Epidemiology of Toxoplasmosis among the Pakistani Population: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Search Strategy
4.2. Data Collection
4.3. Inclusion and Exclusion Criteria
4.4. Meta-Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torgerson, P.R.; Macpherson, C.N. The socioeconomic burden of parasitic zoonoses: Global trends. Vet. Parasitol. 2011, 182, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Vesco, G.; Villari, S.; Buffolano, W. What do we know about risk factors for infection in humans with Toxoplasma gondii and how can we prevent infections? Zoonoses Public Health 2010, 57, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Amjad, Z.; Khan, T.M.; Maalik, A.; Iftikhar, A.; Khan, I.; Ahmed, H. Occurrence of Toxoplasma gondii antibodies and associated risk factors in women in selected districts of Punjab province, Pakistan. Parasitology 2020, 147, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Kruszon-Moran, D.; Sanders-Lewis, K.; Wilson, M. Toxoplasma gondii infection in the United States, 1999–2004, decline from the prior decade. Am. J. Trop. Med. Hyg. 2007, 77, 405–410. [Google Scholar] [CrossRef]
- Bodaghi, B.; Touitou, V.; Fardeau, C.; Paris, L.; LeHoang, P. Toxoplasmosis: New challenges for an old disease. Eye 2012, 26, 241–244. [Google Scholar] [CrossRef]
- Dubey, J.; Jones, J. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- Mohammed, T.K. Seroprevalence of Toxoplasma gondii among pregnant women in Baghdad city. J. Tech. 2011, 24, 21–28. [Google Scholar]
- Shah, M.; Zahid, M.; Alam Sthanadar, A.; Asmat Ali, P. Seroprevalence of Toxoplasma gondii Infection in Human Population of Mohmand Agency Khyber Pakhtunkhwa, Pakistan. Pak. J. Zool. 2014, 46, 1169–1172. [Google Scholar]
- Ahmad, M.; Maqbool, A.; Mahmood-ul-Hassan, M.; Mushtaq-ul-Hassan, M.; Anjum, A. Prevalence of Toxoplasma gondii antibodies in human beings and commensal rodents trapped from Lahore, Pakistan. J. Anim. Plant Sci. 2012, 22, 51–53. [Google Scholar]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [Green Version]
- Alvarado-Esquivel, C.; Rico-Almochantaf, Y.d.R.; Hernández-Tinoco, J.; Quiñones-Canales, G.; Sánchez-Anguiano, L.F.; Torres-González, J.; Schott, B.; Liesenfeld, O.; Dunay, I.R. Toxoplasma gondii exposure and neurological disorders: An age-and gender-matched case-control pilot study. Eur. J. Microbiol. Immunol. 2017, 7, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Spalding, S.M.; Amendoeira, M.R.R.; Klein, C.H.; Ribeiro, L.C. Serological screening and toxoplasmosis exposure factors among pregnant women in South of Brazil. Rev. Soc. Bras. Med. Trop. 2005, 38, 173–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, J.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Cantos, G.A.; Prando, M.D.; Siqueira, M.V.; Teixeira, R.M. Toxoplasmose: Ocorrência de anticorpos antitoxoplasma gondii e diagnóstico. Rev. Assoc. Med. Bras. 2000, 46, 335–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.N.; Khan, S.; Ayaz, S.; Jan, A.H.; Jehangir, S.; Attaullah, S.; Ali, I.; Shams, S. Seroprevalance and risk factors of toxoplasmosis among pregnant women in District Kohat, Khyber Pakhtunkhwa, Pakistan. World Appl. Sci J 2011, 14, 1032–1036. [Google Scholar]
- Fan, C.-K.; Hung, C.-C.; Su, K.-E.; Sung, F.-C.; Chiou, H.-Y.; Gil, V.; da Conceicao dos Reis Ferreira, M.; de Carvalho, J.M.; Cruz, C.; Lin, Y.-K. Seroprevalence of Toxoplasma gondii infection among pre-schoolchildren aged 1–5 years in the Democratic Republic of Sao Tome and Principe, Western Africa. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 446–449. [Google Scholar] [CrossRef]
- Dupont, D.; Fricker-Hidalgo, H.; Brenier-Pinchart, M.-P.; Garnaud, C.; Wallon, M.; Pelloux, H. Serology for Toxoplasma in immunocompromised patients: Still useful? Trends Parasitol. 2021, 37, 205–213. [Google Scholar] [CrossRef]
- Odeniran, P.O.; Omolabi, K.F.; Ademola, I.O. Risk factors associated with seropositivity for Toxoplasma gondii in population-based studies among immunocompromised patients (pregnant women, HIV patients and children) in West African countries, Cameroon and Gabon: A meta-analysis. Acta Trop. 2020, 209, 105544. [Google Scholar] [CrossRef]
- Dogruman-Al, F.; Aslan, S.; Yalcin, S.; Kustimur, S.; Turk, S. A possible relationship between Toxoplasma gondii and schizophrenia: A seroprevalence study. Int. J. Psychiatry Clin. Pract. 2009, 13, 82–87. [Google Scholar] [CrossRef]
- Aldebert, D.; Hypolite, M.; Cavailles, P.; Touquet, B.; Flori, P.; Loeuillet, C.; Cesbron-Delauw, M.-F. Development of high-throughput methods to quantify cysts of Toxoplasma gondii. Cytom. Part A 2011, 79, 952–958. [Google Scholar] [CrossRef]
- Flegr, J.; Preiss, M.; Klose, J.; Havlíček, J.; Vitáková, M.; Kodym, P. Decreased level of psychobiological factor novelty seeking and lower intelligence in men latently infected with the protozoan parasite Toxoplasma gondii Dopamine, a missing link between schizophrenia and toxoplasmosis? Biol. Psychol. 2003, 63, 253–268. [Google Scholar] [CrossRef]
- Khan, M.A.; Islam, Z.; Jan, A.U.; Khan, K.; Shah, A. Seroepidemiology of Toxoplasma gondii Infection in Child Bearing Age Women in Dir Khyberpakhtunkhawa, Pakistan. Pak. J. Zool. 2021, 53, 375. [Google Scholar] [CrossRef]
- Tasawar, Z.; Raza, A.A.; Aziz, F.; Lashari, M.H. Prevalence of human toxoplasmosis in district Muzaffargarh, Punjab, Pakistan. Gomal J. Med. Sci. 2012, 10, 37–41. [Google Scholar]
- Khan, M.Z.; Rahman, S.U.; Gul, N.; Khan, A.A. Toxoplasmosis; seroprevalence, comparative analysis of diagnostic techniques and identification of risk factors in humans in Malakand Agency, Khyber Pakhtunkhwa, Pakistan. Int. J. Biosci. 2014, 5, 1–6. [Google Scholar]
- Awan, U.A.; Khattak, A.A. Has Pakistan failed to roll back HPV? Lancet Oncol. 2022, 23, e204. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ali, M.; Tanveer, F.; Ovais, M.; Idrees, M.; Shinwari, Z.K.; Hollenbeck, J.E. Emerging viral infections in Pakistan: Issues, concerns, and future prospects. Health Secur. 2017, 15, 268–281. [Google Scholar] [CrossRef]
- Tasawar, Z.; Lashari, M.H.; Hanif, M.; Hayat, C. Seroprevalence of Toxoplasma gondii in domestic goats in Multan, Punjab, Pakistan. Pak. J. Life Soc. Sci 2011, 9, 24–27. [Google Scholar]
- Tasawar, Z.; Aziz, F.; Lashari, M.H.; Shafi, S.; Ahmad, M.; Lal, V.; Hayat, C.S. Seroprevalence of Human toxoplasmosis in southern Punjab, Pakistan. Pak. J. Life Soc. Sci. 2012, 10, 48–52. [Google Scholar]
- Hayat, S.; Tasawar, Z.; Akhtar, T. Seroprevalence of Human Toxoplasmosis in Kallarwali, Muzaffar Garh, Pakistan. Gomal J. Med. Sci. 2014, 12, 129–132. [Google Scholar]
- Faisal, I.A.; Khan, A.U.; Waqar, M.; Ahmad, T.; Shah, T.; Khan, M.I.; Ali, N.; Faisal, S.; Saif, I.; Ahmad, W. Distribution of Toxoplasma gondii in the pregnant women of district Swabi Khyber Pakhtunkhwa Pakistan. World Appl. Sci. J. 2014, 29, 77–79. [Google Scholar]
- Majid, A.; Khan, S.; Jan, A.H.; Taib, M.; Adnan, M.; Ali, I.; Khan, S.N. Chronic toxoplasmosis and possible risk factors associated with pregnant women in Khyber Pakhtunkhwa. Biotechnol. Biotechnol. Equip. 2016, 30, 733–736. [Google Scholar] [CrossRef] [Green Version]
- Hussain Shah, M.S.; Naz, F.; Jan, A.; Ullah, R.; Khan, S.F.A.; Haseeb, A.; Ahmad, I.; Younas, M. Seroprevalence and Risk Factors of Toxoplasmosis among Women in District Chitral, Khyber Pakhtunkhwa, Pakistan. World J. Zool. 2016, 11, 135–140. [Google Scholar]
- Khattak, M.; Iltaf, M.; Rehman, A.; Malik, S.; Zahid, M. Prevalence, socio-demographic determinants and risk factors of toxoplasmosis: Case-control study in a rural community of Mardan district, northern Pakistan. J. Anim. Plant Sci. 2017, 27, 617–626. [Google Scholar]
- Nazir, M.; Akhtar, M.; Maqbool, A.; Waheed, A.; Sajid, M.; Ali, M.; Oneeb, M.; Alam, M.; Ahmad, A.; Nazir, N. Antibody prevalence and risk factors for Toxoplasma gondii infection in women from Multan, Pakistan. Zoonoses Public Health 2017, 64, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Zahid, M.; Bibi, B.; Hussain, A.; Haroon, M.; Ali, B. Chromatographic immunoassay based detection of human toxoplasmosis in District Mardan, Khyber Pakhtunkhawa, Pakistan. Pure Appl. Biol. 2017, 6, 1297–1305. [Google Scholar] [CrossRef]
- Shah, N.; Khan, A.; Khisroon, M.; Adnan, M.; Jawad, S.M. Seroprevalence and risk factors of toxoplasmosis in pregnant women of district Swabi. Pure Appl. Biol. 2017, 6, 1306–1313. [Google Scholar] [CrossRef]
- Khan, A.; Naz, K.; Ahmed, H.; Simsek, S.; Afzal, M.S.; Haider, W.; Ahmad, S.S.; Farrakh, S.; Wu, W.; Guan, Y. Knowledge, attitudes and practices related to cystic echinococcosis endemicity in Pakistan. Infect. Dis. Poverty 2018, 7, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Rehman, F.; Ahmad, R.; Jan, S.S. Prevalence of Abortion among Toxoplasma Gondii Seropositive Pregnant Women in Community Hospital of Mardan. J. Saidu Med. Coll. Swat 2018, 8. [Google Scholar] [CrossRef]
- Aleem, U.; Ullah, S.; Qasim, M.; Suliman, M. Seroprevalence of Toxoplasmosis in Pregnant Women in Matta, Upper Swat, Khyber Pakhtunkhwa, Pakistan. J. Saidu Med. Coll. Swat 2018, 8. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Haroon, M.; Khan, M.T.; Jan, F.U.; Iqbal, T.; Khan, A.; Shakeel, M. Seroprevalence of human Toxoplasma gondii infection among pregnant women in Charsadda, KP, Pakistan. J. Parasit. Dis. 2018, 42, 554–558. [Google Scholar] [CrossRef]
- Sadiqui, S.; Shah, S.R.H.; Almugadam, B.S.; Shakeela, Q.; Ahmad, S. Distribution of Toxoplasma gondii IgM and IgG antibody seropositivity among age groups and gestational periods in pregnant women. F1000Research 2018, 7, 1823. [Google Scholar] [CrossRef] [PubMed]
- HasnainJan, M.H.; Faisal, S.; Abid Kamal, M.T.; Khan, K.A.; Muhammad, W. Sero-epidemiology of human Toxoplasma gondii infection among male population in Charsadda, KPK, Pakistan. Int. J. Biosci. 2018, 12, 110–116. [Google Scholar] [CrossRef]
- Ahmad, N.; Khan, I.A.; Iqbal, Z.; Naseem, A.A.; Kayani, A.R.; Afshan, K.; Qayyum, M. Seroepidemiology of Toxoplasmosis in Human Population with Reference to Its Zoonotic Potential in Sub-Tropical Areas of Pakistan. Pak. Vet. J. 2019, 39, 211–215. [Google Scholar] [CrossRef]
- Rehman, F.; Shah, M.; Ali, A.; Ahmad, I.; Sarwar, M.T.; Rapisarda, A.M.C.; Cianci, A. Unpasteurised milk consumption as a potential risk factor for toxoplasmosis in females with recurrent pregnancy loss. J. Obstet. Gynaecol. 2020, 40, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Shah, M.; Ali, A.; Rapisarda, A.; Cianci, A. Seroprevalence and risk factors of Toxoplasma gondii infection in women with recurrent fetal loss from the province of khyber Pakhtunkhwa, Pakistan. J. Neonatal Perinat. Med. 2021, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Nawaz, D.; Shah, M.; Rasool, A.; Akbar, F.; Israr, M. Prevalence of Toxoplasma Gondii in Women Population in Swat, Pakistan. Biomed. J. Sci. Tech. Res. 2020, 30, 23247–23251. [Google Scholar]
- Rostami, A.; Riahi, S.M.; Contopoulos-Ioannidis, D.G.; Gamble, H.R.; Fakhri, Y.; Shiadeh, M.N.; Foroutan, M.; Behniafar, H.; Taghipour, A.; Maldonado, Y.A. Acute Toxoplasma infection in pregnant women worldwide: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007807. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Fons, F.; Vicente, J.; Vidal, D.; Höfle, U.; Villanúa, D.; Gauss, C.; Segalés, J.; Almería, S.; Montoro, V.; Gortázar, C. Seroprevalence of six reproductive pathogens in European wild boar (Sus scrofa) from Spain: The effect on wild boar female reproductive performance. Theriogenology 2006, 65, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.E.; Dubey, J.P. Toxoplasma gondii . In Foodborne Parasites; Springer: Berlin/Heidelberg, Germany, 2018; pp. 119–138. [Google Scholar]
- Cook, E.A.J.; Gitahi, N.; De Glanville, W.A.; Thomas, L.F.; Kariuki, S.; Kang’ethe, E.; Fèvre, E.M. Prevalence and risk factors for exposure to Toxoplasma gondii in slaughterhouse workers in western Kenya. BMC Infect. Dis. 2021, 21, 944. [Google Scholar] [CrossRef]
- Kochanowsky, J.A.; Koshy, A.A. Toxoplasma gondii . Curr. Biol. 2018, 28, R770–R771. [Google Scholar] [CrossRef] [Green Version]
- Minuzzi, C.E.; Fernandes, F.D.a.; Portella, L.P.; Bräunig, P.; Sturza, D.A.F.; Giacomini, L.; Salvagni, E.; Ribeiro, J.d.S.; Silva, C.R.; Difante, C.M. Contaminated water confirmed as source of infection by bioassay in an outbreak of toxoplasmosis in South Brazil. Transbound. Emerg. Dis. 2021, 68, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Awan, U.A.; Zahoor, S.; Ayub, A.; Ahmed, H.; Aftab, N.; Afzal, M.S. COVID-19 and arboviral diseases: Another challenge for Pakistan’s dilapidated healthcare system. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am. J. Epidemiol. 2001, 154, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Studeničová, C.; Benčaiová, G.; Holková, R. Seroprevalence of Toxoplasma gondii antibodies in a healthy population from Slovakia. Eur. J. Intern. Med. 2006, 17, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Hlaváčová, J.; Flegr, J.; Řežábek, K.; Calda, P.; Kaňková, Š. Association between latent toxoplasmosis and fertility parameters of men. Andrology 2021, 9, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Kolbekova, P.; Kourbatova, E.; Novotna, M.; Kodym, P.; Flegr, J. New and old risk-factors for Toxoplasma gondii infection: Prospective cross-sectional study among military personnel in the Czech Republic. Clin. Microbiol. Infect. 2007, 13, 1012–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daryani, A.; Sarvi, S.; Aarabi, M.; Mizani, A.; Ahmadpour, E.; Shokri, A.; Rahimi, M.-T.; Sharif, M. Seroprevalence of Toxoplasma gondii in the Iranian general population: A systematic review and meta-analysis. Acta Trop. 2014, 137, 185–194. [Google Scholar] [CrossRef]
- Rahimi, M.T.; Daryani, A.; Sarvi, S.; Shokri, A.; Ahmadpour, E.; Mizani, A.; Sharif, M.; Teshnizi, S.H. Cats and Toxoplasma gondii: A systematic review and meta-analysis in Iran. Onderstepoort J. Vet. Res. 2015, 82, 1–10. [Google Scholar] [CrossRef]
- Foroutan, M.; Dalvand, S.; Daryani, A.; Ahmadpour, E.; Majidiani, H.; Khademvatan, S.; Abbasi, E. Rolling up the pieces of a puzzle: A systematic review and meta-analysis of the prevalence of toxoplasmosis in Iran. Alex. J. Med. 2018, 54, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Noh, H.-J.; Hwang, O.-S.; Lee, S.-K.; Shin, D.-W. Seroepidemiological study of Toxoplasma gondii infection in the rural area Okcheon-gun, Korea. Korean J. Parasitol. 2000, 38, 251. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Li, Z.; Chen, P.; Chen, L. The seroprevalence of Toxoplasma gondii in Chinese population with cancer: A systematic review and meta-analysis. Medicine 2015, 94, e2274. [Google Scholar] [CrossRef] [PubMed]
- de la Luz Galvan-Ramirez, M.; Troyo, R.; Roman, S.; Calvillo-Sanchez, C.; Bernal-Redondo, R. A systematic review and meta-analysis of Toxoplasma gondii infection among the Mexican population. Parasites Vectors 2012, 5, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadooghian, S.; Mahmoudvand, H.; Mohammadi, M.A.; Sarcheshmeh, N.N.; Kareshk, A.T.; Kamiabi, H.; Zia-Ali, N. Prevalence of Toxoplasma gondii Infection among healthy blood donors in Northeast of Iran. Iran. J. Parasitol. 2017, 12, 554. [Google Scholar] [PubMed]
- Coêlho, R.A.; Kobayashi, M.; Carvalho, L.B., Jr. Prevalence of IgG antibodies specific to Toxoplasma gondii among blood donors in Recife, Northeast Brazil. Rev. Inst. Med. Trop. Sao Paulo 2003, 45, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Karimi, G.; Mardani, A.; Zadsar, M. Prevalence of Toxoplasma gondii among Iranian blood donors: A narrative review article. Iran. J. Parasitol. 2016, 11, 10. [Google Scholar]
- Sohaib, M.; Jamil, F. An insight of meat industry in Pakistan with special reference to halal meat: A comprehensive review. Korean J. Food Sci. Anim. Resour. 2017, 37, 329. [Google Scholar] [CrossRef]
- Boothroyd, J.C.; Grigg, M.E. Population biology of Toxoplasma gondii and its relevance to human infection: Do different strains cause different disease? Curr. Opin. Microbiol. 2002, 5, 438–442. [Google Scholar] [CrossRef]
- Mesquita, R.T.; Ziegler, A.P.; Hiramoto, R.M.; Vidal, J.E.; Pereira-Chioccola, V.L. Real-time quantitative PCR in cerebral toxoplasmosis diagnosis of Brazilian human immunodeficiency virus-infected patients. J. Med. Microbiol. 2010, 59, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Kotresha, D.; Noordin, R. Recombinant proteins in the diagnosis of toxoplasmosis. APMIS 2010, 118, 529–542. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 2015, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Switaj, K.; Master, A.; Skrzypczak, M.; Zaborowski, P. Recent trends in molecular diagnostics for Toxoplasma gondii infections. Clin. Microbiol. Infect. 2005, 11, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostami, A.; Riahi, S.; Gamble, H.; Fakhri, Y.; Shiadeh, M.N.; Danesh, M.; Behniafar, H.; Paktinat, S.; Foroutan, M.; Mokdad, A. Global prevalence of latent toxoplasmosis in pregnant women: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.B.; Costa, A.J.; Jordão Filho, S.; Paim, B.B.; Pinto, F.R.; Di Mauro, D.C. Toxoplasma gondii in semen of experimentally infected swine. Pesqui. Vet. Bras. 2007, 27, 430–434. [Google Scholar] [CrossRef] [Green Version]








| Author | References | Year of Publication | Location | Diagnostic Method | Disease/Species Name | Sex | Total Individuals | Positive Cases | Prevalence (%) |
|---|---|---|---|---|---|---|---|---|---|
| Khan et al., 2011 | [15] | 2011 | Kohat | ELISA | T. gondii | Female | 180 | 26 | 14.40% |
| Tasawar et al., 2011 | [27] | 2011 | Dera Ghazi Khan | LAT | T. gondii | Female | 102 | 31 | 30.39% |
| 2011 | Dera Ghazi Khan | LAT | T. gondii | Male | 98 | 28 | 28.57% | ||
| Tasawar et al., 2012 | [28] | 2012 | Rajanpur, Bahawalnagar, Shujabad, Multan | LAT | T. gondii | Female | 355 | 92 | 25.90% |
| 2012 | Rajanpur, Bahawalnagar, Shujabad, Multan | LAT | T. gondii | Male | 195 | 70 | 35.89% | ||
| Tasawar et al., 2012 | [23] | 2012 | Muzaffargarh | LAT | T. gondii | Female | 50 | 17 | 34% |
| 2012 | Muzaffargarh | LAT | T. gondii | Male | 50 | 20 | 40% | ||
| Ahmad et al., 2012 | [9] | 2012 | Lahore | LAT | T. gondii | Male and Female | 300 | 34 | 11.30% |
| Khan et al., 2014 | [24] | 2014 | Malakand Agency, Batkhela, Bahadur Khan, Rozi Khan Memorial Hospital | ICA, LAT, ELISA | T. gondii | Female | 420 | 276 | 65.71% |
| Hayat et al., 2014 | [29] | 2014 | Kallarwali, Muzaffar Garh | LAT | T. gondii | Female | 75 | 30 | 40% |
| Kallarwali, Muzaffar Garh | LAT | T. gondii | Male | 75 | 33 | 44% | |||
| Faisal et al., 2014 | [30] | 2014 | Swabi | LAT | T. gondii | Female | 805 | 155 | 19.25% |
| Majid et al., 2016 | [31] | 2016 | Upper Dir, Lower Dir, Swat | IFA Kit | T. gondii | Female | 733 | 135 | 18.41% |
| Shah et al., 2016 | [32] | 2016 | Chitral | LFCI | T. gondii | Female | 300 | 74 | 24.70% |
| Khattak et al., 2017 | [33] | 2017 | Mardan, Katlang, Takht Bhal | LAT | T. gondii | Female and Male | 600 | 107 | 17.83% |
| Nazir et al., 2017 | [34] | 2017 | Multan, Muzaffargarh, Dera Ghazi Khan, Layyah | ELISA | T. gondii | Female | 403 | 71 | 17.60% |
| Shah et al., 2017 | [35] | 2017 | Mardan | LFCI, LAT | T. gondii | Female | 225 | 36 | 16.00% |
| Mardan | LFCI, LAT | T. gondii | Male | 375 | 69 | 18.40% | |||
| Shah et al., 2017 | [36] | 2017 | Swabi | LFCI | T. gondii | Female | 100 | 12 | 12% |
| Khan et al., 2018 | [37] | 2018 | District Bannu | RDT | T. gondii | Female | 90 | 3 | 1.77% |
| 2018 | District Bannu | RDT | T. gondii | Male | 80 | 1 | 0.59% | ||
| Rehman et al., 2018 | [38] | 2018 | Mardan | ELISA | T. gondii | Female | 360 | 86 | 23.89% |
| Aleem et al., 2018 | [39] | 2018 | Matta, Upper Swat | LAT | T. gondii | Female | 360 | 170 | 47.20% |
| Faisal et al., 2018 | [40] | 2018 | Charsadda, Shabqadar, Tangi | LAT | T. gondii | Female and Male | 200 | 69 | 34.50% |
| Sadiqui et al., 2018 | [41] | 2018 | Mansehra, Hazara, Abbottabad | ICA, ELISA | T. gondii | Female | 500 | 124 | 24.85% |
| Jan et al., 2018 | [42] | 2018 | Charsadda, Shabqadar, Tangi | LAT | T. gondii | Male | 300 | 63 | 21% |
| Ahmad et al., 2019 | [43] | 2019 | Jhelum, Chakwal, Rawalpindi, Attock, Islamabad Capital Territory | ELISA | T. gondii | Female and Male | 1659 | 338 | 20.37% |
| Ali et al., 2020 | [3] | 2020 | Chiniot, Faisalabad, Jhang and Okara | ELISA | T. gondii | Female | 593 | 44 | 7.42% |
| Rehman et al., 2020 | [44] [45] | 2020 | Mardan | ELISA | T. gondii | Female | 360 | 25 | 6.94% |
| 2020 | Mardan | ELISA | T. gondii | Female | 360 | 86 | 23.90% | ||
| Ullah et al., 2020 | [46] | 2020 | Swat | LFCI | T. gondii | Female | 216 | 26 | 26% |
| Khan et al., 2021 | [22] | 2021 | Lower Dir | ELISA | T. gondii | Female | 405 | 231 | 57.03% |
| Type of Infection | Humans | No. of Studies | Sample Size | Positive Cases | Prevalence (%) | Cochran Q Statistic | I2 Statistic | p-Value † | Tau2 |
|---|---|---|---|---|---|---|---|---|---|
| Toxoplasmosis | Female | 23 | 7985 | 2032 | 25.44 | 931.56 | 97.6% | <0.0001 | 0.0303 |
| Male | 08 | 2039 | 438 | 21.48 | 97.54 | 92.8% | <0.0001 | 0.0148 | |
| Female and Male | 02 | 900 | 141 | 15.67 | 6.68 | 85.0% | 0.0098 | 0.0035 | |
| Detection method | LFCI | 4 | 1216 | 247 | 20.31 | 15.07 | 80.1% | 0.0018 | 0.0038 |
| ELISA | 10 | 5240 | 1306 | 24.92 | 752.36 | 98.8% | <0.0001 | 0.0420 | |
| LAT | 12 | 4585 | 1300 | 28.35 | 500.17 | 97.8% | <0.0001 | 0.0299 | |
| Others (ICA, RDT, IFA) | 2 | 920 | 400 | 43.47 | 398.88 | 99.2% | <0.0001 | 0.0773 | |
| 1 | 170 | 4 | 2.35 | ||||||
| 1 | 733 | 135 | 18.41 | ||||||
| Sample size | <200 | 5 | 700 | 142 | 20.29 | 117.73 | 96.6% | <0.0001 | 0.0513 |
| 200–400 | 10 | 2956 | 722 | 24.42 | 221.35 | 95.9% | <0.0001 | 0.0200 | |
| 400–600 | 8 | 4071 | 1119 | 27.47 | 673.30 | 99.0% | <0.0001 | 0.0469 | |
| >600 | 3 | 3197 | 628 | 19.64 | 1.31 | 0.0% | 0.5199 | 0 | |
| Residence * | Urban | 7 | 1287 | 110 | 8.54 | 13.07 | 54.1% | 0.0420 | 0.1502 |
| Rural | 7 | 1585 | 264 | 16.65 | 13.07 | 54.1% | 0.0420 | 0.1502 | |
| Education * | Literate | 6 | 1935 | 355 | 18.34 | 82.28 | 93.9% | <0.0001 | 1.3105 |
| Illiterate | 6 | 2043 | 449 | 21.97 | 82.28 | 93.9% | <0.0001 | 1.3105 | |
| Contact with animals | Female and Male | 10 | 3043 | 711 | 23.36 | 146.89 | 93.9% | <0.0001 | 0.0130 |
| Undercooked meat and raw vegetable consumption | Female and Male | 989 | 295 | 29.82 | 64.34 | 93.8% | <0.0001 | 0.0286 | |
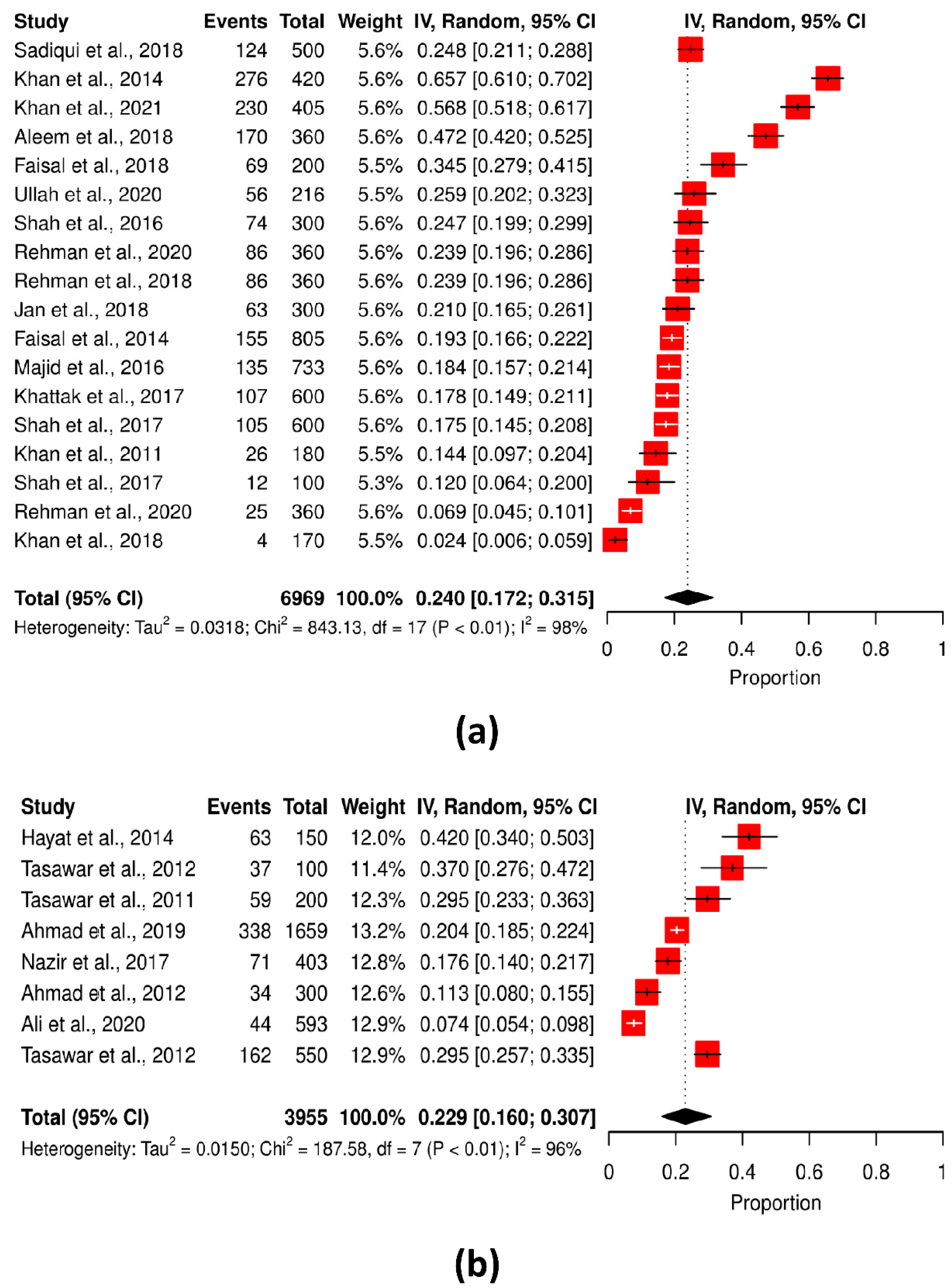
| Geographical region of Pakistan | KPK | 18 | 6969 | 1803 | 25.87 | 843.13 | 98.0% | <0.0001 | 0.0318 |
| Punjab | 8 | 3955 | 808 | 20.42 | 187.58 | 96.3% | <0.0001 | 0.0150 |
| Province | No. of Studies | No. of People Examined | No. of Positive Cases | Prevalence (%) | Heterogeneity Test | |
|---|---|---|---|---|---|---|
| Q | P | |||||
| Khyber Pakhtunkhwa | 18 | 6969 | 1803 | 25.87 | 843.13 | <0.0001 |
| Punjab | 8 | 3955 | 808 | 20.42 | 187.58 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoukat, T.; Awan, U.A.; Mahmood, T.; Afzal, M.S.; Wasif, S.; Ahmed, H.; Cao, J. Epidemiology of Toxoplasmosis among the Pakistani Population: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 675. https://doi.org/10.3390/pathogens11060675
Shoukat T, Awan UA, Mahmood T, Afzal MS, Wasif S, Ahmed H, Cao J. Epidemiology of Toxoplasmosis among the Pakistani Population: A Systematic Review and Meta-Analysis. Pathogens. 2022; 11(6):675. https://doi.org/10.3390/pathogens11060675
Chicago/Turabian StyleShoukat, Tehniat, Usman Ayub Awan, Tahir Mahmood, Muhammad Sohail Afzal, Samia Wasif, Haroon Ahmed, and Jianping Cao. 2022. "Epidemiology of Toxoplasmosis among the Pakistani Population: A Systematic Review and Meta-Analysis" Pathogens 11, no. 6: 675. https://doi.org/10.3390/pathogens11060675








