Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis
Abstract
:1. Introduction
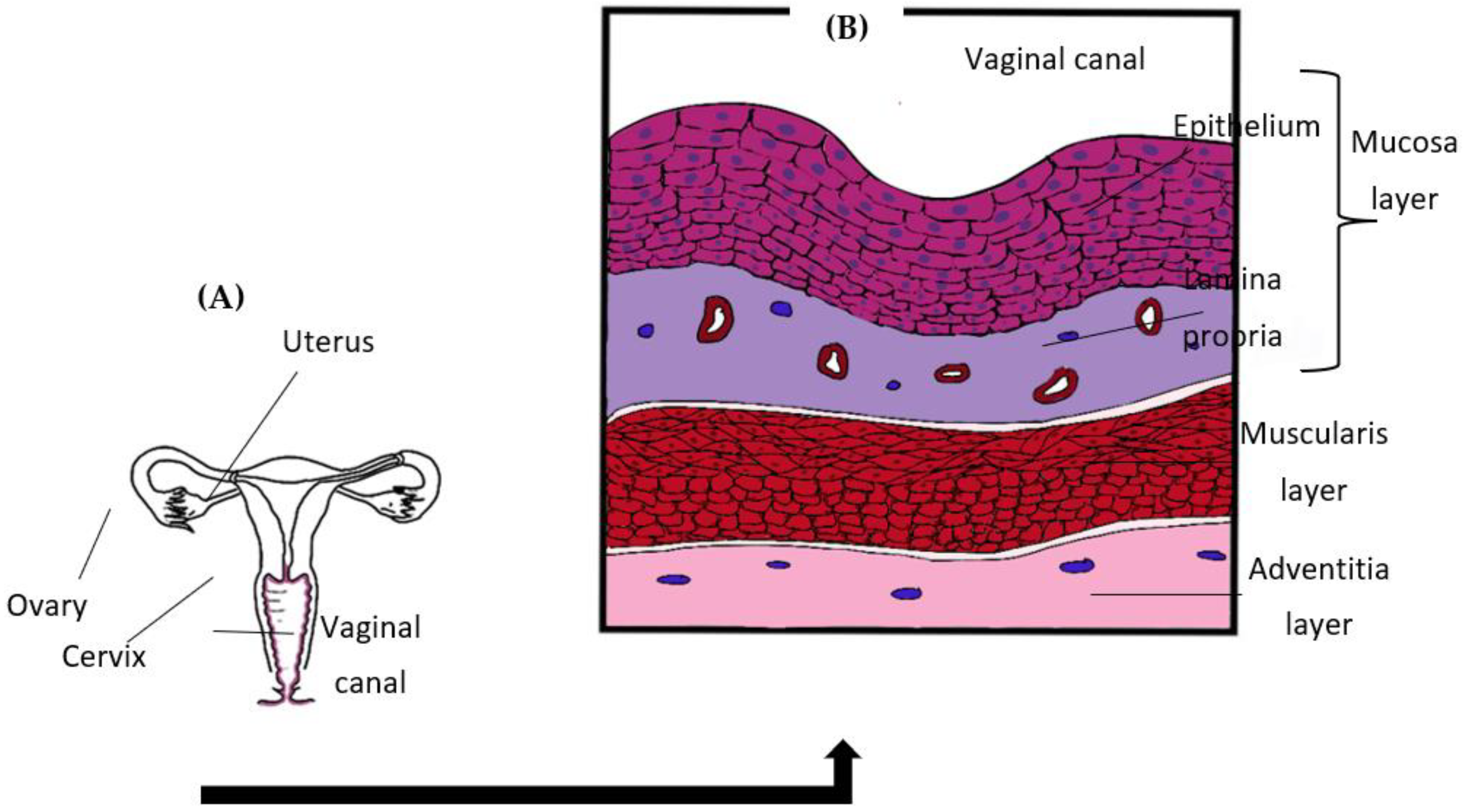
2. Vaginal Mucosa
2.1. Vaginal Structure
2.2. VEC and Its Significance
3. Vaginal Host Immunity
3.1. Role of PRRs in Immune Recognition
3.2. Role of PAMPs in Immune Recognition
3.3. Antimicrobial Defence Mechanism against C. albicans
3.4. Adaptive Immune Responses upon VVC Occurrence
4. Vaginal Microbiota
4.1. Vaginal Microbiota Plays a Predominant Role in VVC Occurrence
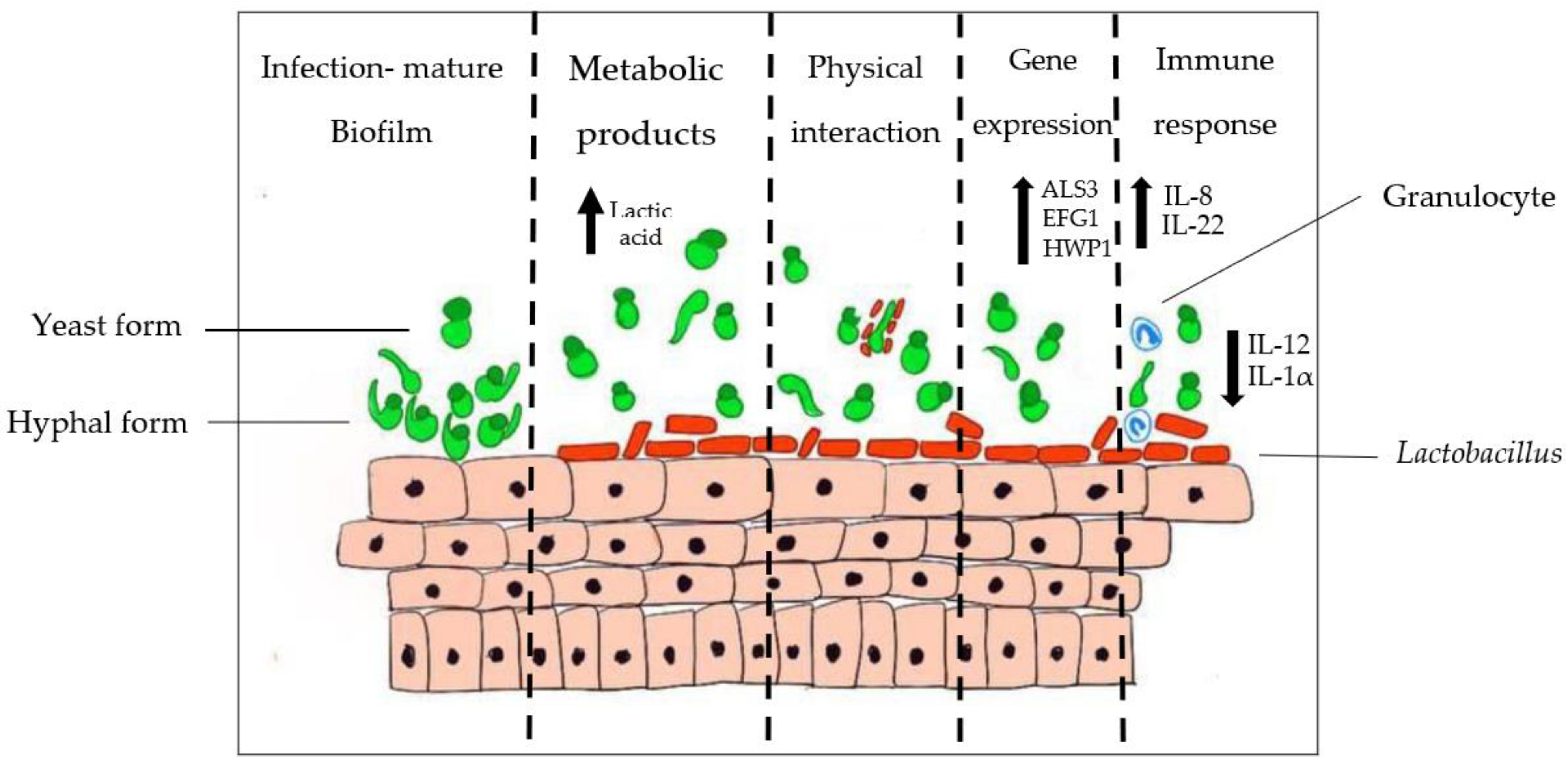
4.2. Interaction between Lactobacillus and Candida
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalia, N.; Singh, J.; Kaur, M. Immunopathology of recurrent vulvovaginal infections: New aspects and research directions. Front. Immunol. 2019, 10, 2034. [Google Scholar] [CrossRef] [PubMed]
- Bitew, A.; Abebaw, Y. Vulvovaginal candidiasis: Species distribution of Candida and their antifungal susceptibility pattern. BMC Women’s Health 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Sasani, E.; Rafat, Z.; Ashrafi, K.; Salimi, Y.; Zandi, M.; Soltani, S.; Hashemi, F.; Hashemi, S.J. Vulvovaginal candidiasis in Iran: A systematic review and meta-analysis on the epidemiology, clinical manifestations, demographic characteristics, risk factors, etiologic agents and laboratory diagnosis. Microb. Pathog. 2021, 154, 104802. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi Nasir, I.; Uchenna, E.; Onyia, J.; Ifunanya, A.L. Prevalence of vulvovaginal candidiasis among nonpregnant women attending a tertiary health care facility in Abuja, Nigeria. Res. Rep. Trop. Med. 2015, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- O’Hanlon, D.E.; Come, R.A.; Moench, T.R. Vaginal pH measured in vivo: Lactobacilli determine pH and lactic acid concentration. BMC Microbiol. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Zhang, Y.; Zhang, T.; Xue, Y.; Xu, H.; An, R. Risk Factors of Vulvovaginal Candidiasis among Women of Reproductive Age in Xi’an: A Cross-Sectional Study. Biomed Res. Int. 2018, 2018, 9703754. [Google Scholar] [CrossRef] [Green Version]
- Lírio, J.; Giraldo, P.C.; Amaral, R.L.; Sarmento, A.C.A.; Costa, A.P.F.; Goncalves, A.K. Antifungal (oral and vaginal) therapy for recurrent vulvovaginal candidiasis: A systematic review protocol. BMJ Open 2019, 9, e027489. [Google Scholar] [CrossRef] [Green Version]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Seidu, L. Chronic Vulvovaginal Candida Hypersensitivity: An Underrecognized and Undertreated Disorder by Allergists. Allergy Rhinol. 2015, 6, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Dovnik, A.; Golle, A.; Novak, D.; Arko, D.; Takač, I. Treatment of vulvovaginal candidiasis: A review of the literature. Acta Dermatovenerol. Alp. Pannonica Adriat. 2015, 24, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, V.; Ballal, M. Distribution of Candida Species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in Southern India. J. Glob. Infect. Dis. 2011, 3, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaffin, W.L. Candida albicans Cell Wall Proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. [Google Scholar] [CrossRef] [Green Version]
- Timmermans, B.; Las Peñas, A.D.; Castaño, I.; Van Dijck, P. Adhesins in candida glabrata. J. Fungi 2018, 4, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaiarasan, K.; Singh, R.; Chaturvedula, L. Changing virulence factors among vaginal non-albicans Candida species. Indian J. Med. Microbiol. 2018, 36, 364–368. [Google Scholar] [CrossRef]
- Nett, J.; Andes, D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr. Opin. Microbiol. 2006, 9, 340–345. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef]
- Ardizzoni, A.; Wheeler, R.T.; Pericolini, E. It Takes Two to Tango: How a Dysregulation of the Innate Immunity, Coupled With Candida Virulence, Triggers VVC Onset. Front. Microbiol. 2021, 12, 692491. [Google Scholar] [CrossRef]
- Ali, A.; Azab, M.; Abdelrahman, A. Distribution of secreted aspartyl protease (SAP) virulence genes and antifungal resistance genes at vulvovaginal candidiasis isolates. GSC Biol. Pharm. Sci. 2018, 5, 86–94. [Google Scholar] [CrossRef]
- Morrow, B.; Srikantha, T.; Soll, D.R. Transcription of the gene for a pepsinogen, PEP1, is regulated by white-opaque switching in Candida albicans. Mol. Cell. Biol. 1992, 12, 2997–3005. [Google Scholar] [PubMed] [Green Version]
- Naglik, J.R.; Rodgers, C.A.; Shirlaw, P.J.; Dobbie, J.L.; Fernandes-Naglik, L.L.; Greenspan, D.; Agabian, N.; Challacombe, S.J. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J. Infect. Dis. 2003, 188, 469–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roselletti, E.; Perito, S.; Gabrielli, E.; Mencacci, A.; Pericolini, E.; Sabbatini, S.; Cassone, A.; Vecchiarelli, A. NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans. Sci. Rep. 2017, 7, 17877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pericolini, E.; Gabrielli, E.; Amacker, M.; Kasper, L.; Roselletti, E.; Luciano, E.; Sabbatini, S.; Kaeser, M.; Moser, C.; Hube, B.; et al. Secretory aspartyl proteinases cause vaginitis and can mediate vaginitis caused by Candida albicans in mice. MBio 2015, 6, e00724-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.P.; Willems, H.M.E.; Moyes, D.L.; Shoaie, S.; Barker, K.S.; Tan, S.L.; Palmer, G.E.; Hube, B.; Naglik, J.R.; Peters, B.M. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect. Immun. 2018, 86, e00645-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.J.; Marathe, J.; Pudney, J. The Structure of the Human Vaginal Stratum Corneum and its Role in Immune Defense. Am. J. Reprod. Immunol. 2014, 71, 618–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patton, D.L.; Thwin, S.S.; Meier, A.; Hooton, T.M.; Stapleton, A.E.; Eschenbach, D.A. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am. J. Obstet. Gynecol. 2000, 183, 967–973. [Google Scholar] [CrossRef]
- Linhares, I.M.; Sisti, G.; Minis, E.; de Freitas, G.B.; Moron, A.F.; Witkin, S.S. Contribution of Epithelial Cells to Defense Mechanisms in the Human Vagina. Curr. Infect. Dis. Rep. 2019, 21, 30. [Google Scholar] [CrossRef]
- Blaskewicz, C.D.; Pudney, J.; Anderson, D.J. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol. Reprod. 2011, 85, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Villar, C.C.; Kashleva, H.; Nobile, C.J.; Mitchell, A.P.; Dongari-Bagtzoglou, A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect. Immun. 2007, 75, 2126–2135. [Google Scholar] [CrossRef] [Green Version]
- Frank, C.F.; Hostetter, M.K. Cleavage of E-cadherin: A mechanism for disruption of the intestinal epithelial barrier by Candida albicans. Transl. Res. 2007, 149, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, A.; Bruce, C.; Anumba, D.O. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum. Reprod. 2005, 20, 1372–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwissa, M.; Nakaya, H.I.; Oluoch, H.; Pulendran, B. Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood 2012, 119, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.R.; Reyes, J.L.; Iannuzzi, J.; Leung, G.; McKay, D.M. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS ONE 2014, 9, e94188. [Google Scholar] [CrossRef]
- Rosati, D.; Bruno, M.; Jaeger, M.; Kullberg, B.-J.; van de Veerdonk, F.; Netea, M.G.; ten Oever, J. An Exaggerated Monocyte-Derived Cytokine Response to Candida Hyphae in Patients With Recurrent Vulvovaginal Candidiasis. J. Infect. Dis. 2020, 23, jiaa444. [Google Scholar] [CrossRef]
- Steele, C.; Fidel, P.L. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect. Immun. 2002, 70, 577–583. [Google Scholar] [CrossRef] [Green Version]
- McClure, R.; Massari, P. TLR-dependent human mucosal epithelial cell responses to microbial pathogens. Front. Immunol. 2014, 5, 386. [Google Scholar] [CrossRef] [Green Version]
- Pudney, J.; Quayle, A.J.; Anderson, D.J. Immunological Microenvironments in the Human Vagina and Cervix: Mediators of Cellular Immunity Are Concentrated in the Cervical Transformation Zone. Biol. Reprod. 2005, 73, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Madanchi, H.; Shoushtari, M.; Kashani, H.H.; Sardari, S. Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases. New Microbes New Infect. 2020, 34, 100627. [Google Scholar] [CrossRef]
- Besold, A.N.; Gilston, B.A.; Radin, J.N.; Ramsoomair, C.; Culbertson, E.M.; Li, C.X.; Cormack, B.P.; Chazin, W.J.; Kehl-Fie, T.E.; Culotta, V.C. Role of Calprotectin in Withholding Zinc and Copper from Candida albicans. Infect. Immun. 2018, 86, e00779-17. [Google Scholar] [CrossRef] [Green Version]
- Curvelo, J.A.D.R.; Barreto, A.L.S.; Portela, M.B.; Alviano, D.S.; Holandino, C.; Souto-Padrón, T.; Soares, R.M.D.A. Effect of the secretory leucocyte proteinase inhibitor (SLPI) on Candida albicans biological processes: A therapeutic alternative? Arch. Oral Biol. 2014, 59, 928–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chairatana, P.; Chiang, I.L.; Nolan, E.M. Human α-Defensin 6 Self-Assembly Prevents Adhesion and Suppresses Virulence Traits of Candida albicans. Biochemistry 2017, 56, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Miró, M.S.; Caeiro, J.P.; Rodriguez, E.; Vargas, L.; Vigezzi, C.; Icely, P.A.; Castillo, G.D.V.; Azcurra, A.I.; Abiega, C.D.; Riera, F.O.; et al. Candida albicans Modulates Murine and Human Beta Defensin-1 during Vaginitis. J. Fungi 2022, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N. Telling apart friend from foe: Discriminating between commensals and pathogens at mucosal sites. Innate Immun. 2010, 16, 391–404. [Google Scholar] [CrossRef]
- Swidergall, M. Candida albicans at Host Barrier Sites: Pattern Recognition Receptors and Beyond. Pathogens 2019, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.; Peters, B.M.; Noverr, M.C.; Fidel, P.L., Jr. Novel mechanism behind the immunopathogenesis of vulvovaginal candidiasis:“neutrophil anergy. Infect. Immun. 2018, 86, e00684-17. [Google Scholar] [CrossRef] [Green Version]
- Wira, C.R.; Fahey, J.V.; Sentman, C.L.; Pioli, P.A.; Shen, L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 2005, 206, 306–335. [Google Scholar] [CrossRef]
- Bojang, E.; Ghuman, H.; Kumwenda, P.; Hall, R.A. Immune sensing of Candida albicans. J. Fungi 2021, 7, 119. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, M.J.; Lee, S.; Choi, S.; Jung, K.H.; Jung, J.; Chong, Y.P.; Kim, S.H.; Choi, S.H.; Kim, Y.S.; et al. Risk factors and outcomes of patients with ocular involvement of candidemia. PLoS ONE 2019, 14, e0222356. [Google Scholar] [CrossRef]
- Fradin, C.; De Groot, P.; MacCallum, D.; Schaller, M.; Klis, F.; Odds, F.C.; Hube, B. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 2005, 56, 397–415. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Luo, G.; Gebremariam, T.; Lee, H.; Schmidt, C.S.; Hennessey, J.P.; French, S.W.; Yeaman, M.R.; Filler, S.G.; Edwards, J.E. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine 2013, 31, 5549–5556. [Google Scholar] [CrossRef] [Green Version]
- Cambi, A.; Gijzen, K.; de Vries, I.J.M.; Torensma, R.; Joosten, B.; Adema, G.J.; Netea, M.G.; Kullberg, B.J.; Romani, L.; Figdor, C.G. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 2003, 33, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A.R. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatinguais, R.; Willment, J.A.; Brown, G.D. PAMPs of the Fungal Cell Wall and Mammalian PRRs. In The Fungal Cell Wall: An Armour and a Weapon for Human Fungal Pathogens, 2nd ed.; Latgé, J.P., Ed.; Springer Nature: Cham, Switzerland, 2020; Volume 425, pp. 187–223. [Google Scholar]
- Chen, S.M.; Shen, H.; Zhang, T.; Huang, X.; Liu, X.Q.; Guo, S.Y.; Zhao, J.J.; Wang, C.F.; Yan, L.; Xu, G.T.; et al. Dectin-1 plays an important role in host defense against systemic Candida glabrata infection. Virulence 2017, 8, 1643–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Toll-Like Receptors and Innate Immunity. Curr. Biol. 2017, 27, R577–R578. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, M.; Plantinga, T.S.; Joosten, L.A.B.; Kullberg, B.J.; Netea, M.G. Genetic basis for recurrent vulvo-vaginal candidiasis. Curr. Infect. Dis. Rep. 2013, 15, 136–142. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Ohno, N.; Murai, T. Suppression by Candida albicans β-glucan of cytokine release from activated human monocytes and from T cells in the presence of monocytes. J. Infect. Dis. 2003, 187, 710–713. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.D.; Gordon, S. Fungal β-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, J.E. N-glycosylation of yeast, with emphasis on Candida albicans. Med. Mycol. Suppl. 2001, 39, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Pericolini, E.; Perito, S.; Castagnoli, A.; Gabrielli, E.; Mencacci, A.; Blasi, E.; Vecchiarelli, A.; Wheeler, R.T. Epitope unmasking in vulvovaginal candidiasis is associated with hyphal growth and neutrophilic infiltration. PLoS ONE 2018, 13, e0201436. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013, 31, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R.; Goud, N.S.; Saraswati, A.P.; Alvala, R.; Alvala, M. Antimicrobial Peptides: A Promising Therapeutic Strategy in Tackling Antimicrobial Resistance. Curr. Med. Chem. 2017, 24, 4303–4314. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G. Antimicrobial peptides of multicellular organisms. Life Puzzle 2017, 415, 51–64. [Google Scholar]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumari, V.; Rangaraj, N.; Nagaraj, R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob. Agents Chemother. 2009, 53, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Oshiro, K.G.N.; Rodrigues, G.; Monges, B.E.D.; Cardoso, M.H.; Franco, O.L. Bioactive Peptides Against Fungal Biofilms. Front. Microbiol. 2019, 10, 2169. [Google Scholar] [CrossRef]
- Tomalka, J.; Azodi, E.; Narra, H.P.; Patel, K.; O’Neill, S.; Cardwell, C.; Hall, B.A.; Wilson, J.M.; Hise, A.G. β-Defensin 1 Plays a Role in Acute Mucosal Defense against Candida albicans. J. Immunol. 2015, 194, 1788–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivarcsi, A.; Nagy, I.; Koreck, A.; Kis, K.; Kenderessy-Szabo, A.; Szell, M.; Dobozy, A.; Kemeny, L. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human β-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005, 7, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factoctum human cathelicidin peptide. Cell. Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M.; Ernst, J.F. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot. Cell 2014, 13, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Levinson, P.; Kaui, R.; Kimani, J.; Ngugi, E.; Moses, S.; MacDonald, K.S.; Broliden, K.; Hirbod, T. Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. Aids 2009, 23, 309–317. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, Y.; Lyu, X.; Dong, N.; Shan, A. Antimicrobial activity, improved cell selectivity and mode of action of short PMAP-36-derived peptides against bacteria and Candida. Sci. Rep. 2016, 6, 27258. [Google Scholar] [CrossRef] [Green Version]
- Woodburn, K.W.; Edward Clemens, L.; Jaynes, J.; Joubert, L.M.; Botha, A.; Nazik, H.; Stevens, D.A. Designed antimicrobial peptides for recurrent vulvovaginal candidiasis treatment. Antimicrob. Agents Chemother. 2019, 63, e02690-18. [Google Scholar] [CrossRef] [Green Version]
- Apalata, T.; Longo-Mbenza, B.; Sturm, A.; Carr, W.; Moodley, P. Factors associated with symptomatic vulvovaginal candidiasis: A study among women attending a primary healthcare clinic in Kwazulu-Natal, South Africa. Ann. Med. Health Sci. Res. 2014, 4, 410. [Google Scholar] [CrossRef] [Green Version]
- Duerr, A.; Heilig, C.M.; Meikle, S.F.; Cu-Uvin, S.; Klein, R.S.; Rompalo, A.; Sobel, J.D. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus-infected women: Risk factors and severity. Obstet. Gynecol. 2003, 101, 548–556. [Google Scholar] [CrossRef]
- Fidel, P.L.; Sobel, J.D. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin. Microbiol. Rev. 1996, 9, 335–348. [Google Scholar] [CrossRef]
- Moragues, M.D.; Omaetxebarria, M.J.; Elguezabal, N.; Sevilla, M.J.; Conti, S.; Polonelli, L.; Pontón, J. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans Activities. Infect. Immun. 2003, 71, 5273–5279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidel, P.L. History and new insights into host defense against vaginal candidiasis. Trends Microbiol. 2004, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Richardson, J.P.; Moyes, D.L. Candida albicans Pathogenicity and Epithelial Immunity. PLoS Pathog. 2014, 10, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Shacklett, B.L.; Anton, P.A. HIV infection and gut mucosal immune function: Updates on pathogenesis with implications for management and intervention. Curr. Infect. Dis. Rep. 2010, 12, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. Mucocutaneous IL-17 immunity in mice and humans: Host defense vs. excessive inflammation. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Coleman, B.M.; Willems, H.M.E.; Barker, K.S.; Aggor, F.E.Y.; Cipolla, E.; Verma, A.H.; Bishu, S.; Huppler, A.H.; Bruno, V.M.; et al. The Interleukin (IL) 17R/IL-22R Signaling Axis Is Dispensable for Vulvovaginal Candidiasis Regardless of Estrogen Status. J. Infect. Dis. 2020, 221, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Łaniewski, P.; Herbst-Kralovetz, M. Vagina. Encycl. Reprod. 2018, 2, 353–359. [Google Scholar]
- Amabebe, E.; Anumba, D.O.C. The vaginal microenvironment: The physiologic role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.A.; Roach, P.J.; Montero, M.; Baroja-Fernández, E.; Muñoz, F.J.; Eydallin, G.; Viale, A.M.; Pozueta-Romero, J. Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol. Rev. 2010, 34, 952–985. [Google Scholar] [CrossRef] [Green Version]
- Mirmonsef, P.; Hotton, A.L.; Gilbert, D.; Gioia, C.J.; Maric, D.; Hope, T.J.; Landay, A.L.; Spear, G.T. Glycogen levels in undiluted genital fluid and their relationship to vaginal pH, estrogen, and progesterone. PLoS ONE 2016, 11, e0153553. [Google Scholar] [CrossRef] [Green Version]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vásquez, A.; Jakobsson, T.; Ahrné, S.; Forsum, U.; Molin, G. Vaginal Lactobacillus Flora of Healthy Swedish Women. J. Clin. Microbiol. 2002, 40, 2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Kakkar, V.; Bhushan, I. Crosstalk between Vaginal Microbiome and Female Health: A review. Microb. Pathog. 2019, 136, 103696. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, L.L.; Ravel, J. The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence 2017, 8, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Seed, P.C. The Human Mycobiome. Cold Spring Harb. Perspect. Med. 2015, 5, a019810. [Google Scholar] [CrossRef] [Green Version]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.E.; Schwartz, J.A.; Robinson, C.K.; O’Hanlon, D.E.; Bradford, L.L.; He, X.; Mark, K.S.; Bruno, V.M.; Ravel, J.; Brotman, R.M. The vaginal microbiota and behavioral factors associated with genital Candida albicans detection in reproductive-age women. Sex. Transm. Dis. 2019, 46, 753. [Google Scholar] [CrossRef]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, Z.; Zhang, X.; Chen, X.; Wang, S. Local probiotic lactobacillus crispatus and lactobacillus delbrueckii exhibit strong antifungal effects against vulvovaginal candidiasis in a rat model. Front. Microbiol. 2019, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Lee, K.; Kwon, B.; You, H.J.; Ko, G.P. Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Sci. Rep. 2019, 9, 8121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allonsius, C.N.; Vandenheuvel, D.; Oerlemans, E.F.M.; Petrova, M.I.; Donders, G.G.G.; Cos, P.; Delputte, P.; Lebeer, S. Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci. Rep. 2019, 9, 2900. [Google Scholar] [CrossRef] [PubMed]
- Paniágua, A.L.; Correia, A.F.; Pereira, L.C.; de Alencar, B.M.; Silva, F.B.A.; Almeida, R.M.; de Medeiros Nóbrega, Y.K. Inhibitory effects of Lactobacillus casei Shirota against both Candida auris and Candida spp. isolates that cause vulvovaginal candidiasis and are resistant to antifungals. BMC Complement. Med. Ther. 2021, 21, 237. [Google Scholar] [CrossRef]
- Vylkova, S.; Carman, A.J.; Danhof, H.A.; Collette, J.R.; Zhou, H.; Lorenz, M.C. The fungal pathogen candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio 2011, 2, e00055-11. [Google Scholar] [CrossRef] [Green Version]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Munoz, R.; Dongari-Bagtzoglou, A. Anticandidal Activities by Lactobacillus Species: An Update on Mechanisms of Action. Front. Oral Health 2021, 2, 47. [Google Scholar] [CrossRef]
- Strus, M.; Brzychczy-Włoch, M.; Gosiewski, T.; Kochan, P.; Heczko, P.B. The in vitro effect of hydrogen peroxide onvaginal microbial communities. FEMS Immunol. Med. Microbiol. 2006, 48, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Strus, M.; Kucharska, A.; Kukla, G.; Brzychczy-Włoch, M.; Maresz, K.; Heczko, P.B. The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect. Dis. Obstet. Gynecol. 2005, 13, 69–75. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, P.R.; Parolin, C.; Abruzzo, A.; Luppi, B.; Protti, M.; Mercolini, L.; Silva, J.A.; Giordani, B.; Marangoni, A.; Nader-Macías, M.E.F.; et al. Biosurfactant from vaginal Lactobacillus crispatus BC1 as a promising agent to interfere with Candida adhesion. Microb. Cell Fact. 2020, 19, 133. [Google Scholar] [CrossRef]
- Morais, I.M.C.; Cordeiro, A.L.; Teixeira, G.S.; Domingues, V.S.; Nardi, R.M.D.; Monteiro, A.S.; Alves, R.J.; Siqueira, E.P.; Santos, V.L. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microb. Cell Fact. 2017, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. In vitro modulation of probiotic bacteria on the biofilm of Candida glabrata. Anaerobe 2015, 34, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial compounds produced by vaginal Lactobacillus crispatus are able to strongly inhibit Candida albicans growth, hyphal formation and regulate virulence-related gene expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosa, Y.; Kwon, D.; de Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10, 467. [Google Scholar] [CrossRef]
- van de Wijgert, J.H.H.M.; Jespers, V. The global health impact of vaginal dysbiosis. Res. Microbiol. 2017, 168, 859–864. [Google Scholar] [CrossRef]
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Iliev, I.D.; Underhill, D.M. Striking a balance: Fungal commensalism versus pathogenesis. Curr. Opin. Microbiol. 2013, 16, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Chen, Y.; Chen, T. Vaginal microbiota transplantation for the treatment of bacterial vaginosis: A conceptual analysis. FEMS Microbiol. Lett. 2019, 366, 25. [Google Scholar] [CrossRef] [Green Version]
- Hameed, S.; Hans, S.; Monasky, R.; Thangamani, S.; Fatima, Z. Understanding Human Microbiota Offers Novel and Promising Therapeutic Options against Candida Infections. Pathogens 2021, 10, 183. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moayyedi, P.; Yuan, Y.; Baharith, H.; Ford, A.C. Faecal microbiota transplantation for Clostridium difficile-associated diarrhoea: A systematic review of randomised controlled trials. Med. J. Aust. 2017, 207, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.F.; et al. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe 2020, 27, 823–829.e3. [Google Scholar] [CrossRef] [PubMed]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Y.; Feng, D.; Wei, D.M.; Mei, L.; Chen, H.; Wang, X.; Fang, F. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst. Rev. 2017, 11, CD010496. [Google Scholar] [CrossRef] [PubMed]
- Kovachev, S.M.; Vatcheva-Dobrevska, R.S. Local Probiotic Therapy for Vaginal Candida albicans Infections. Probiotics Antimicrob. Proteins 2015, 7, 38–44. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakrishnan, S.N.; Yamang, H.; Lorenz, M.C.; Chew, S.Y.; Than, L.T.L. Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis. Pathogens 2022, 11, 618. https://doi.org/10.3390/pathogens11060618
Balakrishnan SN, Yamang H, Lorenz MC, Chew SY, Than LTL. Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis. Pathogens. 2022; 11(6):618. https://doi.org/10.3390/pathogens11060618
Chicago/Turabian StyleBalakrishnan, Subatrra Nair, Haizat Yamang, Michael C. Lorenz, Shu Yih Chew, and Leslie Thian Lung Than. 2022. "Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis" Pathogens 11, no. 6: 618. https://doi.org/10.3390/pathogens11060618
APA StyleBalakrishnan, S. N., Yamang, H., Lorenz, M. C., Chew, S. Y., & Than, L. T. L. (2022). Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis. Pathogens, 11(6), 618. https://doi.org/10.3390/pathogens11060618





