Pyoluteorin Produced by the Biocontrol Agent Pseudomonas protegens Is Involved in the Inhibition of Heterobasidion Species Present in Europe
Abstract
:1. Introduction
2. Results
2.1. Presence and Concentration of Selected Secondary Metabolites in the CFF of P. protegens (Strain DSMZ 13134)
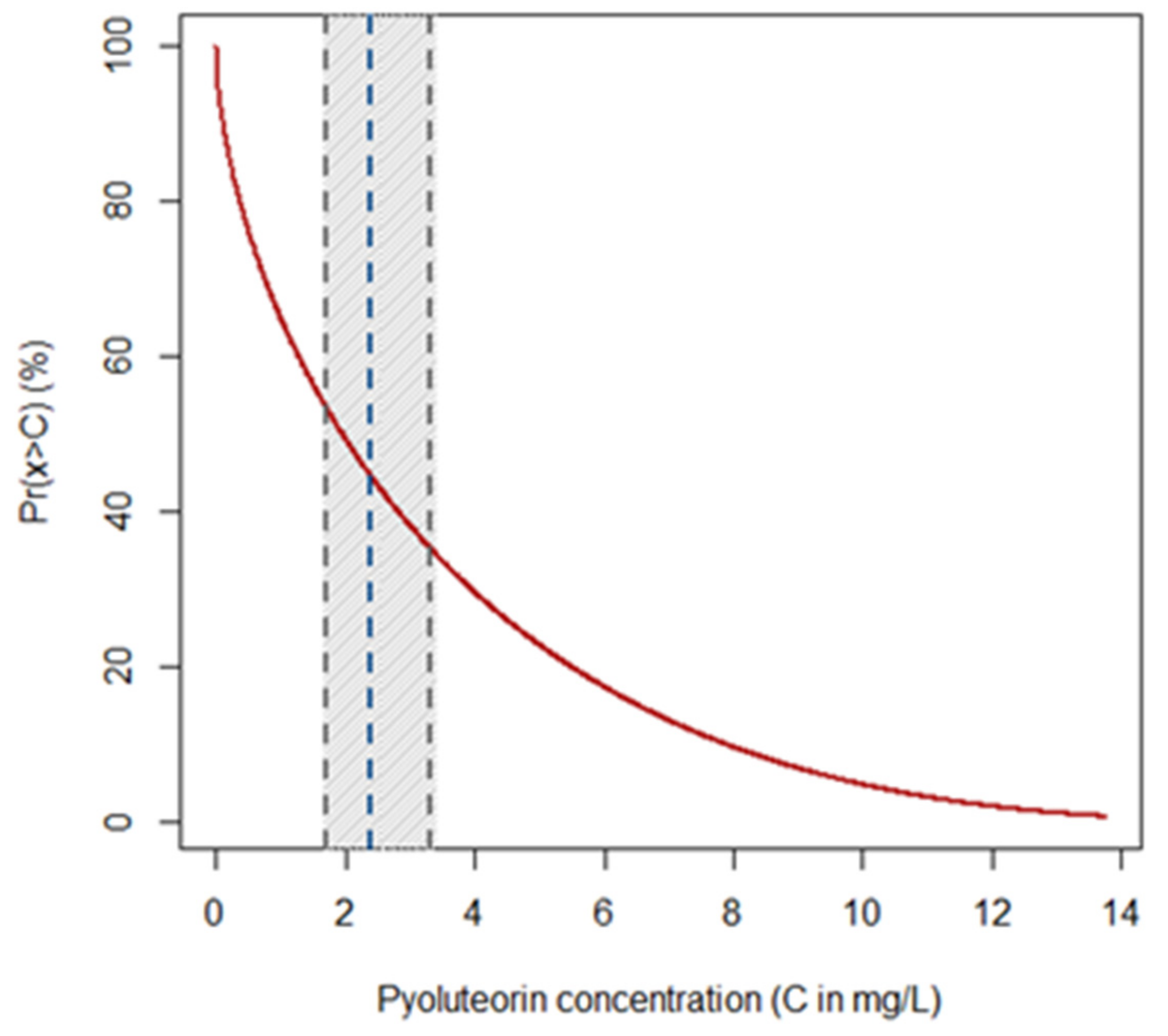
2.2. Effects of PLT on Mycelial Growth of Heterobasidion spp.
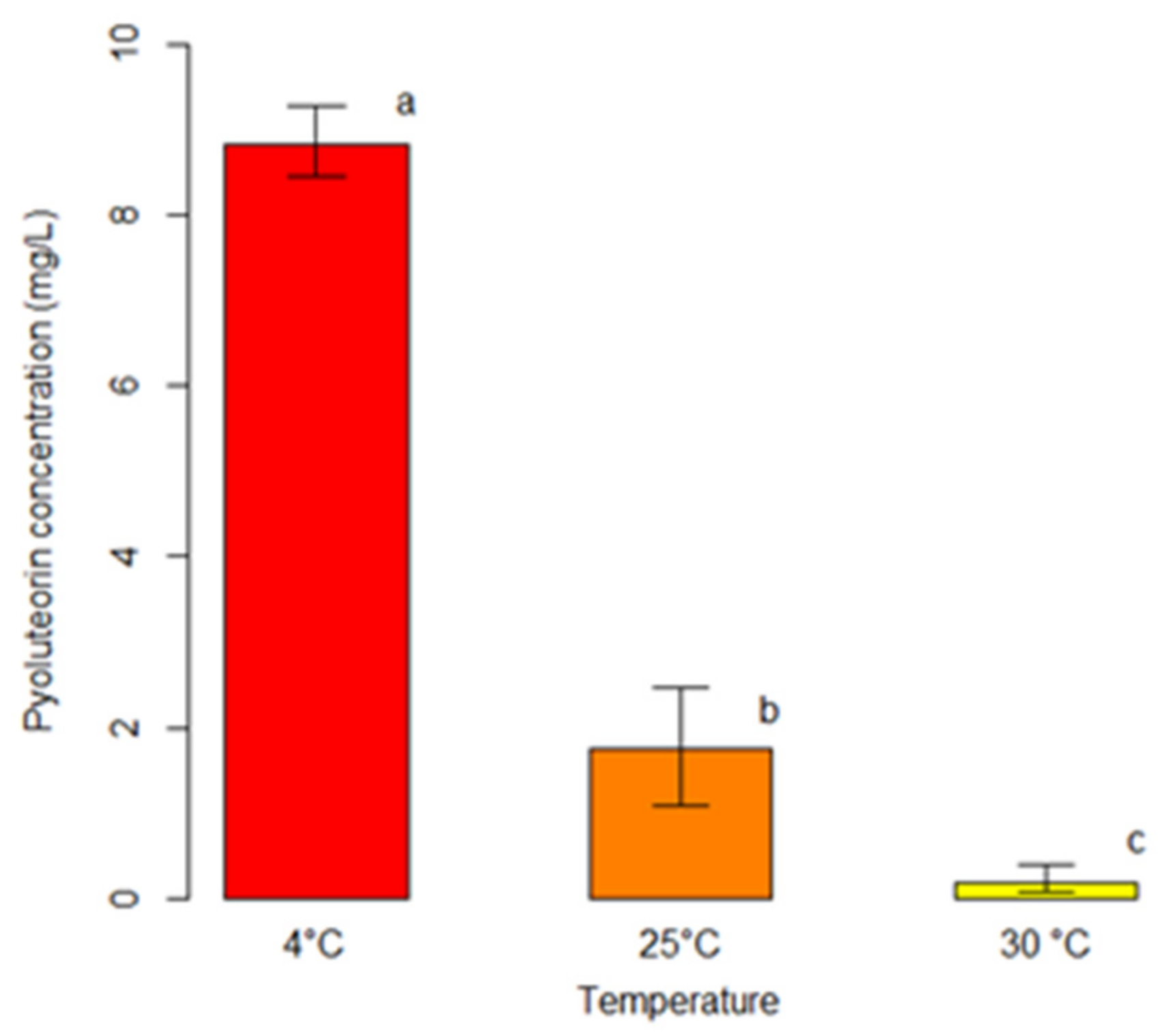
2.3. Effects of Culture Conditions on Secondary Metabolites Production by Monocultures of P. protegens (Strain DSMZ 13134)
2.4. Effects of Co-Cultures of P. protegens (Strain DSMZ 13134) with Heterobasidion spp. on Secondary Metabolites Production by the Bacterium
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Culture Conditions
4.2. Production of Monocultures of P. protegens (Strain DSMZ 13134) for the Quantification of 2,4-DAPG, PLT and PRN
4.3. Production of Co-Cultures of P. protegens (Strain DSMZ 13134) and Heterobasidion spp. for the Quantification of 2,4-DAPG, PLT and PRN
4.4. Quantification of 2,4-DAPG, PLT, PRN Produced by P. protegens (Strain DSMZ 13134)
4.5. Inhibition of Mycelial Growth of Heterobasidion spp. by PLT
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Arora, N.K. Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl. Soil Ecol. 2018, 125, 35–45. [Google Scholar] [CrossRef]
- Shahid, I.; Malik, K.A.; Mehnaz, S. A decade of understanding secondary metabolism in Pseudomonas spp. for sustainable agriculture and pharmaceutical applications. J. Environ. Sustain. 2018, 1, 3–17. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Cesa-Luna, C.; Baez, A.; Aguayo-Acosta, A.; Llano-Villarreal, R.C.; Juárez-González, V.R.; Gaytán, P.; Bustillos-Cristales, M.R.; Rivera-Urbalejo, A.; Muñoz-Rojas, J.; Quintero-Hernández, V. Growth inhibition of pathogenic microorganisms by Pseudomonas protegens EMM-1 and partial characterization of inhibitory substances. PLoS ONE 2020, 15, e0240545. [Google Scholar] [CrossRef]
- Shahid, I.; Han, J.; Hardie, D.; Baig, D.N.; Malik, K.A.; Borchers, C.H.; Mehnaz, S. Profiling of antimicrobial metabolites of plant growth promoting Pseudomonas spp. isolated from different plant hosts. 3 Biotech 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Garbelotto, M.; Gonthier, P. Biology, epidemiology, and control of Heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, P.; Nicolotti, G.; Linzer, R.; Guglielmo, F.; Garbelotto, M. Invasion of European pine stands by a North American forest pathogen and its hybridization with a native interfertile taxon. Mol. Ecol. 2007, 16, 1389–1400. [Google Scholar] [CrossRef]
- EPPO. Pest Risk Analysis for Heterobasidion Irregulare; EPPO: Paris, France, 2015; Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm (accessed on 27 February 2022).
- Gžibovska, Z. Evaluation of Phlebiopsis gigantea and Pseudomonas spp. for Biocontrol of Heterobasidion spp. in Norway Spruce. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2016. [Google Scholar]
- Rönnberg, J.; Magazniece, Z. Potential “new” protective agents for biocontrol of Heterobasidion spp. on Norway spruce. In Proceedings of the LIFE + ELMIAS Ash and Elm, and IUFRO WP 7.02.01 Program & Book of Abstracts Root and Stem Rots Conference (LIFE-IUFRO), Uppsala and Visby, Visby, Sweden, 26 August–1 September 2018; p. 27. [Google Scholar]
- Pellicciaro, M.; Lione, G.; Giordano, L.; Gonthier, P. Biocontrol potential of Pseudomonas protegens against Heterobasidion species attacking conifers in Europe. Biol. Control 2021, 157, 104583. [Google Scholar] [CrossRef]
- Pellicciaro, M.; Lione, G.; Ongaro, S.; Gonthier, P. Comparative efficacy of state-of-the-art and new biological stump treatments in forests infested by the native and the alien invasive Heterobasidion species present in Europe. Pathogens 2021, 10, 1272. [Google Scholar] [CrossRef]
- Roberti, R.; Veronesi, A.; Flamigni, F. Evaluation of microbial products for the control of zucchini foot and root rot caused by Fusarium solani f. sp. cucurbitae race 1. Phytopathol. Mediterr. 2012, 51, 317–331. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Pawar, S.; Chaudhari, A.; Prabha, R.; Shukla, R.; Singh, D.P. Microbial pyrrolnitrin: Natural metabolite with immense practical utility. Biomolecules 2019, 9, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, R. Pseudomonas pigments. III. Derivatives of pyoluteorin. Bull. Agric. Chem. Soc. Jpn. 1959, 23, 126–130. [Google Scholar] [CrossRef]
- Kidarsa, T.A.; Goebel, N.C.; Zabriskie, T.M.; Loper, J.E. Phloroglucinol mediates cross-talk between the pyoluteorin and 2,4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Mol. Microbiol. 2011, 81, 395–414. [Google Scholar] [CrossRef]
- Quecine, M.C.; Kidarsa, T.A.; Goebel, N.C.; Shaffer, B.T.; Henkels, M.D.; Zabriskie, T.M.; Loper, J.E. An interspecies signaling system mediated by fusaric acid has parallel effects on antifungal metabolite production by Pseudomonas protegens strain Pf-5 and antibiosis of Fusarium spp. Appl. Environ. Microbiol. 2016, 82, 1372–1382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, W.; Lu, X.; Xu, Y.; Zhang, X. The stability and degradation of a new biological pesticide, pyoluteorin. Pest Manag. Sci. 2010, 66, 248–252. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef]
- Abdelwahab, M.F.; Kurtán, T.; Mándi, A.; Müller, W.E.; Fouad, M.A.; Kamel, M.S.; Liu, Z.; Ebrahim, W.; Daletos, G.; Proksch, P. Induced secondary metabolites from the endophytic fungus Aspergillus versicolor through bacterial co-culture and OSMAC approaches. Tetrahedron Lett. 2018, 59, 2647–2652. [Google Scholar] [CrossRef] [Green Version]
- Ramette, A.; Frapolli, M.; Fischer-Le Saux, M.; Gruffaz, C.; Meyer, J.M.; Défago, G.; Sutrab, L.; Moënne-Loccoz, Y. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2, 4-diacetylphloroglucinol and pyoluteorin. Syst. Appl. Microbiol. 2011, 34, 180–188. [Google Scholar] [CrossRef]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.; Zubair, M.; Iqbal, M. Biocontrol of bacterial leaf blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar] [CrossRef] [Green Version]
- Prigigallo, M.I.; De Stradis, A.; Anand, A.; Mannerucci, F.; L’Haridon, F.; Weisskopf, L.; Bubici, G. Basidiomycetes are particularly sensitive to bacterial volatile compounds: Mechanistic insight into the case study of Pseudomonas protegens volatilome against Heterobasidion abietinum. Front. Microb. 2021, 12, 684664. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Thompson, B.; Gould, S.J.; Kraus, J.; Loper, J.E. Production of 2,4-diacetylphloroglucinol by the biocontrol agent Pseudomonas fluorescens Pf-5. Can. J. Microbiol. 1994, 40, 1064–1066. [Google Scholar] [CrossRef]
- Kraus, J.; Loper, J.E. Characterization of a genomic region required for production of the antibiotic pyoluteorin by the biological control agent Pseudomonas fluorescens Pf-5. Appl. Environ. Microbiol. 1995, 61, 849–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, B.K.; Défago, G. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 1999, 65, 2429–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodhagen, M.; Henkels, M.D.; Loper, J.E. Positive autoregulation and signaling properties of pyoluteorin, an antibiotic produced by the biological control organism Pseudomonas fluorescens Pf-5. Appl. Environ. Microbiol. 2004, 70, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Philmus, B.; Chang, J.H.; Loper, J.E. Novel mechanism of metabolic co-regulation coordinates the biosynthesis of secondary metabolites in Pseudomonas protegens. eLife 2017, 6, e22835. [Google Scholar] [CrossRef]
- Biessy, A.; Filion, M. Phloroglucinol Derivatives in Plant-Beneficial Pseudomonas spp.: Biosynthesis, Regulation, and Functions. Metabolites 2021, 11, 182. [Google Scholar] [CrossRef]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-cultivation—A powerful emerging tool for enhancing the chemical diversity of microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Moussa, M.; Ebrahim, W.; Bonus, M.; Gohlke, H.; Mándi, A.; Kurtán, T.; Hartmann, R.; Kalscheuer, R.; Lin, W.; Liu, Z.; et al. Co-culture of the fungus Fusarium tricinctum with Streptomyces lividans induces production of cryptic naphthoquinone dimers. RSC Adv. 2019, 9, 1491–1500. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Tang, J.; Karuppiah, V.; Li, Y.; Xu, N.; Chen, J. Co-culture of Trichoderma atroviride SG3403 and Bacillus subtilis 22 improves the production of antifungal secondary metabolites. Biol. Control 2020, 140, 104122. [Google Scholar] [CrossRef]
- Shanahan, P.; O’Sullivan, D.J.; Simpson, P.; Glennon, J.D.; O’Gara, F. Isolation of 2, 4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl. Environ. Microbiol. 1992, 58, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raaijmakers, J.M.; Vlami, M.; de Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Charyulu, E.M.; Gnanamani, A. Condition stabilization for Pseudomonas aeruginosa MTCC 5210 to yield high titers of extra cellular antimicrobial secondary metabolite using response surface methodology. Curr. Res. Bacteriol. 2010, 4, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Horak, I.; Engelbrecht, G.; van Rensburg, P.J.; Claassens, S. Microbial metabolomics: Essential definitions and the importance of cultivation conditions for utilizing Bacillus species as bionematicides. J. Appl. Microbiol. 2019, 127, 326–343. [Google Scholar] [CrossRef] [Green Version]
- Tomm, H.A.; Ucciferri, L.; Ross, A.C. Advances in microbial culturing conditions to activate silent biosynthetic gene clusters for novel metabolite production. J. Ind. Microbiol. 2019, 46, 1381–1400. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Lee, J.Y.; Moon, S.S.; Hwang, B.K. Isolation and antifungal and antioomycete activities of aerugine produced by Pseudomonas fluorescens strain MM-B16. Appl. Environ. Microbiol. 2003, 69, 2023–2031. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, A.; Singh, B.; Joshi, S.; Johri, B.N. Production and characterization of an antifungal compound from Pseudomonas protegens strain W45. Proc. Natl. Acad. Sci. India B Biol. Sci. 2018, 88, 1081–1089. [Google Scholar] [CrossRef]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Zeileis, A. partykit: A modular toolkit for recursive partytioning in R. J. Mach. Learn. Res. 2015, 16, 3905–3909. Available online: http://jmlr.org/papers/v16/hothorn15a.html (accessed on 31 August 2021).
- Lione, G.; Giordano, L.; Turina, M.; Gonthier, P. Hail-induced infections of the chestnut blight pathogen Cryphonectria parasitica depend on wound size and may lead to severe diebacks. Phytopathology 2020, 110, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K. Contributions to the mathematical theory of evolution.-II: Skew variation in homogeneous material. Philos. Trans. R. Soc. Lond. 1895, 186, 343–414. [Google Scholar] [CrossRef] [Green Version]
- Rietz, H.L. Mathematical Statistics; The Open Court Publishing Company: Chicago, IL, USA, 1927. [Google Scholar]
- Lachene, B. On Pearson families of distributions and its applications. Afr. J. Math. Comput. Sci. Res. 2013, 6, 108–117. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, 2–8 September 1971; Petrov, C., Ed.; Akadémiai Kiadó: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Cousineau, D.; Brown, S.; Heathcote, A. Fitting distributions using maximum likelihood: Methods and packages. Behav. Res. Meth. Instrum. Comput. 2004, 36, 742–756. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book, 2nd ed.; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- DiCiccio, T.J.; Efron, B. Bootstrap confidence intervals. Stat. Sci. 1996, 11, 189–228. [Google Scholar] [CrossRef]
- Carsey, T.M.; Harden, J.J. Monte Carlo Simulation and Resampling Methods for Social Science; SAGE Publications Inc.: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 31 August 2021).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap, S original, from StatLib and by Rob Tibshirani. R port by Friedrich Leisch. 2019. Bootstrap: Functions for the Book “An Introduction to the Bootstrap”; R package version 2019.6; CRC Press: Boca Raton, FL, USA, 1994; Available online: https://CRAN.R-project.org/package=bootstrap (accessed on 31 August 2021).
- Zeileis, A.; Leisch, F.; Hornik, K.; Kleiber, C. strucchange: An R Package for Testing for Structural Change in Linear Regression Models. J. Stat. Softw. 2002, 7, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.; Klößner, S. PearsonDS: Pearson Distribution System; R package Version 0.97. 2013. Available online: http://CRAN.R-project.org/package=PearsonDS (accessed on 22 January 2016).
- European Union Commission. Commission Implementing Regulation (EU) No 2021/745 of 6 May 2021 Amending Implementing Regulation (EU) No 540/2011; European Union Commission: Brussels, Belgium, 2021. [Google Scholar]

| Monocultures of P. protegens (Strain DSMZ 13134) | Mean Concentration ± SD 1 (mg/L) | ||||
|---|---|---|---|---|---|
| Culture Medium | Incubation Temperature (°C) | Incubation Period | 2,4-DAPG | PLT | PRN |
| Luria–Bertani broth | 4 | 24 h | 0.17 ± 0.00 | 0.01 ± 0.00 | <LOD 2 |
| 7 days | <LOD | 3.63 ± 0.38 | <LOD | ||
| 25 | 24 h | <LOD | 3.77 ± 0.30 | <LOD | |
| 7 days | <LOD | 0.66 ± 0.00 | <LOD | ||
| 30 | 24 h | 0.26 ± 0.00 | 0.81 ± 0.38 | <LOD | |
| 7 days | <LOD | 0.35 ± 0.24 | <LOD | ||
| King B broth | 4 | 24 h | <LOQ 3 | 0.32 ± 0.00 | <LOD |
| 7 days | <LOQ | 8.03 ± 0.00 | <LOD | ||
| 25 | 24 h | <LOQ | 6.12 ± 0.42 | <LOD | |
| 7 days | <LOQ | 2.34 ± 0.01 | <LOD | ||
| 30 | 24 h | <LOQ | 0.37 ± 0.20 | <LOD | |
| 7 days | <LOQ | 0.49 ± 0.50 | <LOD | ||
| Modified King B broth | 4 | 24 h | <LOD | 0.27 ± 0.00 | <LOD |
| 7 days | <LOD | <LOD | <LOD | ||
| 25 | 24 h | <LOD | <LOD | <LOD | |
| 7 days | <LOD | 1.10 ± 0.02 | <LOD | ||
| 30 | 24 h | <LOD | 4.52 ± 0.49 | <LOD | |
| 7 days | <LOD | 10.21 ± 3.18 | <LOD | ||
| Response Variable | Incubation Temperature | Culture Medium | Incubation Period | Co-Culture Type |
|---|---|---|---|---|
| Concentration of PLT produced by monocultures of P. protegens (strain DSMZ 13134) | c = 0.542 | c = 2.132 | c = 1.985 | - |
| p = 0.987 | p = 0.718 | p = 0.405 | - | |
| Concentration of PLT produced by co-cultures of P. protegens (strain DSMZ 13134) and Heterobasidion spp. | c1= 33.35 * | - | - | c1 = 0.626 |
| p1 = 1.142 × 10−7 | - | - | p1 = 0.988 | |
| c2 = 10.078 * | - | - | c2 = 4.754 | |
| p2 = 2.999 × 10−3 | - | - | p2 = 0.345 |
| Co-Cultures of P. protegens (Strain DSMZ 13134) and Heterobasidion spp. | Mean Concentration ± SD 1 (mg/L) | |||
|---|---|---|---|---|
| Incubation Temperature (°C) | Heterobasidion Species | 2,4-DAPG | PLT | PRN |
| 4 | H. abietinum | <LOQ 2 | 8.05 ± 0.01 | <LOD 3 |
| H. annosum | <LOQ | 9.14 ± 0.01 | <LOD | |
| H. irregulare | <LOQ | 9.90 ± 0.02 | <LOD | |
| H. parviporum | <LOQ | 8.28 ± 0.15 | <LOD | |
| 25 | H. abietinum | <LOQ | 2.52 ± 0.02 | <LOD |
| H. annosum | <LOQ | 0.84 ± 0.00 | <LOD | |
| H. irregulare | <LOQ | 3.31 ± 0.13 | <LOD | |
| H. parviporum | <LOQ | <LOQ | <LOD | |
| 30 | H. abietinum | <LOQ | <LOD | <LOD |
| H. annosum | <LOQ | 0.16 ± 0.18 | <LOD | |
| H. irregulare | <LOQ | 0.07 ± 0.12 | <LOD | |
| H. parviporum | <LOQ | 0.51 ± 0.30 | <LOD | |
| MUT 1 Accession Number | Heterobasidion Species | Isolation Date | Geographic Origin |
|---|---|---|---|
| 6194 * | H. abietinum | 2016 | Nus, AO, Italy |
| 6195 | H. abietinum | 2018 | Chiusa di Pesio, CN, Italy |
| 6196 | H. abietinum | 2018 | Chiusa di Pesio, CN, Italy |
| 6197 | H. abietinum | 2018 | Chiusa di Pesio, CN, Italy |
| 6198 | H. abietinum | 2016 | Chabodey, AO, Italy |
| 3543 * | H. annosum | 2006 | Mesola, FE, Italy |
| 1204 | H. annosum | 2005 | Sabaudia, LT, Italy |
| 3538 | H. annosum | 2006 | Ansedonia, GR, Italy |
| 3656 | H. annosum | 2006 | Sabaudia, LT, Italy |
| 6191 | H. annosum | 2015 | Saint-Denis, AO, Italy |
| 1193 * | H. irregulare | 2005 | Castelfusano, RM, Italy |
| 1151 | H. irregulare | 2005 | Sabaudia, LT, Italy |
| 1197 | H. irregulare | 2005 | Sabaudia, LT, Italy |
| 3627 | H. irregulare | 2005 | Sabaudia, LT, Italy |
| 5666 | H. irregulare | 2006 | Nettuno, RM, Italy |
| 5612 * | H. parviporum | 2006 | Trasquera, VB, Italy |
| 5605 | H. parviporum | 2006 | Druogno, VB, Italy |
| 5615 | H. parviporum | 1999 | Charvensod, AO, Italy |
| 6192 | H. parviporum | 2016 | Chabodey, AO, Italy |
| 6193 | H. parviporum | 2016 | Chabodey, AO, Italy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellicciaro, M.; Padoan, E.; Lione, G.; Celi, L.; Gonthier, P. Pyoluteorin Produced by the Biocontrol Agent Pseudomonas protegens Is Involved in the Inhibition of Heterobasidion Species Present in Europe. Pathogens 2022, 11, 391. https://doi.org/10.3390/pathogens11040391
Pellicciaro M, Padoan E, Lione G, Celi L, Gonthier P. Pyoluteorin Produced by the Biocontrol Agent Pseudomonas protegens Is Involved in the Inhibition of Heterobasidion Species Present in Europe. Pathogens. 2022; 11(4):391. https://doi.org/10.3390/pathogens11040391
Chicago/Turabian StylePellicciaro, Martina, Elio Padoan, Guglielmo Lione, Luisella Celi, and Paolo Gonthier. 2022. "Pyoluteorin Produced by the Biocontrol Agent Pseudomonas protegens Is Involved in the Inhibition of Heterobasidion Species Present in Europe" Pathogens 11, no. 4: 391. https://doi.org/10.3390/pathogens11040391
APA StylePellicciaro, M., Padoan, E., Lione, G., Celi, L., & Gonthier, P. (2022). Pyoluteorin Produced by the Biocontrol Agent Pseudomonas protegens Is Involved in the Inhibition of Heterobasidion Species Present in Europe. Pathogens, 11(4), 391. https://doi.org/10.3390/pathogens11040391








