Insight into Trichinella britovi Infection in Pigs: Effect of Various Infectious Doses on Larvae Density and Spatial Larvae Distribution in Carcasses and Comparison of the Detection of Anti-T. britovi IgG of Three Different Commercial ELISA Tests and Immunoblot Assay
Abstract
:1. Introduction
2. Results
2.1. Intensity of T. britovi ML Infection and Distribution of the Larvae in Muscles of Pigs Experimentally Infected with Different Doses of T. britovi
2.2. Detection of Anti-T. britovi IgG in Pigs Using Three Commercial ELISA Kits
2.3. Variation in the Level of Anti-T. britovi IgG in the Serum of Pigs Infected with T. britovi
2.4. Correlation between Number of Recovered Larvae (lpg) in Particular Muscles of Experimentally Infected Pigs and Anti-T. britovi IgG Level
2.5. Correlations between ELISA Kits
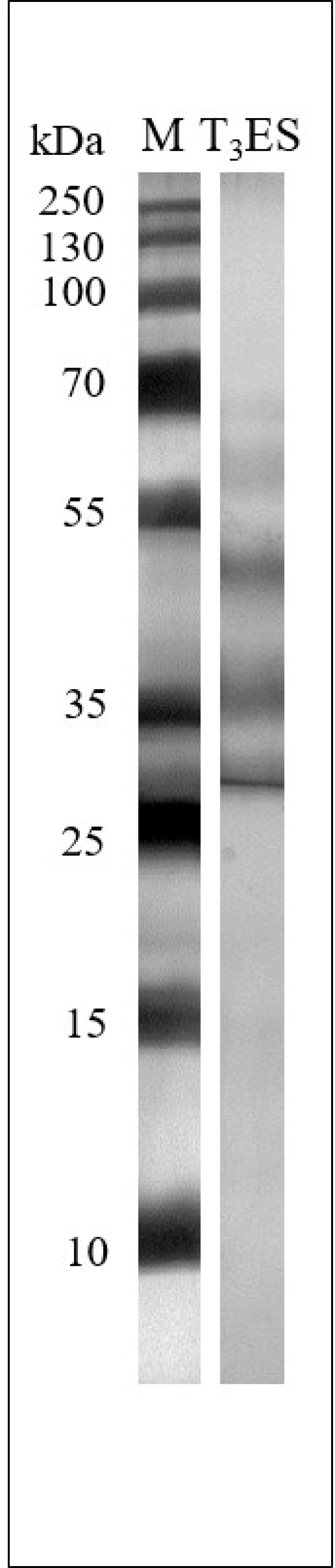
2.6. Recognition of the T. britovi ML ES Antigens by Anti-T. britovi IgG in Western Blot
3. Discussion
4. Materials and Methods
4.1. Parasites, Animals, and Study Design
4.2. Larval Recovery and Counting
4.3. Serological Analyses
4.3.1. Commercial ELISA Tests for Anti-T. britovi IgG Detection
4.3.2. Production of the T. britovi ML ES Antigen and Western Blot
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murrell, K.D.; Pozio, E. Worldwide occurrence and impact of human trichinellosis, 1986–2009. Emerg. Infect. Dis. 2011, 17, 2194–2202. [Google Scholar]
- Pozio, E.; Zarlenga, D.S. Recent advances on the taxonomy, systematics and epidemiology of Trichinella. Int. J. Parasitol. 2005, 35, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Krivokapich, S.J.; Pozio, E.; Gatti, G.M.; Gonzalez Prous, C.L.; Ribicich, M.; Marucci, G.; La Rosa, G.; Confalonieri, V. Trichinella patagoniensis n. sp. (Nematoda), a new encapsulated species infecting carnivorous mammals in South America. Int. J. Parasitol. 2012, 42, 903–909. [Google Scholar] [CrossRef]
- Sharma, R.; Thompson, P.C.; Hoberg, E.P.; Scandrett, W.B.; Konecsni, K.; Harms, N.J.; Kukka, P.M.; Jung, T.S.; Elkin, B.; Mulders, R.; et al. Hiding in plain sight: Discovery and phylogeography of a cryptic species of Trichinella (Nematoda: Trichinellidae) in wolverine (Gulo gulo). Int. J. Parasitol. 2020, 50, 277–287. [Google Scholar] [CrossRef]
- Hurníková, Z.; Šnábel, V.; Pozio, E.; Reiterová, K.; Hrčková, G.; Halásová, D.; Dubinský, P. First record of Trichinella pseudospiralis in the Slovak Republic found in domestic focus. Vet. Parasitol. 2005, 128, 91–98. [Google Scholar] [CrossRef]
- Beck, R.; Beck, A.; Lučinger, S.; Florijančić, T.; Bošković, I.; Marinculić, A. Trichinella pseudospiralis in pig from Croatia. Vet. Parasitol. 2009, 159, 304–307. [Google Scholar] [CrossRef]
- Santrac, V.; Nedic, D.N.; Maric, J.; Nikolic, S.; Stevanovic, O.; Vasilev, S.; Cvetkovic, J.; Sofronic-Milosavljevic, L. The first report of Trichinella pseudospiralis presence in domestic swine and T. britovi in wild boar in Bosnia and Herzegovina. Acta Parasitol. 2015, 60, 471–475. [Google Scholar] [CrossRef]
- Pozio, E.; Rinaldi, L.; Marucci, G.; Musella, V.; Galati, F.; Cringoli, G.; Boireau, P.; La Rosa, G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol. 2009, 39, 71–79. [Google Scholar] [CrossRef]
- Bilska-Zając, E.; Różycki, M.; Grądziel-Krukowska, K.; Bełcik, A.; Mizak, I.; Karamon, J.; Sroka, J.; Zdybel, J.; Cencek, T. Diversity of Trichinella species in relation to the host species and geographical location. Vet. Parasitol. 2020, 279, 109052. [Google Scholar] [CrossRef]
- Bilska-Zając, E.; Różycki, M.; Chmurzyńska, E.; Marucci, G.; Cencek, T.; Karamon, J.; Bocian, Ł. Trichinella species circulating in wild boars (Sus scrofa) populations in Poland. Int. J. Parasitol. Parasites Wildl. 2013, 2, 211–213. [Google Scholar] [CrossRef] [Green Version]
- Pozio, E.; Hoberg, E.; La Rosa, G.; Zarlenga, D.S. Molecular taxonomy, phylogeny and biogeography of nematodes belonging to the Trichinella genus. Infect. Genet. Evol. 2009, 9, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Cabaj, W. Wild and domestic animals as permanent Trichinella reservoir in Poland. Wiad. Parazytol. 2006, 52, 175–179. [Google Scholar] [PubMed]
- Bilska-Zając, E.; Różycki, M.; Karamon, J.; Sroka, J.; Cencek, T. The role of epidemiological investigations in the current epidemiology of trichinellosis in Poland. Życie Wet. 2019, 94, 435–440. [Google Scholar]
- Kapel, C.M.O.; Webster, P.; Lind, P.; Pozio, E.; Henriksen, S.A.; Murrell, K.D.; Nansen, P. Trichinella spiralis, T. britovi, and T. nativa: Infectivity, larval distribution in muscle, and antibody response after experimental infection of pigs. Parasitol. Res. 1998, 84, 264–271. [Google Scholar]
- Malakauskas, A.; Kapel, C.M.O.; Webster, P. Infectivity, persistence and serological response of nine Trichinella genotypes in rats. Parasite 2001, 8, S216–S222. [Google Scholar] [CrossRef] [Green Version]
- Nöckler, K.; Serrano, F.J.; Boireau, P.; Kapel, C.M.O.; Pozio, E. Experimental studies in pigs on Trichinella detection in different diagnostic matrices. Vet. Parasitol. 2005, 132, 85–90. [Google Scholar] [CrossRef]
- Pozio, E.; Meriadli, G.; Licata, E.; Della Casa, G.; Fabiani, M.; Amati, M.; Cherchi, S.; Ramini, M.; Faeti, V.; Interisano, M.; et al. Differences in larval survival and IgG response patterns in long-lasting infections by Trichinella spiralis, Trichinella britovi and Trichinella pseudospiralis in pigs. Parasit. Vectors 2020, 13, 520. [Google Scholar] [CrossRef]
- Gondek, M.; Knysz, P.; Pomorska-Mól, M.; Ziomek, M.; Bień-Kalinowska, J. Acute phase protein pattern and antibody response in pigs experimentally infected with a moderate dose of Trichinella spiralis, T. britovi, and T. pseudospiralis. Vet. Parasitol. 2020, 288, 109277. [Google Scholar] [CrossRef]
- Gomez-Garcia, V.; Hernandez-Quero, J.; Rodriguez-Osorio, M. Short report: Human infection with Trichinella britovi in Granada, Spain. Am. J. Trop. Med. Hyg. 2003, 68, 463–464. [Google Scholar] [CrossRef]
- Akkoc, N.; Kuruuzum, Z.; Akar, S.; Yuce, A.; Onen, F.; Yapar, N.; Ozgenc, O.; Turk, M.; Ozdemir, D.; Avci, M.; et al. A large-scale outbreak of trichinellosis caused by Trichinella britovi in Turkey. Zoonoses Public Health 2009, 56, 65–70. [Google Scholar] [CrossRef]
- Pavic, S.; Andric, A.; Sofronic-Milosavljevic, L.J.; Gnjatovic, M.; Mitić, I.; Vasilev, S.; Sparic, R.; Pavic, A. Trichinella britovi outbreak: Epidemiological, clinical, and biological features. Med. Mal. Infect. 2020, 50, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Cabaj, W.; Moskwa, B.; Pastusiak, K.; Malczewski, A. Trichinellosis in wild animals and domestic pigs in Poland. Med. Weter. 2004, 60, 80–83. [Google Scholar]
- Bień, J.; Moskwa, B.; Goździk, K.; Cybulska, A.; Kornacka, A.; Welc, M.; Popiołek, M.; Cabaj, W. The occurrence of nematodes of the genus Trichinella in wolves (Canis lupus) from the Bieszczady Mountains and Augustowska Forest in Poland. Vet. Parasitol. 2016, 231, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, A.; Kornacka, A.; Moskwa, B. The occurrence and muscle distribution of Trichinella britovi in raccoon dogs (Nyctereutes procyonoides) in wildlife in the Głęboki Bród Forest District, Poland. Int. J. Parasitol. Parasites Wildl. 2019, 9, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, B.; Goździk, K.; Bień, J.; Bogdaszewski, M.; Cabaj, W. Molecular identification of Trichinella britovi in martens (Martes martes) and badgers (Meles meles); new host records in Poland. Acta Parasitol. 2012, 57, 402–405. [Google Scholar] [CrossRef]
- General Veterinary Inspectorate. Veterinary Statistical Reporting. 2015–2020. Available online: https://www.wetgiw.gov.pl/publikacje/rrw-sprawozdawczosc-statystyczna (accessed on 5 April 2022).
- Commission implementing regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat. Off. J. Eur. Union 2015, 212, 7–34.
- ISO 18743: 2015; Microbiology of the Food Chain—Detection of Trichinella Larvae in Meat by Artificial Digestion Method. International Organization for Standardization: Geneva, Switzerland, 2015.
- Prost, E.K.; Nowakowski, Z. Detectability of Trichinella spiralis in muscles by pooled-sample-digestion-method. Fleischwirtschaft 1990, 70, 593–595. [Google Scholar]
- Franssen, F.F.J.; Fonville, M.; Takumi, K.; Vallée, I.; Grasset, A.; Koedam, M.A.; Wester, P.W.; Boireau, P.; van der Giesen, J.W.B. Antibody response against Trichinella spiralis in experimentally infected rats is dose dependent. Vet. Res. 2011, 42, 113. [Google Scholar] [CrossRef] [Green Version]
- Kapel, C.M.O.; Gamble, H.R. Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. in experimentally infected pigs. Int. J. Parasitol. 2000, 30, 215–221. [Google Scholar] [CrossRef]
- Gómez-Morales, M.A.; Ludovisi, A.; Amati, M.; Blaga, R.; Zivojinovic, M.; Ribicich, M.; Pozio, E. A distinctive Western blot pattern to recognize Trichinella infections in humans and pigs. Int. J. Parasitol. 2012, 42, 1017–1023. [Google Scholar] [CrossRef]
- Nöckler, K.; Voigt, W.P.; Protz, D.; Miko, A.; Ziedler, K. Intravitale Diagnostik der Trichinellose beim Schwein mit dem indirekten ELISA. Berl. Münch. Tierärztl. Wschr. 1995, 108, 167–174. [Google Scholar]
- Gondek, M.; Bień, J.; Nowakowski, Z. Use of ELISA and Western blot for serological detection of antibodies to E-S antigens of Trichinella spiralis muscle larvae in sera of swine experimentally infected with Trichinella spiralis. Vet. Immunol. Immunopathol. 2018, 203, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, S.; Stachyra, A.; Stefaniak, J.; Mrówka, K.; Moskwa, B.; Bień-Kalinowska, J. Immunoproteomic analysis of Trichinella spiralis and Trichinella britovi excretory-secretory muscle larvae proteins recognized by sera from humans infected with Trichinella. PLoS ONE 2020, 15, e0241918. [Google Scholar] [CrossRef] [PubMed]
- Zarlenga, D.S.; Chute, M.B.; Martin, A.; Kapel, C.M.O. A multiplex PCR for unequivocal differentiation of all encapsulated and non-encapsulated genotypes of Trichinella. Int. J. Parasitol. 1999, 29, 1859–1867. [Google Scholar] [CrossRef]
- Grzelak, S.; Moskwa, B.; Bień, J. Trichinella britovi muscle larvae and adult worms: Stage-specific and common antigens detected by two-dimensional gel electrophoresis-based immunoblotting. Parasit. Vectors 2018, 11, 584. [Google Scholar] [CrossRef]

| Muscle/Muscle Group | Numbers of Trichinella Larvae/g Muscle (lpg) | |||
|---|---|---|---|---|
| Side | p1 | Left and Right (Combined) | ||
| Left | Right | |||
| Mean ± SD (Median; IQR) | Mean ± SD (Median; IQR) | Mean ± SD (Median; IQR) | ||
| Diaphragm pillars (Crura diaphragmatica) | - | - | - | 41.46 ± 20.28 |
| Tongue (Lingua) | - | - | - | 37.75 ± 19.58 |
| Masseter (M. masseter) | 21.32 ± 11.22 | 21.72 ± 13.44 | 0.956 † | 21.53 ± 12.31 * |
| Neck (Mm. colli) | 12.06 ± 9.00 | 14.53 ± 10.21 | 0.667 † | 13.30 ± 9.36 * |
| Triceps brachii (M. triceps brachii) | 9.71 ± 3.63 | 10.56 ± 6.48 | 0.787 † | 10.14 ± 4.93 * |
| Lateral and medial pterygoid (M. pterygoideus lateralis et medialis) | 17.16 ± 14.15 | 15.34 ± 10.93 | 0.808 † | 16.29 ± 12.73 * |
| Sublumbar (M. psoas major et minor) | 9.42 ± 5.04 | 11.09 ± 8.03 | 0.675 † | 10.25 ± 6.53 * |
| Abdomen (Mm. abdominis) | 12.02 ± 9.03 | 12.26 ± 7.61 | 0.960 † | 12.14 ± 8.32 * |
| Forelimb extensors (Mm. extensores antebrachii) | 8.48 ± 4.96 | 8.65 ± 6.90 | 0.963 † | 8.59 ± 5.91 * |
| Intercostal (M. intercostales) | 6.75 ± 4.32 | 6.18 ± 3.25 | 0.802 † | 6.47 ± 3.77 * |
| Crus flexors (Mm. flexores cruris) | 6.85 ± 4.78 | 7.45 ± 5.23 | 0.840 † | 7.16 ± 4.99 * |
| Forelimb flexors (Mm. flexores antebrachii) | 6.89 ± 2.86 | 6.80 ± 3.43 | 0.959 † | 6.83 ± 2.96 * |
| Femoral biceps (M. biceps femoris) | 6.04 ± 3.78 | 5.15 ± 3.46 | 0.680 † | 5.60 ± 3.54 * |
| Crus extensors (Mm. extensores cruris) | 6.46 ± 3.01 | 6.04 ± 2.71 | 0.801 † | 6.23 ± 2.64 * |
| Back (M. longissimus thoracis) | 4.14 ± 2.44 | 3.69 ± 2.10 | 0.735 † | 3.92 ± 2.26 * |
| p2 | - | - | - | <0.0001 § |
| Tested muscles in total | 9.79 ± 7.94 (7.27; 4.60–12.62) | 9.96 ± 8.28 (7.49; 4.52–12.04) | 0.994‡ | 13.84 ± 14.29 (8.15; 5.04–17.41) |
| Muscle/Muscle Group | Number of Trichinella Larvae/g Muscle (lpg) | |||
|---|---|---|---|---|
| Side | p1 | Left and Right (Combined) | ||
| Left | Right | |||
| Mean ± SD (Median; IQR) | Mean ± SD (Median; IQR) | Mean ± SD (Median; IQR) | ||
| Diaphragm pillars (Crura diaphragmatica) | - | - | - | 43.67 ± 16.57 |
| Tongue (Lingua) | - | - | - | 30.34 ± 11.42 |
| Masseter (M. masseter) | 14.42 ± 5.76 | 17.07 ± 4.52 | 0.324 † | 15.77 ± 4.63 * |
| Neck (Mm. colli) | 14.39 ± 7.40 | 17.21 ± 5.65 | 0.406 † | 15.74 ± 5.47 * |
| Triceps brachii (M. triceps brachii) | 12.68 ± 7.45 | 15.23 ± 7.93 | 0.517 † | 13.96 ± 6.36 * |
| Lateral and medial pterygoid (M. pterygoideus lateralis et medialis) | 12.73 ± 7.58 | 14.78 ± 7.89 | 0.605 † | 13.82 ± 7.18 * |
| Sublumbar (M. psoas major et minor) | 12.23 ± 4.98 | 12.37 ± 4.84 | 0.956 † | 12.30 ± 4.34 * |
| Abdomen (Mm. abdominis) | 11.94 ± 7.83 | 11.67 ± 6.06 | 0.940 † | 11.81 ± 6.29 * |
| Forelimb extensors (Mm. extensores antebrachii) | 7.86 ± 5.66 | 12.69 ± 6.66 | 0.140 † | 10.32 ± 6.00 * |
| Intercostal (Mm. intercostales) | 8.94 ± 6.00 | 9.58 ± 3.61 | 0.800 † | 9.33 ± 4.33 * |
| Crus flexors (Mm. flexores cruris) | 7.64 ± 6.16 | 10.88 ± 7.44 | 0.359 † | 9.19 ± 6.39 * |
| Forelimb flexors (Mm. flexores antebrachii) | 7.47 ± 6.15 | 8.66 ± 5.56 | 0.692 † | 8.06 ± 5.69 * |
| Femoral biceps (M. biceps femoris) | 6.64 ± 3.82 | 8.56 ± 5.02 | 0.404 † | 7.60 ± 3.90 * |
| Crus extensors (Mm. extensores cruris) | 8.85 ± 4.98 | 6.33 ± 3.27 | 0.253 † | 7.50 ± 3.92 * |
| Back (M. longissimus thoracis) | 6.42 ± 3.27 | 5.05 ± 2.36 | 0.355 † | 5.74 ± 2.65 * |
| p2 | - | - | - | <0.0001 § |
| Tested muscles in total | 10.17 ± 6.39 (8.21; 5.61–15.31) | 11.54 ± 6.53 (10.29; 6.54–16.62) | 0.105‡ | 14.34 ± 11.83 (11.16; 6.58–18.29) |
| Muscle/Muscle Group | Pigs Infected with 5000 ML of T. britovi (n = 8) | Pigs Infected with 3000 ML of T. britovi (n = 6) | p1 |
|---|---|---|---|
| Numbers of Trichinella Larvae/g Muscle (lpg) | |||
| Median (IQR) | |||
| Diaphragm pillars (Crura diaphragmatica) | 49.32 (31.33–55.41) | 38.38 (24.27–44.56) | 0.561 |
| Tongue (Lingua) | 33.72 (18.97–39.97) | 35.32 (23.48–37.04) | 0.949 |
| Masseter (M. masseter) | 16.80 (11.82–19.37) | 20.67 (17.24–21.30) | 0.272 |
| Neck (Mm. colli) | 17.43 (10.74–20.27) | 11.65 (7.94 –13.31) | 0.401 |
| Triceps brachii (M. triceps brachii) | 11.79 (8.85–20.60) | 8.75 (7.28–10.70) | 0.220 |
| Lateral and medial pterygoid (M. pterygoideus lateralis et medialis) | 12.67 (6.87–20.77) | 13.27 (6.67–17.41) | 0.897 |
| Sublumbar (M. psoas major et minor) | 13.52 (8.85–15.20) | 9.24 (8.05–9.91) | 0.401 |
| Abdomen (Mm. abdominis) | 11.22 (6.80–14.19) | 10.00 (6.73–13.24) | 0.949 |
| Forelimb extensors (Mm. extensores antebrachii) | 8.58 (5.37–15.21) | 6.92 (5.57–8.74) | 0.561 |
| Intercostal (M. intercostales) | 8.71 (6.48–13.27) | 4.88 (4.38–7.87) | 0.272 |
| Crus flexors (Mm. flexores cruris) | 7.11 (4.07–13.59) | 5.79 (3.66–8.12) | 0.747 |
| Forelimb flexors (Mm. flexores antebrachii) | 7.21 (4.22–10.87) | 5.51 (4.80–8.41) | 0.846 |
| Femoral biceps (M. biceps femoris) | 7.35 (4.28–11.06) | 4.65 (3.07–6.05) | 0.366 |
| Crus extensors (Mm. extensores cruris) | 6.56 (4.13–10.94) | 5.07 (4.31–7.61) | 0.949 |
| Back (M. longissimus thoracis) | 5.85 (3.87–7.75) | 3.08 (2.47–4.54) | 0.220 |
| 15 tested muscles in total | 11.16 (6.58–18.29) | 8.15 (5.04–17.41) | 0.106 |
| Day before/after Infection | ELISA Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PrioCHECK® Trichinella Ab (ThermoFisher Scientific) 1 | ID Screen® Trichinella Indirect Multi-Species (ID Vet) 2 | Pigtype® Trichinella Ab (Qiagen) 3 | ||||||||||
| OD | PP | OD | S/P% | OD | S/P | |||||||
| Mean | ± | SD | Mean | Mean | ± | SD | Mean | Mean | ± | SD | Mean | |
| −4 | 0.065 | ± | 0.004 | 2.258 | 0.097 | ± | 0.012 | 2.660 | 0.104 | ± | 0.031 | 0.041 |
| 3 | 0.073 | ± | 0.009 | 2.526 | 0.104 | ± | 0.020 | 3.148 | 0.121 | ± | 0.031 | 0.056 |
| 6 | 0.089 | ± | 0.056 | 3.089 | 0.171 | ± | 0.191 | 7.507 | 0.197 | ± | 0.082 | 0.121 |
| 9 | 0.096 | ± | 0.076 | 3.319 | 0.179 | ± | 0.189 | 7.436 | 0.221 | ± | 0.088 | 0.142 |
| 13 | 0.102 | ± | 0.065 | 3.548 | 0.172 | ± | 0.158 | 7.621 | 0.250 | ± | 0.085 | 0.166 |
| 15 | 0.123 | ± | 0.068 | 4.288 | 0.171 | ± | 0.134 | 7.511 | 0.251 | ± | 0.108 | 0.173 |
| 17 | 0.149 | ± | 0.083 | 5.199 | 0.192 | ± | 0.142 | 8.829 | 0.237 | ± | 0.089 | 0.166 |
| 20 | 0.117 | ± | 0.066 | 4.126 | 0.169 | ± | 0.128 | 7.033 | 0.231 | ± | 0.124 | 0.160 |
| 24 | 0.113 | ± | 0.061 | 3.979 | 0.167 | ± | 0.110 | 6.958 | 0.234 | ± | 0.108 | 0.163 |
| 27 | 0.157 | ± | 0.117 | 5.512 | 0.215 | ± | 0.169 | 10.093 | 0.295 | ± | 0.104 | 0.194 |
| 29 | 0.245 | ± | 0.275 | 8.602 | 0.324 | ± | 0.292 | 17.405 | 0.376 | ± | 0.267 | 0.256 |
| 36 | 1.034 A | ± | 0.801 | 36.321 | 1.017 A | ± | 0.759 | 63.549 | 1.018 A | ± | 0.761 | 0.802 |
| 41 | 1.733 B | ± | 0.860 | 58.440 | 1.747 B | ± | 0.696 | 111.099 | 2.020 B | ± | 0.973 | 1.654 |
| 45 | 2.038 BC | ± | 0.653 | 67.281 | 1.924 B | ± | 0.389 | 122.164 | 2.339 B | ± | 0.862 | 1.925 |
| 51 | 2.171 BC | ± | 0.621 | 71.688 | 2.023 B | ± | 0.226 | 128.623 | 2.375 B | ± | 0.802 | 1.956 |
| 55 | 2.394 C | ± | 0.528 | 79.028 | 2.016 B | ± | 0.293 | 128.201 | 2.567 B | ± | 0.694 | 2.119 |
| 59 | 2.435 C | ± | 0.485 | 80.402 | 2.059 B | ± | 0.284 | 131.025 | 2.323 B | ± | 0.577 | 1.911 |
| 62 | 2.434 C | ± | 0.481 | 80.816 | 2.145 B | ± | 0.269 | 136.676 | 2.421 B | ± | 0.544 | 1.995 |
| Day before/after Infection | ELISA Test Result | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PrioCHECK® Trichinella Ab (ThermoFisher Scientific) | ID Screen® Trichinella Indirect Multi-Species (ID Vet) | Pigtype® Trichinella Ab (Qiagen) | ||||||||||||||||||||||
| Pig no. | Pig no. | Pig no. | ||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| −4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 3 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 6 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 9 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 13 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 15 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 17 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 20 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 24 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 27 | − | − | − | −1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
| 29 | − | − | − | + | − | − | − | − | − | − | − | + | − | − | − | − | −2 | −2 | − | + | − | − | − | − |
| 36 | + | + | − | + | −1 | + | − | + | + | +/− | − | + | − | + | − | + | + | + | −2 | + | − | + | − | + |
| 41 | + | + | −1 | + | + | + | + | + | + | + | +/− | + | + | + | +/− | + | + | + | + | + | + | + | + | + |
| 45 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 51 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 55 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 59 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 62 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Muscle/Muscle Group | ELISA Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PrioCHECK® Trichinella Ab (ThermoFisher Scientific) | ID Screen® Trichinella Indirect Multi-Species (ID Vet) | Pigtype® Trichinella Ab (QIAGEN) | |||||||
| 41 dpi | 51 dpi | 62 dpi | 41 dpi | 51 dpi | 62 dpi | 41 dpi | 51 dpi | 62 dpi | |
| Diaphragm pillars (Crura diaphragmatica) | −0.276 | −0.273 | −0.699 | −0.216 | 0.080 | −0.142 | −0.220 | −0.070 | −0.478 |
| Tongue (Lingua) | −0.594 | −0.654 | −0.863 | −0.482 | −0.189 | −0.089 | −0.527 | −0.420 | −0.764 |
| Masseter (M. masseter) | 0.139 | 0.040 | −0.382 | 0.101 | −0.01 | −0.380 | 0.164 | 0.228 | −0.229 |
| Neck (Mm. colli) | 0.273 | −0.080 | −0.395 | 0.260 | −0.202 | −0.342 | 0.254 | 0.057 | −0.272 |
| Triceps brachii (M. triceps brachii) | 0.093 | −0.165 | −0.419 | −0.014 | −0.541 | −0.553 | 0.048 | −0.104 | −0.394 |
| Lateral and medial pterygoid (M. pterygoideus lateralis et medialis) | 0.245 | 0.058 | −0.263 | 0.231 | −0.258 | −0.216 | 0.170 | 0.013 | −0.192 |
| Sublumbar (M. psoas major et minor) | 0.388 | 0.019 | −0.260 | 0.385 | 0.167 | −0.198 | 0.434 | 0.277 | −0.041 |
| Abdomen (Mm. abdominis) | 0.486 | 0.249 | 0.040 | 0.335 | 0.023 | −0.436 | 0.502 | 0.408 | 0.144 |
| Forearm extensors (Mm. extensores antebrachii) | 0.706 | 0.411 | 0.214 | 0.622 | 0.120 | −0.124 | 0.722 * | 0.543 | 0.387 |
| Intercostal (Mm. intercostales) | 0.630 | 0.243 | 0.069 | 0.676 | 0.275 | 0.115 | 0.682 | 0.456 | 0.306 |
| Crus flexors (Mm. flexores cruris) | 0.482 | 0.248 | 0.102 | 0.364 | −0.087 | −0.016 | 0.490 | 0.303 | 0.313 |
| Forearm flexors (Mm. flexores antebrachii) | 0.547 | 0.250 | 0.078 | 0.386 | −0.216 | −0.290 | 0.516 | 0.285 | 0.199 |
| Femoral biceps (M. biceps femoris) | 0.485 | 0.228 | −0.021 | 0.463 | 0.116 | −0.185 | 0.536 | 0.456 | 0.146 |
| Crus extensors (Mm. extensores cruris) | 0.559 | 0.271 | 0.084 | 0.536 | −0.006 | 0.080 | 0.541 | 0.297 | 0.272 |
| Back (M. Longissimus thoracis) | 0.328 | −0.042 | −0.323 | 0.287 | −0.029 | −0.193 | 0.332 | 0.109 | −0.103 |
| Pig no. (Serum Samples) | PrioCHECK® Trichinella Ab/ ID Screen® Trichinella Indirect Multi-Species | PrioCHECK® Trichinella Ab/Pigtype® Trichinella Ab | ID Screen® Trichinella Indirect Multi-Species/Pigtype® Trichinella Ab |
|---|---|---|---|
| 1 (n = 18) | 0.915 * | 0.961 * | 0.886 * |
| 2 (n = 18) | 0.950 * | 0.884 * | 0.953 * |
| 3 (n = 18) | 0.862 * | 0.802 * | 0.800 * |
| 4 (n = 18) | 0.928 * | 0.930 * | 0.938 * |
| 5 (n = 18) | 0.757 * | 0.831 * | 0.855 * |
| 6 (n = 18) | 0.963 * | 0.895 * | 0.907 * |
| 7 (n = 18) | 0.934 * | 0.742 * | 0.725 * |
| 8 (n = 18) | 0.777 * | 0.764 * | 0.847 * |
| Whole procedure (n = 144) | 0.903 * | 0.910 * | 0.912 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gondek, M.; Grzelak, S.; Pyz-Łukasik, R.; Knysz, P.; Ziomek, M.; Bień-Kalinowska, J. Insight into Trichinella britovi Infection in Pigs: Effect of Various Infectious Doses on Larvae Density and Spatial Larvae Distribution in Carcasses and Comparison of the Detection of Anti-T. britovi IgG of Three Different Commercial ELISA Tests and Immunoblot Assay. Pathogens 2022, 11, 735. https://doi.org/10.3390/pathogens11070735
Gondek M, Grzelak S, Pyz-Łukasik R, Knysz P, Ziomek M, Bień-Kalinowska J. Insight into Trichinella britovi Infection in Pigs: Effect of Various Infectious Doses on Larvae Density and Spatial Larvae Distribution in Carcasses and Comparison of the Detection of Anti-T. britovi IgG of Three Different Commercial ELISA Tests and Immunoblot Assay. Pathogens. 2022; 11(7):735. https://doi.org/10.3390/pathogens11070735
Chicago/Turabian StyleGondek, Michał, Sylwia Grzelak, Renata Pyz-Łukasik, Przemysław Knysz, Monika Ziomek, and Justyna Bień-Kalinowska. 2022. "Insight into Trichinella britovi Infection in Pigs: Effect of Various Infectious Doses on Larvae Density and Spatial Larvae Distribution in Carcasses and Comparison of the Detection of Anti-T. britovi IgG of Three Different Commercial ELISA Tests and Immunoblot Assay" Pathogens 11, no. 7: 735. https://doi.org/10.3390/pathogens11070735
APA StyleGondek, M., Grzelak, S., Pyz-Łukasik, R., Knysz, P., Ziomek, M., & Bień-Kalinowska, J. (2022). Insight into Trichinella britovi Infection in Pigs: Effect of Various Infectious Doses on Larvae Density and Spatial Larvae Distribution in Carcasses and Comparison of the Detection of Anti-T. britovi IgG of Three Different Commercial ELISA Tests and Immunoblot Assay. Pathogens, 11(7), 735. https://doi.org/10.3390/pathogens11070735





