Identification of the Causal Agent of Brown Leaf Spot on Kiwifruit and Its Sensitivity to Different Active Ingredients of Biological Fungicides
Abstract
:1. Introduction
2. Results
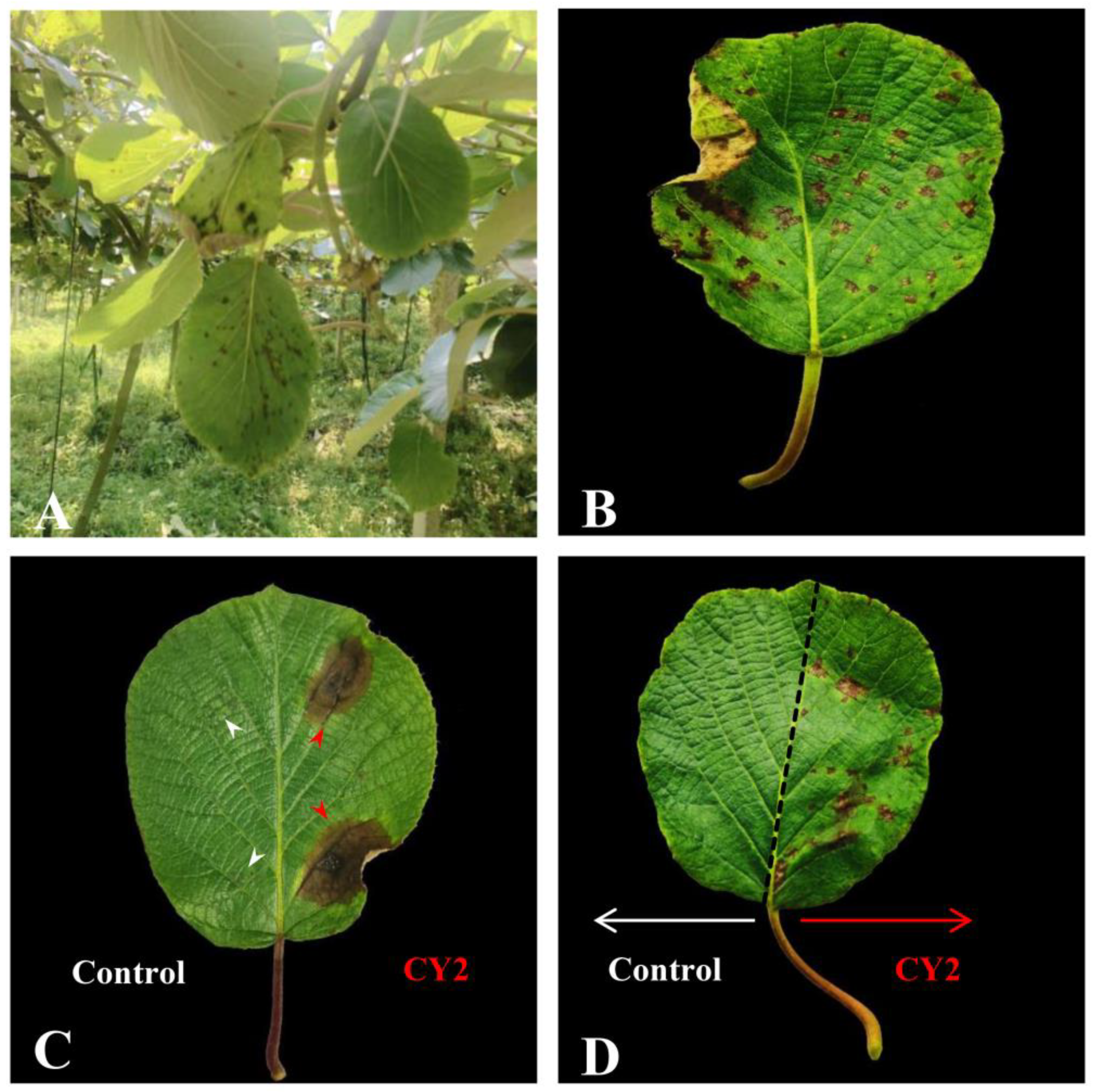
2.1. Pathogenicity Test of the Isolated Strains
2.2. Identification of Isolate CY2
2.3. Biological Characteristics of Isolate CY2
2.4. Toxicity Effects of Several Active Ingredients of Biological Fungicides against F. graminearum CY2
3. Discussion
4. Materials and Methods
4.1. Sampling, Isolation, and Purification
4.2. Pathogenicity Tests
4.3. Morphological Characterization
4.4. DNA Extraction, PCR Amplification, Sequencing, and Phylogenetic Analysis
4.5. Biological Characteristics of Isolate CY2
4.6. In Vitro Toxicity Tests
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Bai, J.; Li, R.; Liu, Z.; Fang, Y.; Wang, D.; Huang, T.; Zhang, L.; Liang, J.; Kou, L. Difference of resistance to postharvest blue mold between Hongyang and Qihong kiwifruits. Food Chem. 2019, 285, 389–396. [Google Scholar] [CrossRef]
- Pereira, C.; Costa, P.; Pinheiro, L.; Balcão, V.M.; Almeida, A. Kiwifruit bacterial canker: An integrative view focused on biocontrol strategies. Planta 2021, 253, 49. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.H.; Lim, M.T.; Kim, G.H.; Han, T.W.; Koh, Y.J. Incidences of leaf spots and blights on kiwifruit in Korea. Plant Pathol. J. 2008, 24, 125–130. [Google Scholar] [CrossRef]
- Hawthorne, B.T.; Rees-George, J.; Samuels, G.J. Fungi associated with leaf spots and post-harvest fruit rots of kiwifruit (Actinidia chinensis) in New Zealand. N. Z. J. Bot. 1982, 20, 143–150. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Chen, M.Y.; Zhang, S.J.; Zhong, C.H. First report of Nigrospora oryzae causing brown/black spot disease of kiwifruit in china. Plant Dis. 2017, 102, 243. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwon, Y.; Kwak, Y.S. First report of kiwifruit brown leaf spot caused by Fusarium tricinctum in South Korea. J. Agric. Life Sci. 2019, 53, 135–140. [Google Scholar] [CrossRef]
- Dean, R.; Kan, J.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? New Phytol. 2018, 217, 1203–1212. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Liu, R.L.; Gao, H.Y.; Han, Y.C.; Wu, W.J.; Chen, H.J. Identification and biological characterization of dominant spoilage fungi from postharvest water bamboo shoot. J. Nucl. Agric. Sci. 2019, 33, 2354–2361. [Google Scholar] [CrossRef]
- Keller, M.D.; Bergstrom, G.C.; Shields, E.J. The aerobiology of Fusarium graminearum. Aerobiologia 2014, 30, 123–136. [Google Scholar] [CrossRef]
- Yin, J.; Hao, C.; Niu, G.; Wang, W.; Wang, G.; Xiang, P.; Xu, J.R.; Zhang, X. FgPal1 regulates morphogenesis and pathogenesis in Fusarium graminearum. Environ. Microbiol. 2020, 22, 5373–5386. [Google Scholar] [CrossRef]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A major player in the multifaceted response of Fusarium to its environment. Toxins 2013, 6, 1–19. [Google Scholar] [CrossRef]
- Kaur, S.; Barakat, R.; Kaur, J.; Epstein, L. The effect of temperature on disease severity and growth of Fusarium oxysporum f. sp. apii races 2 and 4 in celery. Phytopathology 2022, 112, 364–372. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zuo, W.; He, D. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Rasiukevičiūtė, N.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Kupčinskienė, A.; Duchovskis, P.; Samuolienė, G.; Valiuškaitė, A. The effect of monochromatic LED light wavelengths and photoperiods on Botrytis cinerea. J. Fungi 2021, 7, 970. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y. Simultaneous sulfamethoxazole biodegradation and nitrogen conversion by Achromobacter sp. JL9 using with different carbon and nitrogen sources. Bioresour. Technol. 2019, 293, 122061. [Google Scholar] [CrossRef]
- Tateishi, H.; Miyake, T.; Mori, M.; Kimura, R.; Sakuma, Y.; Saishoji, T. Sensitivity of Japanese Fusarium graminearum species complex isolates to metconazole. J. Pestic. Sci. 2010, 35, 419–430. [Google Scholar] [CrossRef]
- Yu, W.Y.; Zhang, L.G.; Qiu, J.B.; Wang, J.X.; Chen, C.J. Effect of carbendazim-8- oxyquinoline-copper, a novel chelate fungicide against Fusarium graminearum. J. Pestic. Sci. 2011, 36, 385–391. [Google Scholar] [CrossRef]
- Khaskheli, M.A.; Wu, L.; Chen, G.; Chen, L.; Hussain, S.; Song, D.; Liu, S.; Feng, G. Isolation and characterization of root-associated bacterial endophytes and their biocontrol potential against major fungal phytopathogens of rice (Oryza sativa L.). Pathogens 2020, 9, 172. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, UK, 2006; pp. 179–182. [Google Scholar] [CrossRef]
- Saleh, A.A.; Sharafaddin, A.H.; El_Komy, M.H.; Ibrahim, Y.E.; Hamad, Y.K. Molecular and physiological characterization of Fusarium strains associated with different diseases in date palm. PLoS ONE 2021, 16, e0254170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.B.; Zhu, Q.M.; Zhi, T.H.; Li, Q.Y.; Fan, R. First report of fruit blotch on kiwifruit caused by Fusarium avenaceum in china. Plant Dis. 2020, 104, 1545. [Google Scholar] [CrossRef]
- Li, H.H.; Tang, W.; Liu, K.; Zhang, L.; Liu, Y.S. First report of Fusarium fujikuroi causing brown leaf spot on kiwifruit. Plant Dis. 2019, 104, 1560. [Google Scholar] [CrossRef]
- Chen, T.T.; Wu, X.; Dai, Y.Y.; Yin, X.H.; Zhao, Z.B.; Zhang, Z.Z.; Li, W.Z.; He, L.N.; Long, Y.H. Sensitivity testing of natural antifungal agents on Fusarium fujikuroi to investigate the potential for sustainable control of kiwifruit leaf spot disease. J. Fungi 2022, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ai, J.; Fan, S.; Lv, H.; Qin, H.; Yang, Y.; Liu, Y. Fusarium acuminatum: A new pathogen causing postharvest rot on stored kiwifruit in China. Plant Dis. 2015, 99, 1644. [Google Scholar] [CrossRef]
- Beyer, M.; Rding, S.; Ludewig, A.; Verreet, J.A. Germination and survival of Fusarium graminearum macroconidia as affected by environmental factors. J. Phytopathol. 2004, 152, 92–97. [Google Scholar] [CrossRef]
- Mongrain, D.; Couture, L.; Comeau, A. Natural occurrence of Fusarium graminearum on adult wheat midge and transmission to wheat spikes. Cereal Res. Commun. 2000, 28, 173–180. [Google Scholar] [CrossRef]
- Trail, F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef]
- Arseniuk, E.; Foremska, T.E.; Goral, J.; Chelowski, J. Fusarium head blight reactions and accumulation of deoxynivalenol (DON) and some of its derivates in kernels of wheat, triticale and rye. J. Phytopathol. 1999, 147, 577–590. [Google Scholar] [CrossRef]
- Atanassov, Z.; Nakamura, C.; Mori, N.; Kaneda, C.; Kato, H.; Jin, Y.Z. Mycotoxin production and pathogenicity of Fusarium species and wheat resistance to fusarium head blight. Can. J. Bot. 1994, 72, 161–167. [Google Scholar] [CrossRef]
- Eudes, F.; Comeau, A.; Rioux, S.; Collin, J. Impact of trichothecenes on Fusarium head blight (Fusarium graminearum) development in spring wheat (Triticum aestivum). Can. J. Plant Pathol. 2001, 23, 318–322. [Google Scholar] [CrossRef]
- Gilbert, J.; Abramson, D.; McCallum, B.; Clear, R. Comparison of Canadian Fusarium graminearum isolates for aggressiveness, vegetative compatibility, and production of ergosterol and mycotoxins. Mycopathologia 2002, 153, 209–215. [Google Scholar] [CrossRef]
- Stack, R.W. A comparison of the inoculum potential of ascospores and conidia of Gibberella zea. Can. J. Plant Pathol. 1989, 11, 137–142. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y. Biological characteristics and isolation of Pythium ultimum causing rot of Chinese cabbage. Australas. Plant Pathol. 2020, 49, 2. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Sun, Q.; Li, C.M.; Chen, H.F.; Lan, B. Biological characteristics and genetic diversity of Phomopsis asparagi, causal agent of asparagus stem blight. Plant Dis. 2020, 104, 2898–2904. [Google Scholar] [CrossRef]
- Borges Neto, C.B.; Gorgati, C.Q.; Pitelli, R.A. Influence of the photoperiod and temperature on the intensity of disease caused by Fusarium graminearum in Egeria densa and E. najas. Planta Daninha 2005, 23, 449–456. [Google Scholar] [CrossRef]
- Valcke, M.; Bourgault, M.H.; Rochette, L.; Normandin, L.; Samuel, O.; Belleville, D.; Blanchet, C.; Phaneuf, D. Human health risk assessment on the consumption of fruits and vegetables containing residual pesticides: A cancer and non-cancer risk/benefit perspective. Environ. Int. 2017, 108, 63–74. [Google Scholar] [CrossRef]
- Gonalves, D.; Ribeiro, W.R.; Gonalves, D.C.; Menini, L.; Costa, H. Recent advances and future perspective of essential oils in control Colletotrichum spp.: A sustainable alternative in postharvest treatment of fruits. Food Res. Int. 2021, 150, 110758. [Google Scholar] [CrossRef]
- García-Machado, F.J.; García-García, A.L.; Borges, A.A.; Jiménez-Arias, D. Root treatment with a vitamin k3 derivative: A promising alternative to synthetic fungicides against botrytis cinerea in tomato plants. Pest Manag. Sci. 2022, 78, 974–981. [Google Scholar] [CrossRef]
- Zhao, Y.; Chi, M.; Sun, H.; Qian, H.; Yang, J.; Huang, J. The FgCYP51B Y123H mutation confers reduced sensitivity to prochloraz and is important for conidiation and ascospore development in Fusarium graminearum. Phytopathology 2021, 111, 1420–1427. [Google Scholar] [CrossRef]
- Rekanović, E.; Mihajlović, M.; Potočnik, I. In vitro sensitivity of Fusarium graminearum (schwabe) to difenoconazole, prothioconazole and thiophanate-methyl. Pestic. Fitomed. 2010, 25, 325–333. [Google Scholar] [CrossRef]
- Sevastos, A.; Markoglou, A.; Labrou, N.E.; Flouri, F.; Malandrakis, A. Molecular characterization, fitness and mycotoxin production of Fusarium graminearum laboratory strains resistant to benzimidazoles. Pestic. Biochem. Physiol. 2016, 128, 1–9. [Google Scholar] [CrossRef]
- Yerkovich, N.; Cantoro, R.; Palazzini, J.M.; Torres, A.; Chulze, S.N. Fusarium head blight in Argentina: Pathogen aggressiveness, triazole tolerance and biocontrol-cultivar combined strategy to reduce disease and deoxynivalenol in wheat. Crop Prot. 2020, 137, 105300. [Google Scholar] [CrossRef]
- Chaves, M.; Reginatto, P.; Costa, B.; Paschoal, R.; Teixeira, M.L.; Fuentefria, A.M. Fungicide resistance in Fusarium graminearum species complex. Curr. Microbiol. 2022, 79, 62. [Google Scholar] [CrossRef]
- Gao, Y.; He, L.; Li, X.; Lin, J.; Mu, W.; Liu, F. Toxicity and biochemical action of the antibiotic fungicide tetramycin on Colletotrichum scovillei. Pestic. Biochem. Phys. 2018, 147, 51–58. [Google Scholar] [CrossRef]
- Ma, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Tian, S. Honokiol suppresses mycelial growth and reduces virulence of botrytis cinerea by inducing autophagic activities and apoptosis. Food Microbiol. 2020, 88, 103411. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Zhao, J.; Chen, C.; Lin, J.; Jayawardena, R.S.; Xiang, M.; Manawasinghe, I.S.; You, C. Fusarium elaeidis causes stem and root rot on Alocasia longiloba in south China. Pathogens 2021, 10, 1395. [Google Scholar] [CrossRef]
- Ghaderi, F.; Habibi, A. Morphological and molecular characterization of Phytophthora species associated with root and crown rot of Pomegranate in Iran. Plant Pathol. 2020, 70, 615–629. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M.; Anderson, J.B. Patterns of descent in clonal lineages and their multilocus fingerprints are resolved with combined gene genealogies. Evolution 1999, 53, 11–21. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef]
- Kumar, S.; Glen, S.; Koichiro, T. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Afroz, T.; Choi, H.W.; Lee, H.S.; Assefa, A.D.; Hahn, B.S. First report of Fusarium wilt caused by Fusarium equiseti on cabbage (Brassica oleracea var. capitate) in Korea. Plant Dis. 2020, 105, 1198. [Google Scholar] [CrossRef]
- Song, S.Q.; Lü, Z.; Wang, J.; Zhu, J.; Gu, M.Y.; Tang, Q.Y.; Zhang, Z.D.; Wang, W.; Zhang, L.J.; Wang, B. First report of a new potato disease caused by Galactomyces candidum F12 in china. J. Integr. Agric. 2020, 19, 2470–2476. [Google Scholar] [CrossRef]
- Xing, K.; Liu, Y.F.; Shen, X.Q.; Zhu, X.; Li, X.Y.; Miao, X.M.; Feng, Z.Z.; Peng, X.; Qin, S. Effect of o-chitosan nanoparticles on the development and membrane permeability of Verticillium dahliae. Carbohydr. Polym. 2017, 165, 334–343. [Google Scholar] [CrossRef]
- Xu, S.; Zeng, X.; Dai, S.; Wang, J.; Chen, Y.; Song, J.; Shi, Y.; Cheng, X.; Liao, S.; Zhao, Z. Turpentine derived secondary amines for sustainable crop protection: Synthesis, activity evaluation and QSAR study. J. Agric. Food Chem. 2020, 68, 11829–11838. [Google Scholar] [CrossRef]
- Gopal, M.; Ramappa, M.; Mohamed, A.; Dhakal, R.; Chaurasia, S. Quantitative corneal neural imaging using in vivo confocal microscopy in cases of congenital corneal anesthesia: A prospective analysis and clinical correlation. Indian J. Ophthalmol. 2021, 69, 262–266. [Google Scholar] [CrossRef]



| Species | Culture Accession | GenBank Accession | ||
|---|---|---|---|---|
| ITS | TEF-1α | RPB2 | ||
| Fusarium graminearum | NRRL 31084 | - | MW233103 | MW233447 |
| Fusarium graminearum | LC 13775 | - | MW620072 | MW474597 |
| Fusarium asiaticum | NRRL 13818 | - | MW233069 | MW233412 |
| Fusarium acuminatum | NRRL 54218 | HM068326 | HM068316 | HM068336 |
| Fusarium equiseti | NRRL 26419 | GQ505688 | GQ505599 | GQ505777 |
| Fusarium equiseti | NRRL 43636 | GQ505752 | GQ505663 | GQ505841 |
| Fusarium langsethiae | NRRL 54940 | - | MW233138 | MW233482 |
| Fusarium langsethiae | CBS 36236 | - | MW233114 | MW233458 |
| Fusarium duofalcatisporum | NRRL 36401 | - | GQ505651 | GQ505829 |
| Fusarium duofalcatisporum | NRRL 36448 | GQ505741 | GQ505652 | GQ505830 |
| Fusarium kyushuense | NRRL 3509 | NR152943 | MW233056 | MW233399 |
| Fusarium kyushuense | NRRL 6491 | - | MW233057 | MW233400 |
| Fusarium multiceps | NRRL 43639 | GQ505755 | GQ505666 | GQ505844 |
| Fusarium gracilipes | NRRL 43635 | GQ505751 | GQ505662 | GQ505840 |
| Active Ingredients of Biological Fungicides | Regression Equation | Determination Coefficient (R2) | EC50 (μg mL−1) | 95% Confidence Interval |
|---|---|---|---|---|
| 98% Honokiol DP | Y = 3.4834 + 1.5742x | 0.9876 | 9.26 ± 0.11 | 7.97–10.60 |
| 98% Matrine DP | Y = 3.1989 + 1.5251x | 0.9831 | 15.2 ± 0.31 | 13.11–17.54 |
| 1.5% Tetramycin AS | Y = 4.3763 + 1.0306x | 0.9817 | 4.02 ± 0.05 | 3.10–5.23 |
| 97% Citral AS | Y = 2.1413 + 1.974x | 0.9825 | 28.6 ± 0.99 | 23.99–32.84 |
| 98% Baicalein DP | Y = 2.457 + 1.5172x | 0.9730 | 47.3 ± 0.12 | 39.31–57.23 |
| Target Region/Gene | Description | Primer | Sequence 5′ → 3′ | Reference |
|---|---|---|---|---|
| ITS | Region with ribosomal RNA genes and two internal transcribed spacers | ITS1 | TCCGTAGGTGAACCTGCGG | [49] |
| ITS4 | TCCTCCGCTTATTGATATGC | |||
| TEF-1α | Translation elongation factor 1-α gene | EF1-728F | CATCGAGAAGTTCGAGAAGG | [50] |
| EF1-986R | TACTTGAAGGAACCCTTACC | |||
| RPB2 | Second largest subunit of RNA polymerase II | fRPB2-7cR | CCCATRGCTTGTYYRCCCAT | [51] |
| RPB2-5F2 | GGGGWGAYCAGAAGAAGGC | [52] |
| Active Ingredients of Biological Fungicides | Manufacturer | Concentration Gradient (μg mL−1) | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | ||
| 98% Honokiol DP | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China | 4 | 8 | 16 | 32 | 64 |
| 98% Matrine DP | Aladdin Industrial Corporation, Shanghai, China | 4 | 8 | 16 | 32 | 64 |
| 1.5% Tetramycin AS | Liaoning Wkioc Bioengineering Co., Ltd., Shenyang, China | 4 | 8 | 16 | 32 | 64 |
| 97% Citral AS | Aladdin Industrial Corporation, Shanghai, China | 5 | 10 | 20 | 40 | 80 |
| 98% Baicalein DP | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China | 10 | 20 | 40 | 80 | 160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Ran, F.; Shi, J.; Chen, T.; Zhao, Z.; Zhang, Z.; He, L.; Li, W.; Wang, B.; Chen, X.; et al. Identification of the Causal Agent of Brown Leaf Spot on Kiwifruit and Its Sensitivity to Different Active Ingredients of Biological Fungicides. Pathogens 2022, 11, 673. https://doi.org/10.3390/pathogens11060673
Chen J, Ran F, Shi J, Chen T, Zhao Z, Zhang Z, He L, Li W, Wang B, Chen X, et al. Identification of the Causal Agent of Brown Leaf Spot on Kiwifruit and Its Sensitivity to Different Active Ingredients of Biological Fungicides. Pathogens. 2022; 11(6):673. https://doi.org/10.3390/pathogens11060673
Chicago/Turabian StyleChen, Jia, Fei Ran, Jinqiao Shi, Tingting Chen, Zhibo Zhao, Zhuzhu Zhang, Linan He, Wenzhi Li, Bingce Wang, Xuetang Chen, and et al. 2022. "Identification of the Causal Agent of Brown Leaf Spot on Kiwifruit and Its Sensitivity to Different Active Ingredients of Biological Fungicides" Pathogens 11, no. 6: 673. https://doi.org/10.3390/pathogens11060673
APA StyleChen, J., Ran, F., Shi, J., Chen, T., Zhao, Z., Zhang, Z., He, L., Li, W., Wang, B., Chen, X., Wang, W., & Long, Y. (2022). Identification of the Causal Agent of Brown Leaf Spot on Kiwifruit and Its Sensitivity to Different Active Ingredients of Biological Fungicides. Pathogens, 11(6), 673. https://doi.org/10.3390/pathogens11060673






