Co-Circulation of Different Hepatitis E Virus Genotype 3 Subtypes in Pigs and Wild Boar in North-East Germany, 2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Wild Boar and Pig Samples
2.2. RNA Isolation
2.3. Quantitative Real-Time RT-PCR and Sequence Analysis
2.4. Phylogenetic Analysis
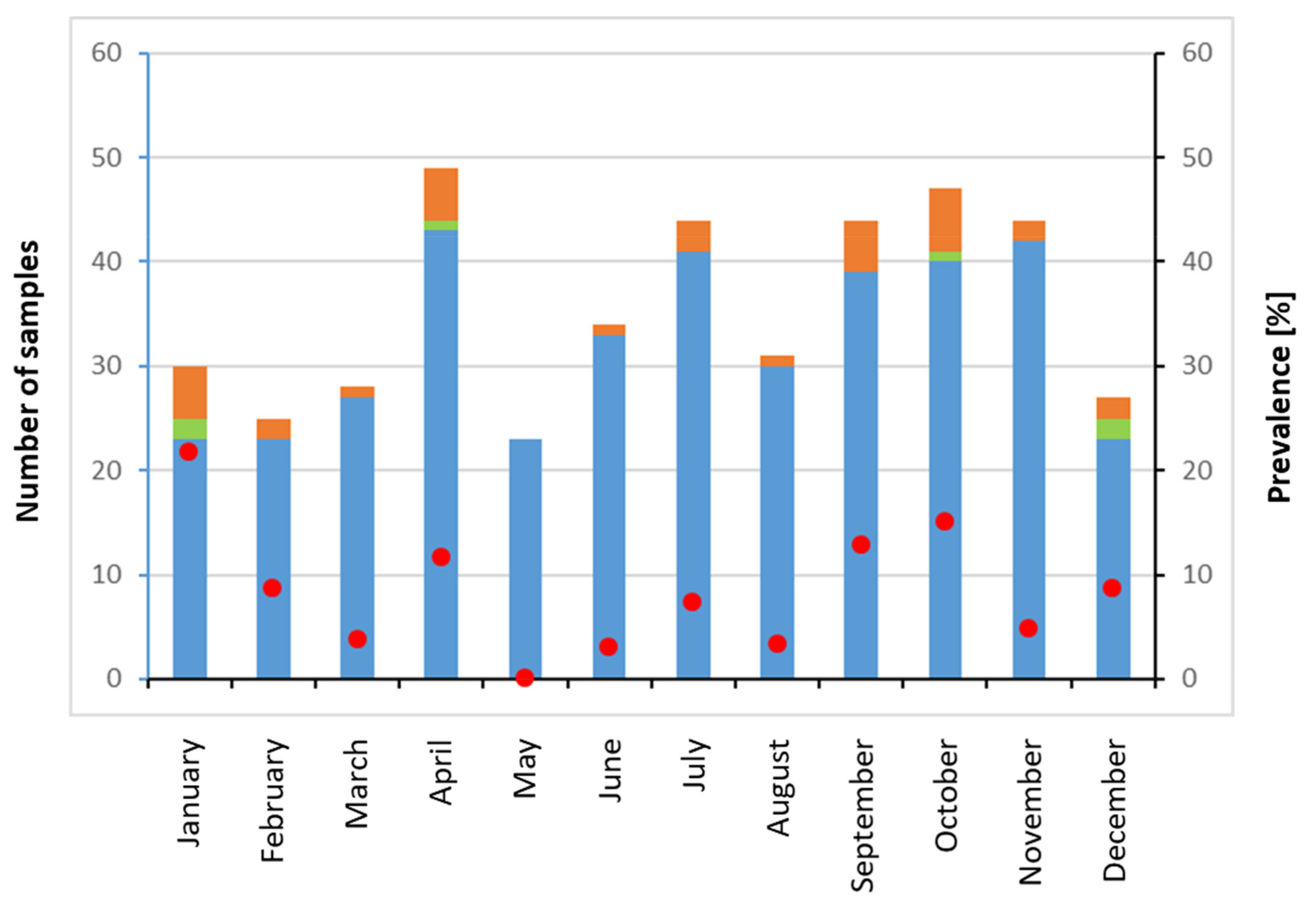
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ankcorn, M.J.; Tedder, R.S. Hepatitis E: The current state of play. Transfus. Med. 2017, 27, 84–95. [Google Scholar] [CrossRef] [PubMed]
- WHO. Hepatitis E. 2022. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-e (accessed on 10 March 2022).
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.M.; Purdy, M.A.; International Committee on the Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Hakze-van der Honing, R.W.; van Coillie, E.; Antonis, A.F.; van der Poel, W.H. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS ONE 2011, 6, e22673. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, O.; Schimanski, S.; Koch, J.; Kohler, M.; Rothe, C.; Plentz, A.; Jilg, W.; Stark, K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J. Infect. Dis. 2008, 198, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Garbuglia, A.R.; Scognamiglio, P.; Petrosillo, N.; Mastroianni, C.M.; Sordillo, P.; Gentile, D.; La Scala, P.; Girardi, E.; Capobianchi, M.R. Hepatitis E virus genotype 4 outbreak, Italy, 2011. Emerg Infect. Dis. 2013, 19, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Bouamra, Y.; Gerolami, R.; Arzouni, J.P.; Grimaud, J.C.; Lafforgue, P.; Nelli, M.; Tivoli, N.; Ferretti, A.; Motte, A.; Colson, P. Emergence of autochthonous infections with hepatitis E virus of genotype 4 in Europe. Intervirology 2014, 57, 43–48. [Google Scholar] [CrossRef]
- Lee, G.H.; Tan, B.H.; Teo, E.C.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016, 150, 355–357.e3. [Google Scholar] [CrossRef]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hatchette, T.; Stueck, A.; Deschambault, Y.; Murnaghan, K.; Varga, J.; Johnston, L. Rat hepatitis E virus linked to severe acute hepatitis in an immunocompetent patient. J. Infect. Dis 2019, 220, 951–955. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.; Wu, S.; Chew, N.F.; Leung, K.H.; Chan, J.F.; Zhao, P.S.; Chan, W.M.; Poon, R.W.; Tsoi, H.W.; et al. Transmission of rat hepatitis E virus infection to humans in Hong Kong: A clinical and epidemiological analysis. Hepatology 2021, 73, 10–22. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Perez, A.B.; Pineda, J.A.; Reina, G.; Fuentes-Lopez, A.; Freyre-Carrillo, C.; Ramirez-Arellano, E.; Alados, J.C.; Rivero, A.; et al. Orthohepevirus C infection as an emerging cause of acute hepatitis in Spain: First report in Europe. J. Hepatol. 2022, in press. [Google Scholar] [CrossRef]
- Dalton, H.R.; Izopet, J. Transmission and epidemiology of hepatitis E virus genotype 3 and 4 infections. Cold Spring Harb. Perspect. Med. 2018, 8, a032144. [Google Scholar] [CrossRef] [PubMed]
- Treagus, S.; Wright, C.; Baker-Austin, C.; Longdon, B.; Lowther, J. The foodborne transmission of hepatitis E virus to humans. Food Environ. Virol. 2021, 13, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Pallerla, S.R.; Johne, R.; Todt, D.; Steinmann, E.; Schemmerer, M.; Wenzel, J.J.; Hofmann, J.; Shih, J.W.K.; Wedemeyer, H.; et al. Hepatitis E: An update on One Health and clinical medicine. Liver Int. 2021, 41, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Meng, X.J. Molecular biology and replication of hepatitis E virus. Emerg. Microbes Infect. 2012, 1, e17. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef]
- Vina-Rodriguez, A.; Schlosser, J.; Becher, D.; Kaden, V.; Groschup, M.H.; Eiden, M. Hepatitis E virus genotype 3 diversity: Phylogenetic analysis and presence of subtype 3b in wild boar in Europe. Viruses 2015, 7, 2704–2726. [Google Scholar] [CrossRef]
- Wang, B.; Meng, X.J. Hepatitis E virus: Host tropism and zoonotic infection. Curr. Opin. Microbiol. 2021, 59, 8–15. [Google Scholar] [CrossRef]
- Eiden, M.; Vina-Rodriguez, A.; Schlosser, J.; Schirrmeier, H.; Groschup, M.H. Detection of hepatitis E virus in archived rabbit serum samples, Germany 1989. Food Environ. Virol. 2016, 8, 105–107. [Google Scholar] [CrossRef]
- Hammerschmidt, F.; Schwaiger, K.; Dahnert, L.; Vina-Rodriguez, A.; Hoper, D.; Gareis, M.; Groschup, M.H.; Eiden, M. Hepatitis E virus in wild rabbits and European brown hares in Germany. Zoonoses Public Health 2017, 64, 612–622. [Google Scholar] [CrossRef]
- Ryll, R.; Eiden, M.; Heuser, E.; Weinhardt, M.; Ziege, M.; Hoper, D.; Groschup, M.H.; Heckel, G.; Johne, R.; Ulrich, R.G. Hepatitis E virus in feral rabbits along a rural-urban transect in Central Germany. Infect. Genet. Evol 2018, 61, 155–159. [Google Scholar] [CrossRef]
- Abravanel, F.; Lhomme, S.; El Costa, H.; Schvartz, B.; Peron, J.M.; Kamar, N.; Izopet, J. Rabbit hepatitis E virus infections in humans, France. Emerg. Infect. Dis. 2017, 23, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Hackl, S.S.; Piepenschneider, M.; Vina-Rodriguez, A.; Dremsek, P.; Ulrich, R.G.; Groschup, M.H.; Eiden, M. Serologic and molecular survey of hepatitis E virus in German deer populations. J. Wildl Dis 2016, 52, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ninove, L.; Nougairede, A.; Gazin, C.; Thirion, L.; Delogu, I.; Zandotti, C.; Charrel, R.N.; De Lamballerie, X. RNA and DNA bacteriophages as molecular diagnosis controls in clinical virology: A comprehensive study of more than 45,000 routine PCR tests. PLoS ONE 2011, 6, e16142. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.J.; Norder, H.; Okamoto, H.; van der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Moshiri, N. ViralMSA: Massively scalable reference-guided multiple sequence alignment of viral genomes. Bioinformatics 2021, 37, 714–716. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Ugene Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation, R package version 1.0.7.; Available online: https://CRAN.R-project.org/package=dplyr (accessed on 30 May 2022). [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.Y.; McInerny, G. ggtree: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2016, 8, 28–36. [Google Scholar] [CrossRef]
- Yu, G.; Lam, T.T.; Zhu, H.; Guan, Y. Two methods for mapping and visualizing associated data on phylogeny using ggtree. Mol. Biol. Evol. 2018, 35, 3041–3043. [Google Scholar] [CrossRef] [PubMed]
- Yu, G. Using ggtree to visualize data on tree-like structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Lam, T.T.; Xu, S.; Dai, Z.; Zhou, L.; Feng, T.; Guo, P.; Dunn, C.W.; Jones, B.R.; Bradley, T.; et al. Treeio: An R package for phylogenetic tree input and output with richly annotated and associated data. Mol. Biol. Evol. 2020, 37, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Nicot, F.; Dimeglio, C.; Migueres, M.; Jeanne, N.; Latour, J.; Abravanel, F.; Ranger, N.; Harter, A.; Dubois, M.; Lameiras, S.; et al. Classification of the zoonotic hepatitis E virus genotype 3 into distinct subgenotypes. Front. Microbiol. 2021, 11, 634430. [Google Scholar] [CrossRef]
- Dremsek, P.; Joel, S.; Baechlein, C.; Pavio, N.; Schielke, A.; Ziller, M.; Durrwald, R.; Renner, C.; Groschup, M.H.; Johne, R.; et al. Hepatitis E virus seroprevalence of domestic pigs in Germany determined by a novel in-house and two reference ELISAs. J. Virol. Methods 2013, 190, 11–16. [Google Scholar] [CrossRef]
- Baechlein, C.; Schielke, A.; Johne, R.; Ulrich, R.G.; Baumgaertner, W.; Grummer, B. Prevalence of hepatitis E virus-specific antibodies in sera of German domestic pigs estimated by using different assays. Vet. Microbiol. 2010, 144, 187–191. [Google Scholar] [CrossRef][Green Version]
- Krumbholz, A.; Joel, S.; Neubert, A.; Dremsek, P.; Durrwald, R.; Johne, R.; Hlinak, A.; Walther, M.; Lange, J.; Wutzler, P.; et al. Age-related and regional differences in the prevalence of hepatitis E virus-specific antibodies in pigs in Germany. Vet. Microbiol. 2013, 167, 394–402. [Google Scholar] [CrossRef]
- Denzin, N.; Borgwardt, J. Occurrence and geographical distribution of antibodies to hepatitis E virus in wild boars of Saxony-Anhalt, Germany (2011). Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 230–235. [Google Scholar]
- Schielke, A.; Ibrahim, V.; Czogiel, I.; Faber, M.; Schrader, C.; Dremsek, P.; Ulrich, R.G.; Johne, R. Hepatitis E virus antibody prevalence in hunters from a district in Central Germany, 2013: A cross-sectional study providing evidence for the benefit of protective gloves during disembowelling of wild boars. BMC Infect. Dis. 2015, 15, 440. [Google Scholar] [CrossRef]
- De Oya, N.J.; de Blas, I.; Blazquez, A.B.; Martin-Acebes, M.A.; Halaihel, N.; Girones, O.; Saiz, J.C.; Escribano-Romero, E. Widespread distribution of hepatitis E virus in Spanish pig herds. BMC Res. Notes 2011, 4, 412. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Angeloni, G.; Salata, C.; Vonesch, N.; D’Amico, W.; Campagna, G.; Natale, A.; Zuliani, F.; Ceglie, L.; Monne, I.; et al. Hepatitis E virus infection in North Italy: High seroprevalence in swine herds and increased risk for swine workers. Epidemiol. Infect. 2017, 145, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Feurer, C.; Le Roux, A.; Rossel, R.; Barnaud, E.; Dumarest, M.; Garry, P.; Pavio, N. High load of hepatitis E viral RNA in pork livers but absence in pork muscle at French slaughterhouses. Int. J. Food Microbiol. 2018, 264, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; Bouwknegt, M.; van der Giessen, J.W.; de Roda Husman, A.M.; Reusken, C.B. Seroprevalence of hepatitis E virus in pigs from different farming systems in The Netherlands. J. Food Prot. 2014, 77, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Grierson, S.; Heaney, J.; Cheney, T.; Morgan, D.; Wyllie, S.; Powell, L.; Smith, D.; Ijaz, S.; Steinbach, F.; Choudhury, B.; et al. Prevalence of hepatitis E virus infection in pigs at the time of slaughter, United Kingdom, 2013. Emerg. Infect. Dis. 2015, 21, 1396–1401. [Google Scholar] [CrossRef]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Kubacki, J.; Fraefel, C.; Jermini, M.; Giannini, P.; Martinetti, G.; Ripellino, P.; Bernasconi, E.; Sidler, X.; Stephan, R.; Bachofen, C. Complete genome sequences of two swiss hepatitis E virus isolates from human stool and raw pork sausage. Genome Announc. 2017, 5, e00888-17. [Google Scholar] [CrossRef]
- Berto, A.; Grierson, S.; Hakze-van der Honing, R.; Martelli, F.; Johne, R.; Reetz, J.; Ulrich, R.G.; Pavio, N.; Van der Poel, W.H.; Banks, M. Hepatitis E virus in pork liver sausage, France. Emerg. Infect. Dis. 2013, 19, 264–266. [Google Scholar] [CrossRef]
- Faber, M.; Askar, M.; Stark, K. Case-control study on risk factors for acute hepatitis E in Germany, 2012 to 2014. Eurosurveillance 2018, 23, 17-00469. [Google Scholar] [CrossRef] [PubMed]
- Hriskova, K.; Marosevic, D.; Belting, A.; Wenzel, J.J.; Carl, A.; Katz, K. Epidemiology of hepatitis E in 2017 in Bavaria, Germany. Food Environ. Virol. 2021, 13, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Wolf, A.; Meisel, H.; Kaiser, M.; Ellerbrok, H.; Pauli, G. High HEV presence in four different wild boar populations in East and West Germany. Vet. Microbiol. 2009, 139, 270–278. [Google Scholar] [CrossRef]
- Baechlein, C.; Seehusen, F.; Nathues, H.; Baumgartner, W.; Grummer, B. Molecular detection of hepatitis E virus in German domestic pigs. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Trojnar, E.; Anheyer-Behmenburg, H.; Binder, A.; Schotte, U.; Ellerbroek, L.; Klein, G.; Johne, R. Detection of hepatitis E virus RNA in raw sausages and liver sausages from retail in Germany using an optimized method. Int. J. Food Microbiol. 2015, 215, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Inoue, J.; Tsuruoka, M.; Nishizawa, T.; Nagashima, S.; Takahashi, M.; Shimosegawa, T.; Okamoto, H. Full-length genomic sequence analysis of new subtype 3k hepatitis E virus isolates with 99.97% nucleotide identity obtained from two consecutive acute hepatitis patients in a city in northeast Japan. J. Med. Virol. 2017, 89, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Dähnert, L.; Eiden, M.; Schlosser, J.; Fast, C.; Schröder, C.; Lange, E.; Gröner, A.; Schäfer, W.; Groschup, M.H. High sensitivity of domestic pigs to intravenous infection with HEV. BMC Vet. Res. 2018, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Eiden, M.; Vina-Rodriguez, A.; Fast, C.; Dremsek, P.; Lange, E.; Ulrich, R.G.; Groschup, M.H. Natural and experimental hepatitis E virus genotype 3-infection in European wild boar is transmissible to domestic pigs. Vet. Res. 2014, 45, 121. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.; Hernandez, M.; Gutierrez-Boada, M.; Valero, A.; Navarro, A.; Munoz-Chimeno, M.; Fernandez-Manzano, A.; Escobar, F.M.; Martinez, I.; Barcena, C.; et al. Occurrence of hepatitis E virus in pigs and pork cuts and organs at the time of slaughter, Spain, 2017. Front. Microbiol. 2020, 10, 2990. [Google Scholar] [CrossRef]
- Di Bartolo, I.; Diez-Valcarce, M.; Vasickova, P.; Kralik, P.; Hernandez, M.; Angeloni, G.; Ostanello, F.; Bouwknegt, M.; Rodriguez-Lazaro, D.; Pavlik, I.; et al. Hepatitis E virus in pork production chain in Czech Republic, Italy, and Spain, 2010. Emerg. Infect. Dis. 2012, 18, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Chelli, E.; Suffredini, E.; De Santis, P.; De Medici, D.; Di Bella, S.; D’Amato, S.; Gucciardi, F.; Guercio, A.; Ostanello, F.; Perrone, V.; et al. Hepatitis E virus occurrence in pigs slaughtered in Italy. Animals 2021, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Gomez, J.; Garcia Bocanegra, I.; Gomez-Guillamon, F.; Camacho-Sillero, L.; Zorrilla, I.; Lopez-Lopez, P.; Cano-Terriza, D.; Jimenez-Ruiz, S.; Frias, M.; Rivero-Juarez, A. Absence of hepatitis E virus circulation in wild rabbits (Oryctolagus cuniculus) and Iberian hares (Lepus granatensis) in Mediterranean ecosystems in Spain. Transbound. Emerg. Dis. 2020, 67, 1422–1427. [Google Scholar] [CrossRef]
- Leblanc, D.; Poitras, E.; Gagne, M.J.; Ward, P.; Houde, A. Hepatitis E virus load in swine organs and tissues at slaughterhouse determined by real-time RT-PCR. Int. J. Food Microbiol. 2010, 139, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Krog, J.S.; Larsen, L.E.; Breum, S.O. Tracing hepatitis E virus in pigs from birth to slaughter. Front. Vet. Sci. 2019, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Anheyer-Behmenburg, H.E.; Szabo, K.; Schotte, U.; Binder, A.; Klein, G.; Johne, R. Hepatitis E virus in wild boars and spillover infection in red and roe deer, Germany, 2013–2015. Emerg. Infect. Dis. 2017, 23, 130–133. [Google Scholar] [CrossRef]
- De Sabato, L.; Amoroso, M.G.; Ianiro, G.; Esposito, C.; De Grossi, L.; Fusco, G.; Barone, A.; Martini, E.; Ostanello, F.; Di Bartolo, I. Detection of hepatitis E virus in livers and muscle tissues of wild boars in Italy. Food Environ. Virol. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Berto, A.; Martelli, F.; Grierson, S.; Banks, M. Hepatitis E virus in pork food chain, United Kingdom, 2009–2010. Emerg. Infect. Dis. 2012, 18, 1358–1360. [Google Scholar] [CrossRef] [PubMed]
- Forzan, M.; Pacini, M.I.; Periccioli, M.; Mazzei, M. Hepatitis E virus RNA presence in wild boar carcasses at slaughterhouses in Italy. Animals 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Dimeglio, C.; Castanier, M.; Peron, J.M.; Kamar, N.; Lhomme, S.; Izopet, J. Does HEV-3 subtype play a role in the severity of acute hepatitis E? Liver Int. 2020, 40, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Subissi, L.; Peeters, M.; Lamoral, S.; Klamer, S.; Suin, V.; Van Gucht, S. Subtype-specific differences in the risk of hospitalisation among patients infected with hepatitis E virus genotype 3 in Belgium, 2010–2018. Epidemiol. Infect. 2019, 147, e224. [Google Scholar] [CrossRef] [PubMed]
- Garbuglia, A.R.; Bruni, R.; Villano, U.; Vairo, F.; Lapa, D.; Madonna, E.; Picchi, G.; Binda, B.; Mariani, R.; De Paulis, F.; et al. Hepatitis E outbreak in the central part of Italy sustained by multiple HEV genotype 3 strains, June–December 2019. Viruses 2021, 13, 1159. [Google Scholar] [CrossRef] [PubMed]

| No. | Sample ID | Species | Tissue (ct-Value) | Genotype | Subtype | Accession | ||
|---|---|---|---|---|---|---|---|---|
| Liver | Faeces | Muscle | Number | |||||
| 1 | MWP2019-9 | wb | 32.7 | n.a. | neg | HEV-3 | 3i-like | ON240936 |
| 2 | MWP2019-14 | pig | 21.3 | n.a. | neg | HEV-3 | 3c | ON240935 |
| 3 | MWP2019-22 | wb | 22.86 | n.a. | neg | HEV-3 | 3i-like | ON240934 |
| 4 | MWP2019-23 | pig | 26.52 | n.a. | neg | HEV-3 | 3f | ON240933 |
| 5 | MWP2019-24 | pig | 24.14 | n.a. | neg | HEV-3 | 3f | ON240932 |
| 6 | MWP2019-33 | pig | 32.36 | n.a. | neg | no sequence | - | - |
| 7 | MWP2019-35 | pig | 19.23 | n.a. | neg | HEV-3 | 3c | ON240931 |
| 8 | MWP2019-53 | pig | 27 | n.a. | neg | HEV-3 | 3f | ON240930 |
| 9 | MWP2019-97 | pig | 26.2 | n.a. | neg | HEV-3 | 3a | ON240929 |
| 10 | MWP2019-100 | pig | 22.2 | n.a. | neg | HEV-3 | 3a | ON240928 |
| 11 | MWP2019-104 | pig | 33.1 | n.a. | neg | HEV-3 | 3a | ON240927 |
| 12 | MWP2019-113 | pig | 32.8 | n.a. | neg | HEV-3 | 3f | ON240926 |
| 13 | MWP2019-117 | wb | 28.1 | n.a. | neg | HEV-3 | 3c | ON240925 |
| 14 | MWP2019-170 | pig | 23.1 | n.a. | 20.7 | HEV-3 | 3f | ON240924 |
| 15 | MWP2019-190 | pig | 27.4 | n.a. | neg | HEV-3 | 3c | ON240923 |
| 16 | MWP2019-208 | pig | 23.5 | n.a. | neg | HEV-3 | 3f | ON240922 |
| 17 | MWP2019-209 | pig | 32.4 | n.a. | neg | HEV-3 | 3f | ON240921 |
| 18 | MWP2019-242 | pig | 30.4 | 29.19 | neg | HEV-3 | 3f | ON240949 |
| 19 | MWP2019-255 | pig | 31.34 | 38.20 | neg | HEV-3 | 3k | ON240948 |
| 20 | MWP2019-256 | pig | 34.62 | 34.19 | neg | HEV-3 | 3k | ON240947 |
| 21 | MWP2019-257 | pig | 36.65 | 40.71 | neg | HEV-3 | 3k | ON240946 |
| 22 | MWP2019-276 | pig | 21.26 | 20.26 | neg | HEV-3 | 3e | ON240945 |
| 23 | MWP2019-277 | pig | n.a. | 34.68 | neg | no sequence | - | - |
| 24 | MWP2019-288 | pig | 22.86 | 24.56 | neg | HEV-3 | 3e | ON240943 |
| 25 | MWP2019-292 | wb | 23.95 | 27 | neg | HEV-3 | 3i-like | ON240942 |
| 26 | MWP2019-314 | pig | 22.8 | 22.69 | neg | HEV-3 | 3c | ON240950 |
| 27 | MWP2019-315 | pig | 22.1 | 22.39 | neg | HEV-3 | 3i-like | ON240941 |
| 28 | MWP2019-316 | pig | 23.29 | 21.19 | neg | HEV-3 | 3c | ON240940 |
| 29 | MWP2019-317 | pig | 20.18 | 22.1 | neg | HEV-3 | 3c | ON240939 |
| 30 | MWP2019-354 | pig | 23.56 | 24.19 | neg | HEV-3 | 3a | ON240938 |
| 31 | MWP2019-366 | pig | 28.05 | n.a | neg | HEV-3 | 3k | ON240937 |
| 32 | MWP2019-385 | wb | n.a. | 23.51 | neg | HEV-3 | 3c | ON240944 |
| 33 | MWP2019-386 | wb | 16.3 | n.a. | neg | HEV-3 | 3c | ON240944 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priemer, G.; Cierniak, F.; Wolf, C.; Ulrich, R.G.; Groschup, M.H.; Eiden, M. Co-Circulation of Different Hepatitis E Virus Genotype 3 Subtypes in Pigs and Wild Boar in North-East Germany, 2019. Pathogens 2022, 11, 773. https://doi.org/10.3390/pathogens11070773
Priemer G, Cierniak F, Wolf C, Ulrich RG, Groschup MH, Eiden M. Co-Circulation of Different Hepatitis E Virus Genotype 3 Subtypes in Pigs and Wild Boar in North-East Germany, 2019. Pathogens. 2022; 11(7):773. https://doi.org/10.3390/pathogens11070773
Chicago/Turabian StylePriemer, Grit, Filip Cierniak, Carola Wolf, Rainer G. Ulrich, Martin H. Groschup, and Martin Eiden. 2022. "Co-Circulation of Different Hepatitis E Virus Genotype 3 Subtypes in Pigs and Wild Boar in North-East Germany, 2019" Pathogens 11, no. 7: 773. https://doi.org/10.3390/pathogens11070773
APA StylePriemer, G., Cierniak, F., Wolf, C., Ulrich, R. G., Groschup, M. H., & Eiden, M. (2022). Co-Circulation of Different Hepatitis E Virus Genotype 3 Subtypes in Pigs and Wild Boar in North-East Germany, 2019. Pathogens, 11(7), 773. https://doi.org/10.3390/pathogens11070773






