Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Periprosthetic Joint Infections
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of Patients with MRSA-Induced PJIs
2.2. Molecular Typing of MRSA Isolates from PJIs
2.3. Association of Antibiotic Resistance and Genotypes of MRSA in PJIs
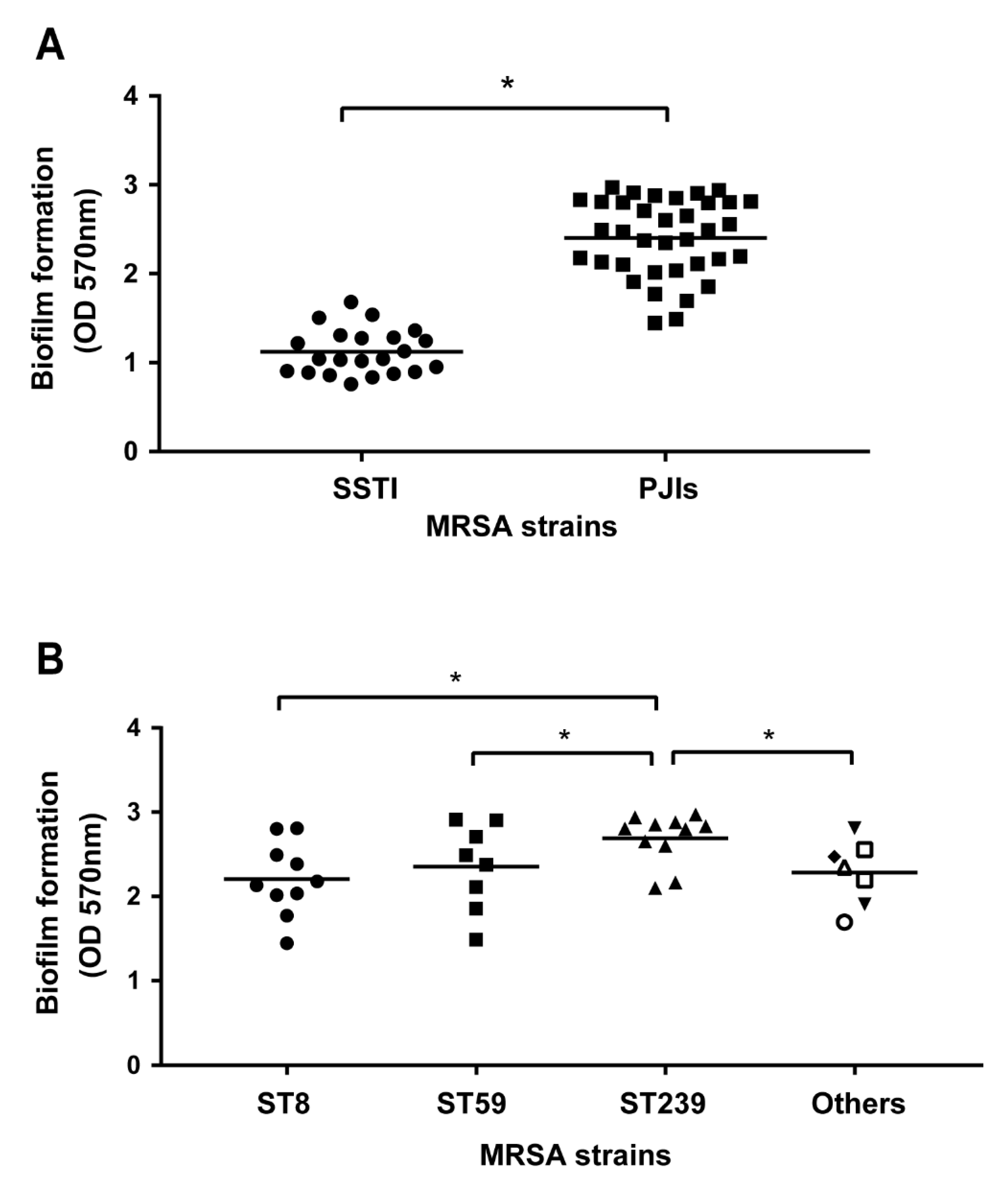
2.4. Association of Biofilm Formation and Genotypes of MRSA in PJIs
2.5. Correlation between MRSA Genotypes and Clinical Phenotypes
2.6. Epidemiological Characteristics of MRSA-Induced PJIs
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates and Patient Characteristics
4.2. Genomic DNA Extraction
4.3. SCCmec Typing, Multilocus Sequence Typing (MLST), and Spa Typing
4.4. Detection of Panton–Valentine Leukocidin (pvl)
4.5. Antibiotic Susceptibility Test
4.6. Biofilm Formation Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pivec, R.; Johnson, A.J.; Mears, S.C.; Mont, M.A. Hip arthroplasty. Lancet 2012, 380, 1768–1777. [Google Scholar] [CrossRef]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sotelo, J.; Berry, D.J.; Hanssen, A.D.; Cabanela, M.E. Midterm to long-term followup of staged reimplantation for infected hip arthroplasty. Clin. Orthop. Relat. Res. 2009, 467, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.J.; Abdel, M.P.; Sanders, T.L.; Fitzgerald, S.F.; Hanssen, A.D.; Berry, D.J. Irrigation and Debridement with Component Retention for Acute Infection after Hip Arthroplasty: Improved Results with Contemporary Management. J. Bone Jt. Surg. Am. 2017, 99, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Lora-Tamayo, J.; Murillo, O.; Iribarren, J.A.; Soriano, A.; Sanchez-Somolinos, M.; Baraia-Etxaburu, J.M.; Rico, A.; Palomino, J.; Rodriguez-Pardo, D.; Horcajada, J.P.; et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin. Infect. Dis. 2013, 56, 182–194. [Google Scholar] [CrossRef]
- Nodzo, S.R.; Boyle, K.K.; Spiro, S.; Nocon, A.A.; Miller, A.O.; Westrich, G.H. Success rates, characteristics, and costs of articulating antibiotic spacers for total knee periprosthetic joint infection. Knee 2017, 24, 1175–1181. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macia, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr. Rev. Musculoskelet. Med. 2018, 11, 389–3440. [Google Scholar] [CrossRef]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Katayama, Y.; Yuzawa, H.; Ito, T. Molecular genetics of methicillin-resistant Staphylococcus aureus. Int. J. Med. Microbiol. 2002, 292, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Aires-de-Sousa, M.; Boyce, J.; Tiemersma, E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368, 874–885. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; El-Naenaeey, E.-S.Y.; Kandeel, T.M.; Hegazy, W.A.H.; Mosbah, R.A.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 667. [Google Scholar] [CrossRef]
- Marschall, J.; Lane, M.A.; Beekmann, S.E.; Polgreen, P.M.; Babcock, H.M. Current management of prosthetic joint infections in adults: Results of an Emerging Infections Network survey. Int. J. Antimicrob. Agents 2013, 41, 272–277. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Role of Rifampin against Staphylococcal Biofilm Infections In Vitro, in Animal Models, and in Orthopedic-Device-Related Infections. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Dubee, V.; Zeller, V.; Lhotellier, L.; Kitzis, M.D.; Ziza, J.M.; Mamoudy, P.; Desplaces, N. Continuous high-dose vancomycin combination therapy for methicillin-resistant staphylococcal prosthetic hip infection: A prospective cohort study. Clin. Microbiol. Infect. 2013, 19, E98–E105. [Google Scholar] [CrossRef]
- Spellberg, B.; Lipsky, B.A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef]
- Kim, B.N.; Kim, E.S.; Oh, M.D. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J. Antimicrob. Chemother. 2014, 69, 309–322. [Google Scholar] [CrossRef]
- Nathwani, D.; Davey, P.G.; Marwick, C.A. MRSA: Treating people with infection. BMJ Clin. Evid. 2010, 2010, 922. [Google Scholar]
- Leijtens, B.; Weerwag, L.; Schreurs, B.W.; Kullberg, B.J.; Rijnen, W. Clinical Outcome of Antibiotic Suppressive Therapy in Patients with a Prosthetic Joint Infection after Hip Replacement. J. Bone Jt. Infect. 2019, 4, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, C.; Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. 1), i37–i40. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chowdhury, R.; Datta, M.; Chowdhury, G.; Mukhopadhyay, A.K. Characterization of the clonal profile of methicillin resistant Staphylococcus aureus isolated from patients with early post-operative orthopedic implant based infections. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 8. [Google Scholar] [CrossRef]
- Saleh, A.; George, J.; Faour, M.; Klika, A.K.; Higuera, C.A. Serum biomarkers in periprosthetic joint infections. Bone Joint. Res. 2018, 7, 85–93. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Iwao, Y.; Ishii, R.; Tomita, Y.; Shibuya, Y.; Takano, T.; Hung, W.C.; Higuchi, W.; Isobe, H.; Nishiyama, A.; Yano, M.; et al. The emerging ST8 methicillin-resistant Staphylococcus aureus clone in the community in Japan: Associated infections, genetic diversity, and comparative genomics. J. Infect. Chemother. 2012, 18, 228–240. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lai, C.C.; Chiang, H.T.; Lu, M.C.; Wang, L.F.; Tsai, T.L.; Kang, M.Y.; Jan, Y.N.; Lo, Y.T.; Ko, W.C.; et al. Predominance of methicillin-resistant Staphylococcus aureus in the residents and environments of long-term care facilities in Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 62–74. [Google Scholar] [CrossRef]
- Peng, K.T.; Huang, T.Y.; Chiang, Y.C.; Hsu, Y.Y.; Chuang, F.Y.; Lee, C.W.; Chang, P.J. Comparison of Methicillin-Resistant Staphylococcus aureus Isolates from Cellulitis and from Osteomyelitis in a Taiwan Hospital, 2016–2018. J. Clin. Med. 2019, 8, 816. [Google Scholar] [CrossRef]
- Bhatta, D.R.; Cavaco, L.M.; Nath, G.; Kumar, K.; Gaur, A.; Gokhale, S.; Bhatta, D.R. Association of Panton Valentine Leukocidin (PVL) genes with methicillin resistant Staphylococcus aureus (MRSA) in Western Nepal: A matter of concern for community infections (a hospital based prospective study). BMC Infect. Dis. 2016, 16, 199. [Google Scholar] [CrossRef]
- Chen, F.J.; Siu, L.K.; Lin, J.C.; Wang, C.H.; Lu, P.L. Molecular typing and characterization of nasal carriage and community-onset infection methicillin-susceptible Staphylococcus aureus isolates in two Taiwan medical centers. BMC Infect. Dis. 2012, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, T.; Coelho, C.; Silva, I.S.; de Lencastre, H.; Aires-de-Sousa, M. Staphylococcus aureus in former Portuguese colonies from Africa and the Far East: Missing data to help fill the world map. Clin. Microbiol. Infect. 2015, 21, 842.e1–842.e10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsao, F.Y.; Kou, H.W.; Huang, Y.C. Dissemination of methicillin-resistant Staphylococcus aureus sequence type 45 among nursing home residents and staff in Taiwan. Clin. Microbiol. Infect. 2015, 21, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Ko, W.C.; Ho, M.W.; Lin, H.H.; Yang, Y.L.; Lin, J.N.; Huang, I.W.; Wang, H.Y.; Lai, J.F.; Shiau, Y.R.; et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization among human immunodeficient virus-infected outpatients in Taiwan: Oral Candida colonization as a comparator. J. Oral. Microbiol. 2017, 9, 1322446. [Google Scholar] [CrossRef]
- Nakaminami, H.; Hirai, Y.; Nishimura, H.; Takadama, S.; Noguchi, N. Arthritis Caused by MRSA CC398 in a Patient without Animal Contact, Japan. Emerg. Infect. Dis. 2020, 26, 795–797. [Google Scholar] [CrossRef]
- Williamson, D.A.; Bakker, S.; Coombs, G.W.; Tan, H.; Monecke, S.; Heffernan, H. Emergence and molecular characterization of clonal complex 398 (CC398) methicillin-resistant Staphylococcus aureus (MRSA) in New Zealand. J. Antimicrob. Chemother. 2014, 69, 1428–1430. [Google Scholar] [CrossRef][Green Version]
- DeLeo, F.R.; Otto, M.; Kreiswirth, B.N.; Chambers, H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010, 375, 1557–1568. [Google Scholar] [CrossRef]
- De Sousa, M.A.; Conceicao, T.; Simas, C.; De Lencastre, H. Comparison of genetic backgrounds of methicillin-resistant and-susceptible Staphylococcus aureus isolates from Portuguese hospitals and the community. J. Clin. Microbiol. 2005, 43, 5150–5157. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef]
- Kassim, A.; Omuse, G.; Premji, Z.; Revathi, G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: A cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 21. [Google Scholar] [CrossRef]



| Characteristic | PJIs (n = 36) |
|---|---|
| Sex | |
| Female | 16 (44%) |
| Male | 20 (56%) |
| Age (year) | |
| Mean ± SD | 64 ± 18 |
| Range | 26–98 |
| Surgical sites | |
| Elbow | 1 (3%) |
| Hip | 29 (81%) |
| Knee | 6 (17%) |
| Underlying diseases | |
| CHC | 17 (47%) |
| CKD | 3 (8%) |
| DM | 9 (25%) |
| Gout | 3 (8%) |
| Cancer | 2 (6%) |
| SCCmec Type, No. (%) | MLST, No. (%) | Spa Type, No. (%) | PVL Positive, No. (%) | |||||
|---|---|---|---|---|---|---|---|---|
| II | 1 (3%) | ST5 | 2 (6%) | t002 | 2 (6%) | 22 (61%) | ||
| III | 13 (36%) | ST7 | 1 (3%) | t008 | 9 (25%) | |||
| IV | 13 (36%) | ST8 | 10 (28%) | t015 | 2 (6%) | |||
| V | 9 (25%) | ST45 | 1 (3%) | t034 | 1 (3%) | |||
| ST59 | 8 (22%) | t037 | 11 (31%) | |||||
| ST239 | 11 (31%) | t091 | 1 (3%) | |||||
| ST508 | 2 (6%) | t437 | 8 (22%) | |||||
| ST1232 | 1 (3%) | t441 | 1 (3%) | |||||
| t1081 | 1 (3%) | |||||||
| Genogroups | Lineages | MRSA Isolates, % |
|---|---|---|
| ST8 (n = 10) | t008-SCCmecIV-PVL+ | 6 (17%) |
| t008-SCCmecIV-PVL- | 1 (3%) | |
| t008-SCCmecV-PVL+ | 2 (6%) | |
| t441-SCCmecIV-PVL+ | 1 (3%) | |
| ST59 (n = 8) | t437-SCCmecIV-PVL+ | 1 (3%) |
| t437-SCCmecIV-PVL- | 2 (6%) | |
| t437-SCCmecV-PVL+ | 5 (14%) | |
| ST239 (n = 11) | t037-SCCmecIII-PVL+ | 5 (14%) |
| t037-SCCmecIII-PVL- | 6 (17%) | |
| Others (n = 7) | ||
| ST5 | t002-SCCmecII-PVL- | 1 (3%) |
| t002-SCCmecIII-PVL- | 1 (3%) | |
| ST7 | t091-SCCmecIII-PVL+ | 1 (3%) |
| ST45 | t1081-SCCmecV-PVL- | 1 (3%) |
| ST508 | t015-SCCmecIV-PVL- | 2 (6%) |
| ST1232 | t034-SCCmecV-PVL+ | 1 (3%) |
| Antibiotic | MIC a (µg/mL) | Total Strains (n = 36) | MRSA Genogroups (Resistance, %) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ST8 (n = 10) | ST59 (n = 8) | ST239 (n = 11) | ST5 (n = 2) | ST7 (n = 1) | ST45 (n = 1) | ST508 (n = 2) | ST1232 (n = 1) | |||
| Ciprofloxacin | ≥4 | 24 (67%) | 8 (80) | 2 (25) | 11 (100) | 2 (100) | 0 | 1 (100) | 0 | 0 |
| Gentamicin | ≥16 | 17 (44%) | 1 (10) | 3 (38) | 11(100) | 2 (100) | 0 | 0 | 0 | 0 |
| TMP-SMX | ≥4 | 11 (31%) | 0 | 0 | 11 (100) | 0 | 0 | 0 | 0 | 0 |
| Fusidic acid | ≥1 | 5 (14%) | 1 (10) | 2 (25) | 1 (9) | 0 | 1 (100) | 0 | 0 | 0 |
| Rifampicin | ≥4 | 2 (6%) | 0 | 0 | 0 | 2 (100) | 0 | 0 | 0 | 0 |
| Vancomycin | ≥2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MLST | Strain | MICof Antibiotics (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| Ciprofloxacin | Gentamicin | TMP-SMX | Fusidic Acid | Rifampicin | Vancomycin | ||
| ST8 | Sta-25 | 12 | 12 | 0.064 | 3 | 0.008 | 1.5 |
| Sta-30 | 12 | 0.5 | 0.064 | 0.064 | 0.006 | 1 | |
| Sta-581 | 32 | 0.38 | 0.064 | 0.047 | 0.006 | 0.5 | |
| Sta-582 | 0.19 | 0.38 | 0.047 | 0.047 | 0.006 | 0.5 | |
| Sta-596 | 0.19 | 0.25 | 0.047 | 0.032 | 0.006 | 0.5 | |
| Sta-695 | 16 | 0.25 | 0.047 | 0.032 | 0.002 | 1 | |
| Sta-1595 | 6 | 0.25 | 0.047 | 0.19 | 0.004 | 0.5 | |
| Sta-1629 | 8 | 32 | 0.047 | 0.125 | 0.004 | 0.5 | |
| Sta-1671 | 8 | 12 | 0.047 | 0.094 | 0.004 | 0.75 | |
| Sta-1708 | 8 | 0.38 | 0.047 | 0.047 | 0.004 | 0.5 | |
| ST59 | P2 | 0.25 | 256 | 0.064 | 3 | 0.008 | 1 |
| P11 | 0.25 | 16 | 0.094 | 0.032 | 0.016 | 1.5 | |
| P16 | 0.25 | 0.38 | 0.032 | 0.064 | 0.008 | 1.5 | |
| P65 | 0.25 | 64 | 0.064 | 0.047 | 0.006 | 1 | |
| Sta-329 | 32 | 0.38 | 0.047 | 0.032 | 0.008 | 1.5 | |
| Sta-414 | 0.25 | 4 | 0.032 | 0.032 | 0.004 | 0.5 | |
| Sta-468 | 32 | 0.5 | 0.032 | 0.047 | 0.006 | 1.5 | |
| Sta-790 | 0.25 | 8 | 0.047 | 1.5 | 0.004 | 0.38 | |
| ST239 | P61 | 32 | 256 | 8 | 0.064 | 0.006 | 1.5 |
| Sta-271 | 32 | 256 | 8 | 0.047 | 0.004 | 1 | |
| Sta-297 | 32 | 256 | 8 | 0.032 | 0.004 | 1 | |
| Sta-311 | 32 | 256 | 12 | 0.032 | 0.004 | 1 | |
| Sta-314 | 32 | 256 | 6 | 0.032 | 0.004 | 0.75 | |
| Sta-373 | 32 | 256 | 4 | 2 | 0.004 | 1 | |
| Sta-390 | 32 | 256 | 32 | 0.047 | 0.004 | 1.5 | |
| Sta-436 | 32 | 256 | 4 | 0.094 | 0.004 | 1 | |
| Sta-635 | 32 | 256 | 12 | 0.19 | 0.006 | 1 | |
| Sta-1597 | 32 | 256 | 32 | 0.064 | 0.006 | 1.5 | |
| Sta-1730 | 32 | 256 | 32 | 0.064 | 0.006 | 1 | |
| ST5 | Sta-697 | 32 | 96 | 0.047 | 0.047 | 32 | 0.75 |
| Sta-1709 | 32 | 128 | 0.047 | 0.094 | 32 | 0.5 | |
| ST7 | P9 | 0.25 | 12 | 0.064 | 3 | 0.004 | 1 |
| ST45 | Sta-1628 | 12 | 0.38 | 0.047 | 0.19 | 0.004 | 0.38 |
| ST508 | Sta-516 | 0.125 | 0.25 | 0.047 | 0.047 | 0.006 | 0.38 |
| Sta-1411 | 0.125 | 0.19 | 0.047 | 0.125 | 0.006 | 0.75 | |
| ST1232 | Sta-1505 | 0.25 | 0.19 | 0.047 | 0.19 | 0.006 | 0.5 |
| Variables | ST8 (n = 10) | ST59 (n = 8) | ST239 (n = 11) | ST5 (n = 2) | ST7 (n = 1) | ST45 (n = 1) | ST508 (n = 2) | ST1232 (n = 1) |
|---|---|---|---|---|---|---|---|---|
| CRP:mg/dL | 66.8 ± 57.56 | 184.29 ± 90.60 | 279.16 ± 109.53 | 160.3 ± 0 | 78.52 | 218.92 | 97.54 ± 135.18 | 14.84 |
| WBC:/μL | 9.79 ± 3.86 | 14.1 ± 4.02 | 18.49 ± 5.32 | 12.8 ± 0 | N.A | 13.4 ± 4.02 | 14.6 ± 8.49 | 10.1 |
| ESR:mm/hr | 78.29 ± 29.53 | 92.75 ± 45.83 | 95.33 ± 21.21 | 86 ± 0 | 140 | N.A | 15.15 ± 5.44 | 60 |
| Fever (no./total no.) | 1/10 | 0/8 | 7/11 | 0/2 | 1/1 | 0/1 | 0/2 | 0/1 |
| Characteristic | MRSA Isolates | |||
|---|---|---|---|---|
| ST8 (n = 10) | ST59 (n = 8) | ST239 (n = 11) | Others (n = 7) | |
| Sex | ||||
| Female | 2 (20%) | 4 (50%) | 7 (64%) | 3 (43%) |
| Male | 8 (80%) | 4 (50%) | 4 (36%) | 4 (57%) |
| Age (year) | ||||
| Mean ± SD | 62 ± 19 | 70 ± 17 | 56 ± 16 | 71 ± 17 |
| Range | 26–86 | 40–86 | 29–98 | 41–87 |
| Underlying diseases | ||||
| CHC | 4 (40%) | 2 (25%) | 9 (82%) | 2 (29%) |
| CKD | 0 | 0 | 1 (9%) | 2 (29%) |
| DM | 2 (20%) | 3 (38%) | 1 (9%) | 3 (43%) |
| Gout | 0 | 2 (25%) | 0 | 1 (14%) |
| Cancer | 0 | 0 | 2 (18%) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-L.; Huang, T.-Y.; Hsu, W.-B.; Lee, C.-W.; Chiang, Y.-C.; Chang, P.-J.; Peng, K.-T. Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Periprosthetic Joint Infections. Pathogens 2022, 11, 719. https://doi.org/10.3390/pathogens11070719
Chen J-L, Huang T-Y, Hsu W-B, Lee C-W, Chiang Y-C, Chang P-J, Peng K-T. Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Periprosthetic Joint Infections. Pathogens. 2022; 11(7):719. https://doi.org/10.3390/pathogens11070719
Chicago/Turabian StyleChen, Jiun-Liang, Tsung-Yu Huang, Wei-Bin Hsu, Chiang-Wen Lee, Yao-Chang Chiang, Pey-Jium Chang, and Kuo-Ti Peng. 2022. "Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Periprosthetic Joint Infections" Pathogens 11, no. 7: 719. https://doi.org/10.3390/pathogens11070719
APA StyleChen, J.-L., Huang, T.-Y., Hsu, W.-B., Lee, C.-W., Chiang, Y.-C., Chang, P.-J., & Peng, K.-T. (2022). Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Periprosthetic Joint Infections. Pathogens, 11(7), 719. https://doi.org/10.3390/pathogens11070719







