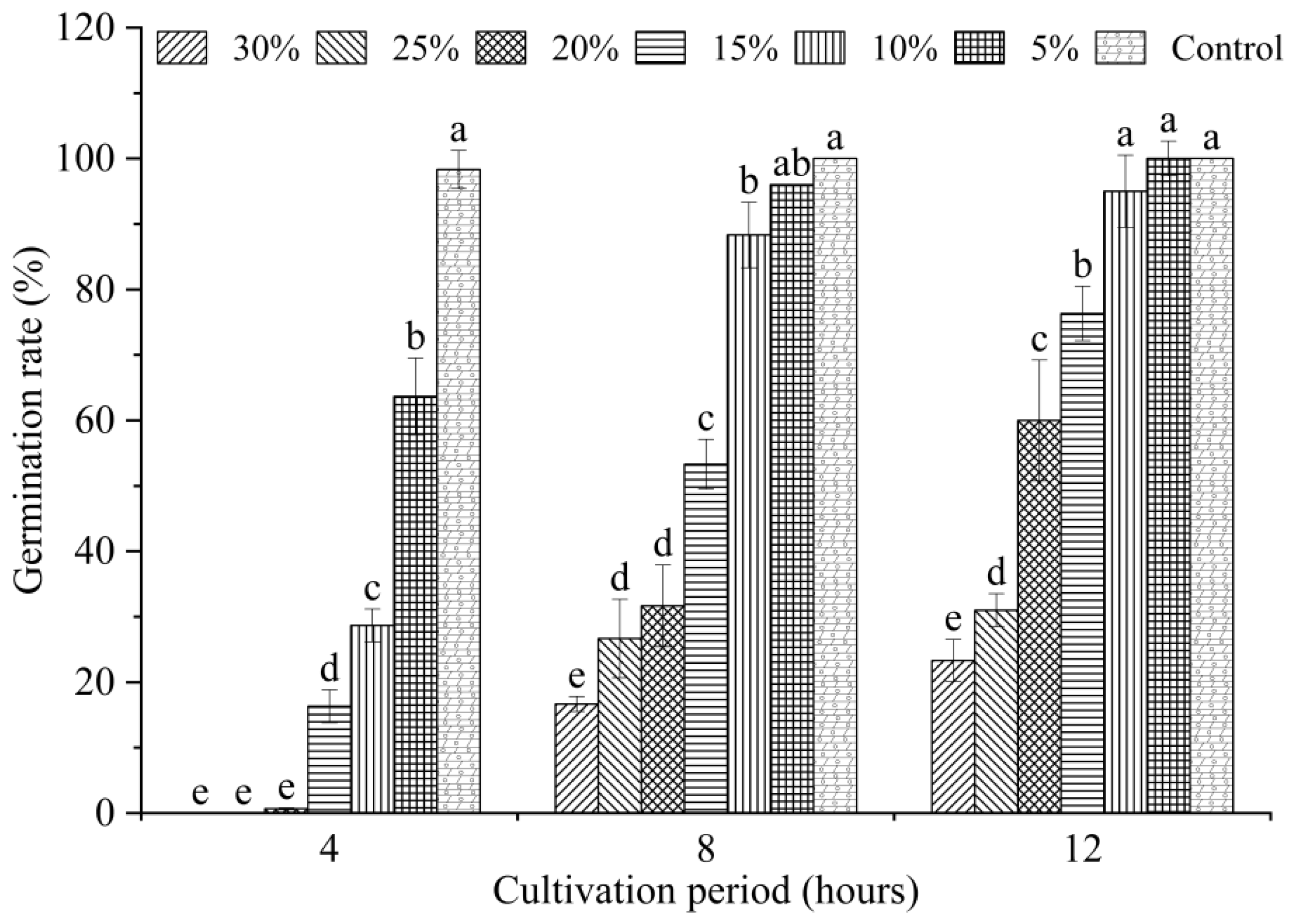
2.3. Effects of Bacterial Strain QTH8 on Conidial Germination
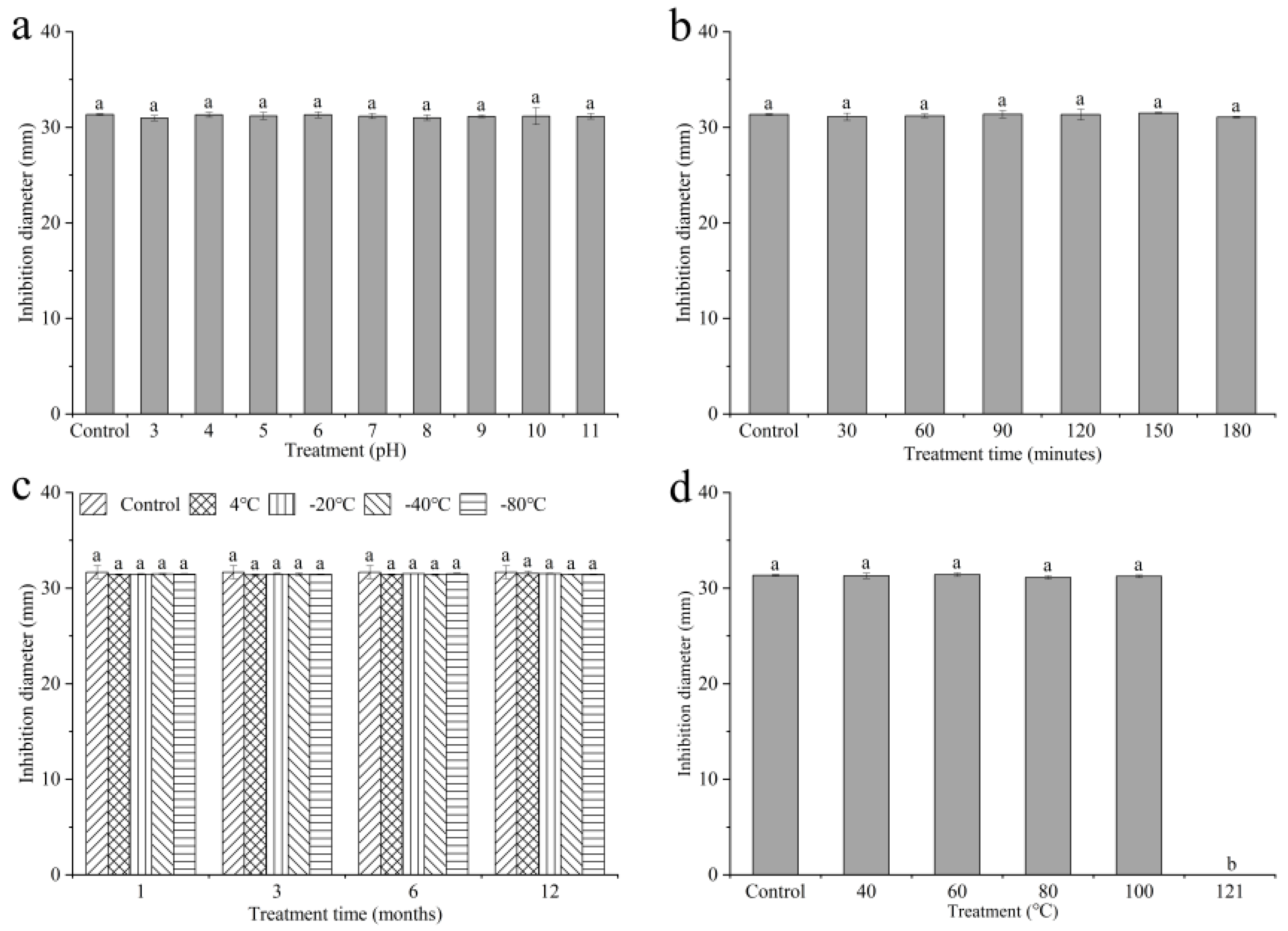
2.4. Active Stability of Culture Filtrates of QTH8
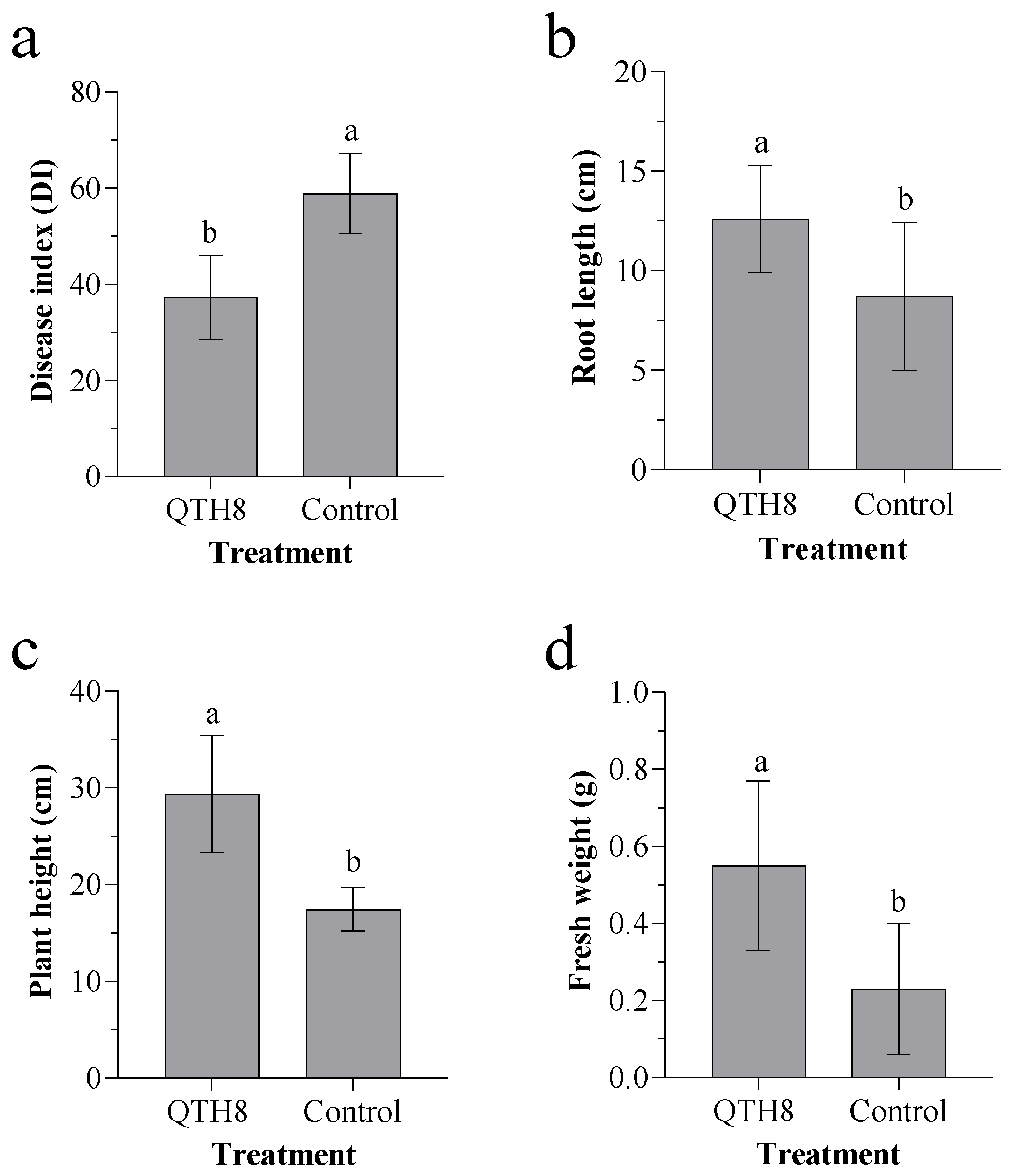
2.5. Effect of Bacterial Strain QTH8 on Wheat Coleoptiles Infected Fusarium seudograminearum
2.6. Effect of Bacterial Strain QTH8 on Fusarium pseudograminearum in Glasshouse
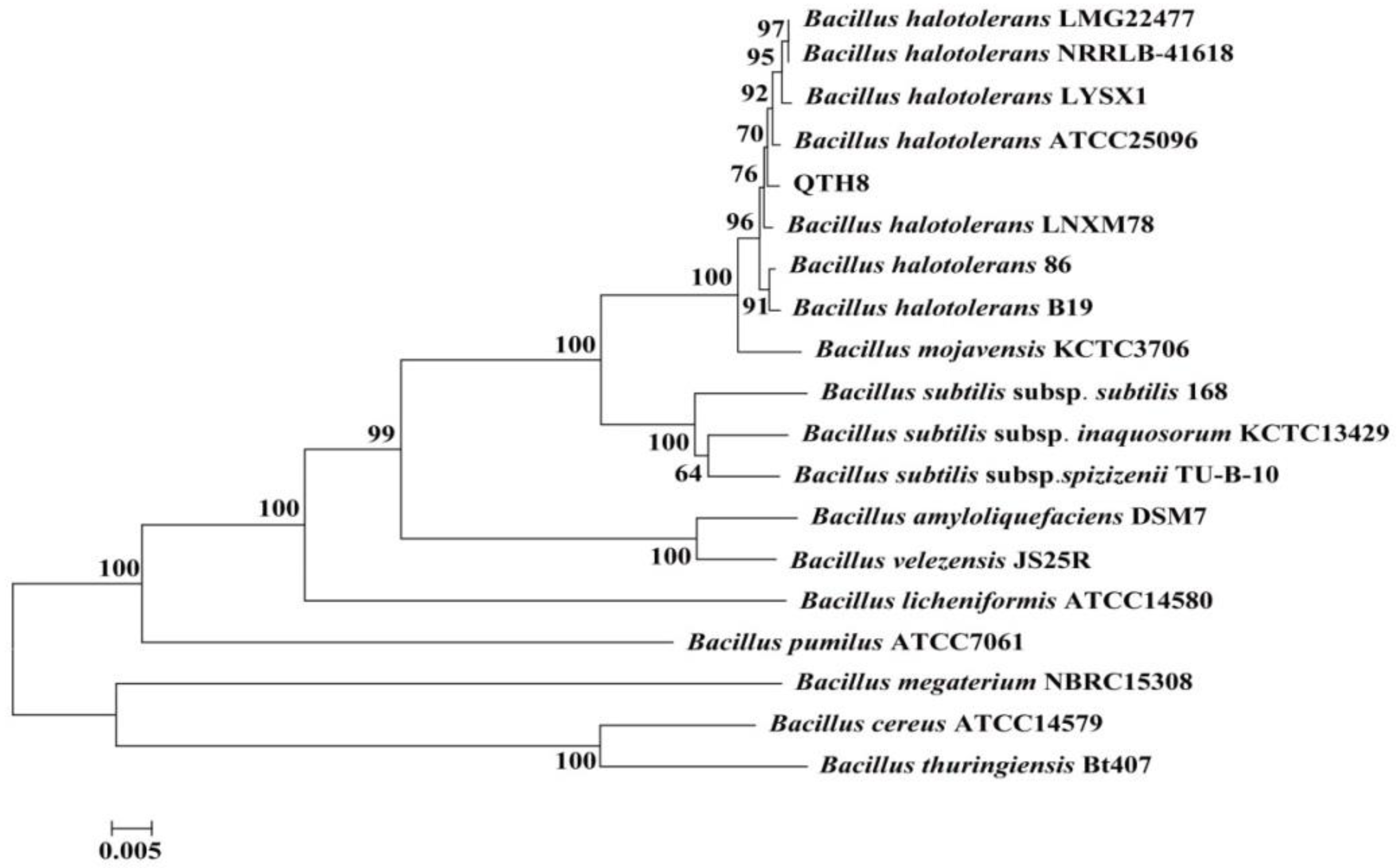
2.7. Identification of Bacterial Strain QTH8
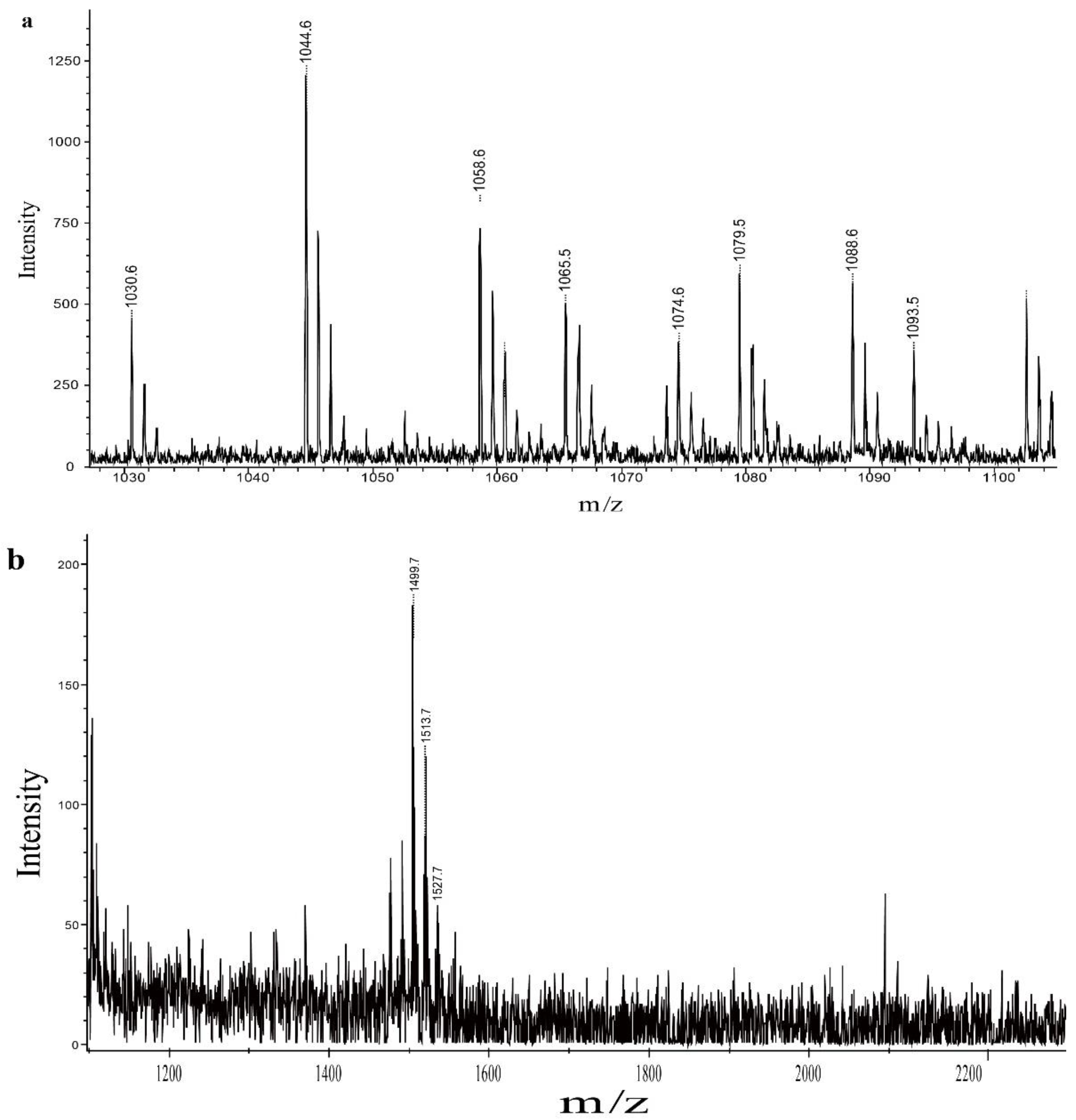
2.8. Analysis of QTH8 Lipopeptides
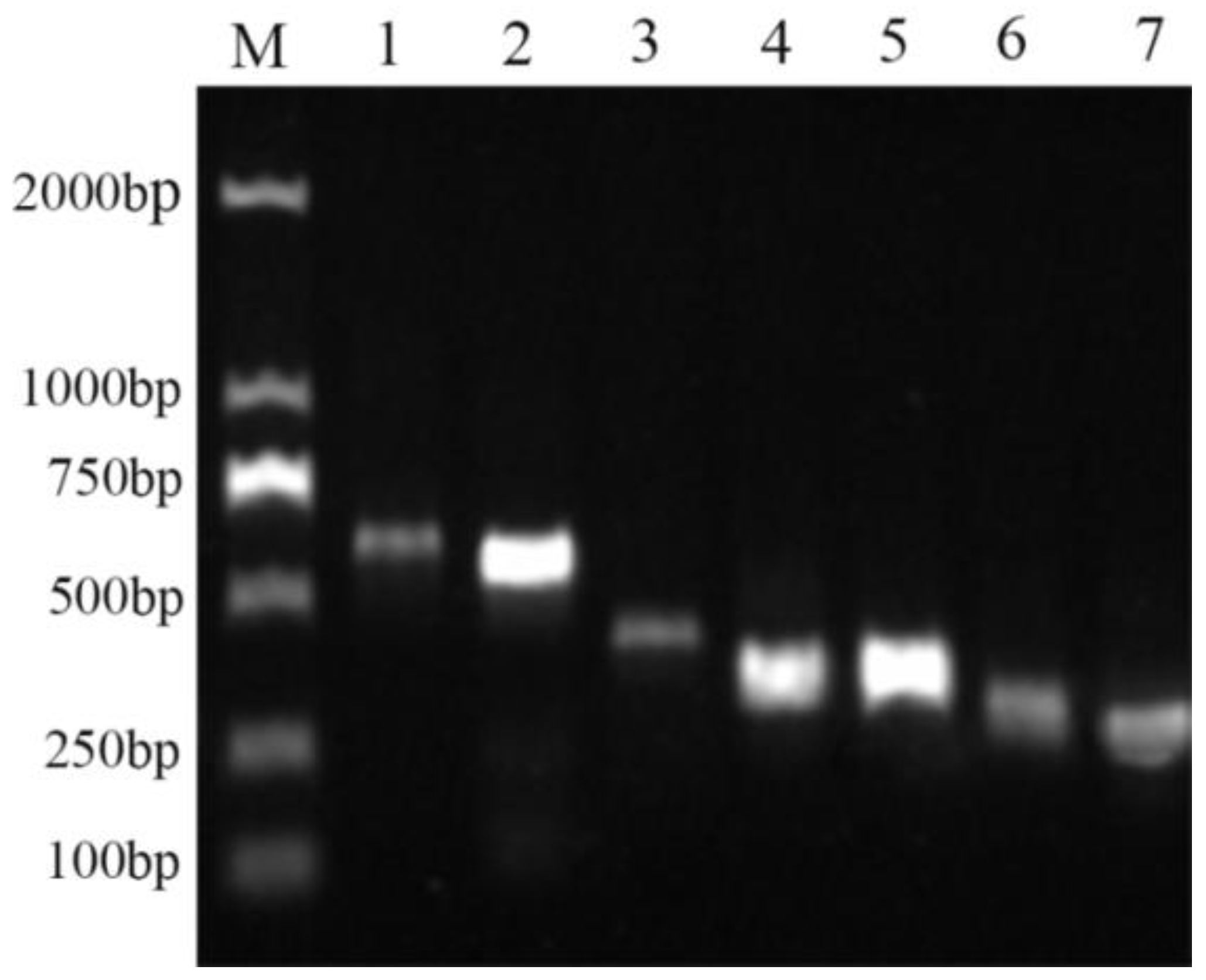
2.9. Functional Gene Analysis of Bacterial Strain QTH8
3. Discussion
4. Materials and Methods
4.1. Fungal Isolates, Bacterial Strains, and Growth Conditions
4.2. Anagonism of Bacterial Strains against Fusarium pseudograminearum In Vitro
4.3. Determination of Antimicrobial Spectrum of Bacterial Strain QTH8 Culture Filtrates
4.4. Effects of Bacterial Strain QTH8 Culture Filtrate on Conidia Germination of Fusarium pseudograminearum
4.5. Treatment of QTH8 Culture Filtrates
4.6. Assessment of the Effect of QTH8 Culture Filtrates on Fusarium pseudograminearum-Infected Seedlings by Coleoptile Inoculation
4.7. Antagonism of Bacterial Strain QTH8 against Fusarium pseudograminearum In Vivo
4.8. Identification of QTH8
4.9. Identification of Lipopeptides
4.10. Detection of Antimicrobial Peptide Biosynthetic Genes
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moya-Elizondo, E.A.; Rew, L.J.; Jacobsen, B.J.; Hogg, A.C.; Dyer, A.T. Ditribution and prevalence of Fusarium crown rot and common root rot pathogens of wheat in Montana. Plant Dis. 2011, 95, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; He, X.; Wang, S.; Ma, Q.; Sun, B.; Ding, S.; Chen, L.; Zhang, M.; Li, H. Diversity of the Fusarium pathogens associated with crown rot in the Huanghuai wheat-growing region of China. Environ. Microbiol. 2019, 21, 2740–2754. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, H.; Fu, B.; Xing, X.; Sun, B.; Tang, W. First report of Fusarium pseudograminearum causing crown rot of wheat in Henan, China. Plant Dis. 2012, 96, 1065. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, W.; Zhang, P.; Sun, H.; Zhang, X.; Zhang, A.; Chen, H. Fusarium pseudograminearum as an emerging pathogen of crown rot of wheat in eastern China. Plant Pathol. 2019, 69, 240–248. [Google Scholar] [CrossRef]
- Malfanova, N.V. Endophytic Bacteria with Plant Growth Promoting and Biocontrol Abilities. In Microbial Control of Plant Diseases; Lugtenberg, B., Malfanova, N., Kamilova, F., Berg, G., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 67–91. [Google Scholar]
- Fira, D.; Dimkic, I.; Beric, T.; Lozo, J.; Stankovic, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Zhao, Q.; Ran, W.; Wang, H.; Li, X.; Shen, Q.; Shen, S.; Xu, Y. Biocontrol of Fusarium wilt disease in muskmelon with Bacillus subtilis Y-IVI. BioControl 2012, 58, 283–292. [Google Scholar] [CrossRef]
- Kumar, K.V.K.; Yellareddygari, S.K.R.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Zhou, X.G.; Sudini, H.; Groth, D.E.; Krishnam Ratu, S.; Miller, M.E. Efficacy of Bacillus subtilis MBI 600 against sheath blight caused by Rhizoctonia solani and on growth and yield of rice. Rice Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef] [Green Version]
- De Curtis, F.; Lima, G.; Vitullo, D.; De Cicco, V. Biocontrol of Rhizoctonia solani and Sclerotium rolfsii on tomato by delivering antagonistic bacteria through a drip irrigation system. Crop Prot. 2010, 29, 663–670. [Google Scholar] [CrossRef]
- Mercado-Flores, Y.; Cárdenas-Álvarez, I.O.; Rojas-Olvera, A.V.; Pérez-Camarillo, J.P.; Leyva-Mir, S.G.; Anducho-Reyes, M.A. Application of Bacillus subtilis in the biological control of the phytopathogenic fungus Sporisorium reilianum. Biol. Control 2014, 76, 36–40. [Google Scholar] [CrossRef]
- Li, S.; Zhang, N.; Zhang, Z.; Luo, J.; Shen, B.; Zhang, R.; Shen, Q. Antagonist Bacillus subtilis HJ5 controls Verticillium wilt of cotton by root colonization and biofilm formation. Biol. Fertil. Soils 2012, 49, 295–303. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, X.; Yu, J.; Guo, Z.; Wu, J.; Li, X.; Chi, Y.; Wan, S. Biological control of peanut southern blight (Sclerotium rolfsii) by the strain Bacillus pumilus LX11. Biocontrol Sci.Technol. 2020, 30, 485–489. [Google Scholar] [CrossRef]
- Kong, Q.; Shan, S.; Liu, Q.; Wang, X.; Yu, F. Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int. J. Food Microbiol. 2010, 139, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Slama, H.B.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for Fusarium antagonistic bacteria from contrasting niches designated the endophyte Bacillus halotolerans as plant warden against Fusarium. Front. Microbiol. 2018, 9, 3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kefi, A.; Ben Slimene, I.; Karkouch, I.; Rihouey, C.; Azaeiz, S.; Bejaoui, M.; Belaid, R.; Cosette, P.; Jouenne, T.; Limam, F. Characterization of endophytic Bacillus strains from tomato plants (Lycopersicon esculentum) displaying antifungal activity against Botrytis cinerea Pers. World J. Microbiol. Biotechnol. 2015, 31, 1967–1976. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mould in postharvest strawberry fruit. Postharvest Biol. Technol. 2021, 174, 111456. [Google Scholar] [CrossRef]
- Sendi, Y.; Pfeiffer, T.; Koch, E.; Mhadhbi, H.; Mrabet, M. Potential of common bean (Phaseolus vulgaris L.) root microbiome in the biocontrol of root rot disease and traits of performance. J. Plant Dis. Prot. 2020, 127, 453–462. [Google Scholar] [CrossRef]
- Liu, G.; Lin, X.; Xu, S.; Liu, G.; Liu, F.; Mu, W. Screening, identification and application of soil bacteria with nematicidal activity against root-knot nematode (Meloidogyne incognita) on tomato. Pest Manag. Sci. 2020, 76, 2217–2224. [Google Scholar] [CrossRef]
- Riaz, R.; Khan, A.; Khan, W.J.; Jabeen, Z.; Yasmin, H.; Naz, R.; Nosheen, A.; Hassan, M.N. Vegetable associated Bacillus spp. suppress the pea (Pisum sativum L.) root rot caused by Fusarium solani. Biol. Control 2021, 158, 104610. [Google Scholar] [CrossRef]
- Wang, T.; Liang, Y.; Wu, M.; Chen, Z.; Lin, J.; Yang, L. Natural products from Bacillus subtilis with antimicrobial properties. Chin. J. Chem. Eng. 2015, 23, 744–754. [Google Scholar] [CrossRef]
- Deleu, M.; Bouffioux, O.; Razafindralambo, H.; Paquot, M.; Hbid, C.; Thonart, P.; Jacques, P.; Brasseur, R. Interaction of surfactin with membranes: A computational approach. Langmuir 2003, 19, 3377–3385. [Google Scholar] [CrossRef]
- Heerklotz, H.; Wieprecht, T.; Seelig, J. Membrane perturbation by the lipopeptide surfactin and detergents asstudied by Deuterium NMR. J. Phys. Chem. B 2004, 108, 4909–4915. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mnif, I.; Ghribi, D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Biopolymers 2015, 104, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, F.; Lu, Y.; Deng, J. Combination of chitosan and salicylic acid to control postharvest green mold caused by Penicillium digitatum in grapefruit fruit. Sci. Hortic. 2018, 233, 54–60. [Google Scholar] [CrossRef]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Antimicrobial peptide genes in Bacillus strains from plant environments. Int. Microbiol. 2011, 14, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.A.; Ren, H.; Ahmed, T.; Luo, J.; An, Q.; Qi, X.; Li, B. Antifungal effects of rhizospheric Bacillus species against bayberry twig blight pathogen Pestalotiopsis versicolor. Agronomy 2020, 10, 1811. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, L.; Nie, Q.; Hsiang, T.; Sun, Z.; Zhou, Y. Isolation, identification and biocontrol mechanisms of endophytic bacterium D61-A from Fraxinus hupehensis against Rhizoctonia solani. Biol. Control 2021, 158, 104621. [Google Scholar] [CrossRef]
- Choub, V.; Maung, C.E.H.; Won, S.J.; Moon, J.H.; Kim, K.Y.; Han, Y.S.; Cho, J.Y.; Ahn, Y.S. Antifungal activity of cyclic tetrapeptide from Bacillus velezensis CE 100 against plant pathogen Colletotrichum gloeosporioides. Pathogens 2021, 10, 209. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Wang, R.; Wang, Z.; Zhang, Y.; Gu, Q.; Farzand, A.; Yang, X.; Semenov, M.; Borriss, R.; et al. Genomic features and molecular function of a novel stress-tolerant Bacillus halotolerans strain isolated from an extreme environment. Biology 2021, 10, 1030. [Google Scholar] [CrossRef]
- Xia, Y.; Li, S.; Liu, X.; Zhang, C.; Xu, J.; Chen, Y. Bacillus halotolerans strain LYSX1-induced systemic resistance against the root-knot nematode Meloidogyne javanica in tomato. Ann. Microbiol. 2019, 69, 1227–1233. [Google Scholar] [CrossRef]
- Dheepa, R.; Vinodkumar, S.; Renukadevi, P.; Nakkeeran, S. Phenotypic and molecular characterization of chrysanthemum white rust pathogen Puccinia horiana (Henn) and the effect of liquid based formulation of Bacillus spp. for the management of chrysanthemum white rust under protected cultivation. Biol. Control 2016, 103, 172–186. [Google Scholar] [CrossRef]
- Ahmad, B. Chemical composition and antifungal, phytotoxic, brine shrimp cytotoxicity, insecticidal and antibacterial activities of the essential oils of Acacia modesta. J. Med. Plants Res. 2012, 6, 4653–4659. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Yu, Q.; Li, J.; Zhang, X. Inhibitory Effect of Bacillus amyloliquefuciens ZJ01 against Alternaria alternata in Chinese Jujube. J. Hebei Agric. Sci. 2018, 22, 40–42. [Google Scholar]
- Xu, Z.; Zhang, R.; Wang, D.; Qiu, M.; Feng, H.; Zhang, N.; Shen, Q. Enhanced control of cucumber wilt disease by Bacillus amyloliquefaciens SQR9 by altering the regulation of its DegU phosphorylation. Appl. Environ. Microbiol. 2014, 80, 2941–2950. [Google Scholar] [CrossRef] [Green Version]
- Zeriouh, H.; de Vicente, A.; Perez-Garcia, A.; Romero, D. Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ. Microbiol. 2014, 16, 2196–2211. [Google Scholar] [CrossRef]
- Chaabouni, I.; Guesmi, A.; Cherif, A. Secondary metabolites of Bacillus: potentials in biotechnology in biotechnology. In Bacillus Thuringiensis Biotechnology; Sansinenea, E., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 347–366. [Google Scholar] [CrossRef]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive secondary metabolites from Bacillus subtilis: A comprehensive review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 2011, 33, 1523–1538. [Google Scholar] [CrossRef]
- Vater, J.; Kablitz, B.; Wilde, C.; Franke, P.; Mehta, N.; Cameotra, S.S. Matrix-assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl. Environ. Microbiol. 2002, 68, 6210–6219. [Google Scholar] [CrossRef] [Green Version]
- Koumoutsi, A.; Chen, X.H.; Henne, A.; Liesegang, H.; Hitzeroth, G.; Franke, P.; Vater, J.; Borriss, R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 2004, 186, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Vater, J.; Gao, X.; Hitzeroth, G.; Wilde, C.; Franke, P. “Whole cell”—Matrix-assisted laser desorption ionization-time of flight-mass spectrometry, an emerging technique for efficient screening of biocombinatorial libraries of natural compounds-present state of research. Comb. Chem. High. Throughput. Screen. 2003, 6, 557–567. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Shao, D.; Jiang, C.; Shi, J.; Li, Q.; Huang, Q.; Rajoka, M.S.R.; Yang, H.; Jin, M. Biological activity of lipopeptides from Bacillus. Appl. Microbiol. Biotechnol. 2017, 101, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; McSpadden Gardener, B.B. Identification and characterization of novel genetic markers associated with biological control activities in Bacillus subtilis. Phytopathology 2006, 96, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Quan, X.; Xue, B.; Goodwin, P.H.; Lu, S.; Wang, J.; Du, W.; Wu, C. Isolation and identification of Bacillus subtilis strain YB-05 and its antifungal substances showing antagonism against Gaeumannomyces graminis var. tritici. Biol. Control 2015, 85, 52–58. [Google Scholar] [CrossRef]
- Landy, M.; Warren, G.H.; RosenmanM, S.B.; Colio, L.G. Bacillomycin; an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc. Soc. Exp. Biol. Med. 1948, 67, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Comby, M.; Gacoin, M.; Robineau, M.; Rabenoelina, F.; Ptas, S.; Dupont, J.; Profizi, C.; Baillieul, F. Screening of wheat endophytes as biological control agents against Fusarium head blight using two different in vitro tests. Microbiol. Res. 2017, 202, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ao, J.; Yang, W.; Jiao, L.; Zheng, T.; Chen, X. Purification and characterization of a novel antifungal protein secreted by Penicillium chrysogenum from an Arctic sediment. Appl. Microbiol. Biotechnol. 2013, 97, 10381–10390. [Google Scholar] [CrossRef]
- Sarven, M.S.; Hao, Q.; Deng, J.; Yang, F.; Wang, G.; Xiao, Y.; Xiao, X. Biological control of tomato gray mold caused by Botrytis Cinerea with the entomopathogenic fungus Metarhizium Anisopliae. Pathogens 2020, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Cao, S.; Wang, Z.; Xu, H.; Liang, J.; Liu, H.; Wang, G.; Ding, M.; Wang, Q.; Gong, C.; et al. An expanded subfamily of G-protein-coupled receptor genes in Fusarium graminearum required for wheat infection. Nat. Microbiol. 2019, 4, 1582–1591. [Google Scholar] [CrossRef]
- Bovill, W.D.; Horne, M.; Herde, D.; Davis, M.; Wildermuth, G.B.; Sutherland, M.W. Pyramiding QTL increases seedling resistance to crown rot (Fusarium pseudograminearum) of wheat (Triticum aestivum). Theor. Appl. Genet. 2010, 121, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhu, K.; Sun, Y.; Zheng, W.; Hou, Y.; Xu, J. Inhibitory effect of kresoxim-methyl on Fusarium pseudograminearum and control effect on crown root of wheat. Acta Phytopathol. Sin. 2022, 52. [Google Scholar] [CrossRef]
- Logan, N.A.; De Vos, P. Bacillus Cohn 1872, 174AL. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; De Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., Whitman, W., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 21–128. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Yao, S.; Pham, H.; Vater, J.; Wang, J. Lipopeptide antibiotics produced by the engineered strain Bacillus subtilis GEB3 and detection of its bioactivity. Sci. Agric. Sin. 2003, 36, 1496–1501. [Google Scholar] [CrossRef]
| Strain | Inhibition Diameter (mm) |
|---|---|
| QTH8 | 31.67 ± 0.68 a |
| QTH2 | 28.61 ± 0.49 b |
| QTH5 | 25.23 ± 0.25 d |
| QTH1 | 22.37 ± 0.26 e |
| QTH7 | 19.53 ± 0.31 f |
| Phytopathogenic Fungi | Inhibition Diameter (mm) |
|---|---|
| Hainesia lythri | 34.09 ± 0.14 a |
| Pestalotiopsis sp. | 31.58 ± 0.95 b |
| Botrytis cinerea | 31.25 ± 0.64 b |
| Curvularia lunata | 31.03 ± 1.03 b |
| Phyllosticta theaefolia | 30.98 ± 1.45 b |
| Fusarium graminearum | 30.97 ± 0.68 b |
| Phytophthora nicotianae | 21.27 ± 0.05 c |
| Sclerotinia sclerotiorum | 21.23 ± 1.45 c |
| Treatment | Root Length (cm) | Plant Height (cm) | Fresh Weight (g) | Disease Index | Efficacy (%) |
|---|---|---|---|---|---|
| Control | 15.49 ± 1.12 c | 12.87 ± 1.50 a | 0.18 ± 0.01 a | 70.24 ± 3.76 a | |
| Treatment 2 | 18.57 ± 1.96 b | 13.45 ± 1.87 a | 0.17 ± 0.01 a | 58.82 ± 6.32 b | 16.26 |
| Treatment 1 | 21.73 ± 0.38 a | 12.20 ± 0.41 a | 0.18 ± 0.01 a | 26.43 ± 5.18 c | 62.37 |
| Result | Characteristic | Result | |
|---|---|---|---|
| Shape | rod | Acid from: | |
| Endospore | + | D-Glucose | + |
| Gram stain | + | D-Mannitol | + |
| Citrate utilization | + | Glycerine | + |
| Nitrate reduction | + | Sucrose | + |
| MR reaction | − | Lactose | − |
| Indole production | − | Gas from: | |
| Starch hydrolysis | + | D-Glucose | − |
| Casein hydrolysis | + | D-Mannitol | − |
| Anaerobic growth | − | Glycerine | − |
| V-P test | + | Sucrose | + |
| Catalase test | + | Lactose | − |
| Litmus milk test: | Growth at: | ||
| Acid reaction | − | 5 °C | + |
| Alkaline reaction | − | 10 °C | + |
| Curd formation | − | 20 °C | + |
| Peptonization | + | 45 °C | + |
| Growth in 10% NaCl | + | 50 °C | − |
| Isolates | Plant Disease | Sources |
|---|---|---|
| Fusarium pseudograminearum | Wheat crown rot | This lab |
| Hainesia lythri | Peony coelomycete leaf spot | This lab |
| Pestalotiopsis sp. | Moonflower leaf spot | This lab |
| Botrytis cinerea | Tomato gray mold | This lab |
| Curvularia lunata | Maize leaf spot | This lab |
| Phyllosticta theaefolia | Tea white scab | This lab |
| Fusarium graminearum | Fusarium head blight | This lab |
| Phytophthora nicotianae | Tobacco black shank | This lab |
| Sclerotinia sclerotiorum | Rape sclerotinia stem rot | This lab |
| Primer | Sequence (5′-3′) | Use of Primers |
|---|---|---|
| bmyB-F | TGAAACAAAGGCATATGCTC | bmyB gene amplification |
| bmyB-R | AAAAATGCATCTGCCGTTCC | |
| ituC-F | TTCACTTTTGATCTGGCGAT | ituC gene amplification |
| ituC-R | CGTCCGGTACATTTTCAC | |
| srfAB-F | GTTCTCGCAGTCCAGCAGAAG | srfAB gene amplification |
| srfAB-R | GCCGAGCGTATCCGTACCGAG | |
| srfAA-F | GAAAGAGCGGCTGCTGAAAC | srfAA gene amplification |
| srfAA-R | CCCAATATTGCCGCAATGAC | |
| spaS-F | GGTTTGTTGGATGGAGCTGT | spaS gene amplification |
| spaS-R | GCAAGGAGTCAGAGCAAGGT | |
| bacA-F | CAGCTCATGGGAATGCTTTT | bacA gene amplification |
| bacA-R | CTCGGTCCTGAAGGGACAAG | |
| fenD-F | CCTGCAGAAGGAGAAGTGAAG | fenD gene amplification |
| fenD-R | TGCTCATCGTCTTCCGTTTC | |
| 27F | AGAGTTTGATCMTGGCTCAG | 16S rDNA gene amplification |
| 1492R | GGYTACCTTGTTACGCACTT | |
| gyrB 1 | GAAGTCATCATGACCGTTCTGCAYGCNGGNGGNAARTTYGA | gyrB gene amplification |
| gyrB 2 | AGCAGGGTACGGATGTGCGAGCCRTCNACRTCNGCRTCNGTCAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xu, J.; Fu, L.; Xu, G.; Lin, X.; Qiao, J.; Xia, Y. Biocontrol of Wheat Crown Rot Using Bacillus halotolerans QTH8. Pathogens 2022, 11, 595. https://doi.org/10.3390/pathogens11050595
Li S, Xu J, Fu L, Xu G, Lin X, Qiao J, Xia Y. Biocontrol of Wheat Crown Rot Using Bacillus halotolerans QTH8. Pathogens. 2022; 11(5):595. https://doi.org/10.3390/pathogens11050595
Chicago/Turabian StyleLi, Shen, Jianqiang Xu, Liming Fu, Guohui Xu, Xiaomin Lin, Junqing Qiao, and Yanfei Xia. 2022. "Biocontrol of Wheat Crown Rot Using Bacillus halotolerans QTH8" Pathogens 11, no. 5: 595. https://doi.org/10.3390/pathogens11050595
APA StyleLi, S., Xu, J., Fu, L., Xu, G., Lin, X., Qiao, J., & Xia, Y. (2022). Biocontrol of Wheat Crown Rot Using Bacillus halotolerans QTH8. Pathogens, 11(5), 595. https://doi.org/10.3390/pathogens11050595





