In Vitro Analysis of Extracts of Plant Used in Mexican Traditional Medicine, Which Are Useful to Combat Clostridioides difficile Infection
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis
2.2. Antibacterial Activity Assay and Minimal Inhibitory Concentration (MIC) Determination
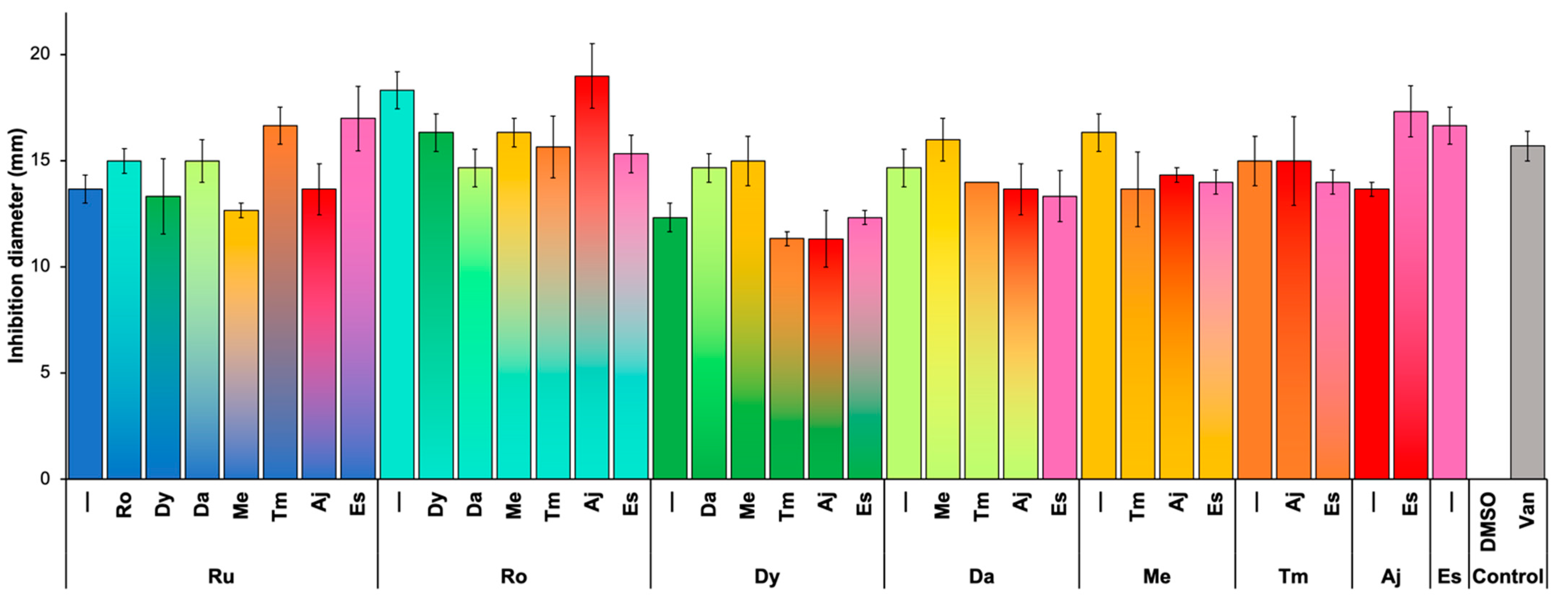
2.3. Synergism between Plant Extracts
2.4. Action Mechanism of Plant Extracts
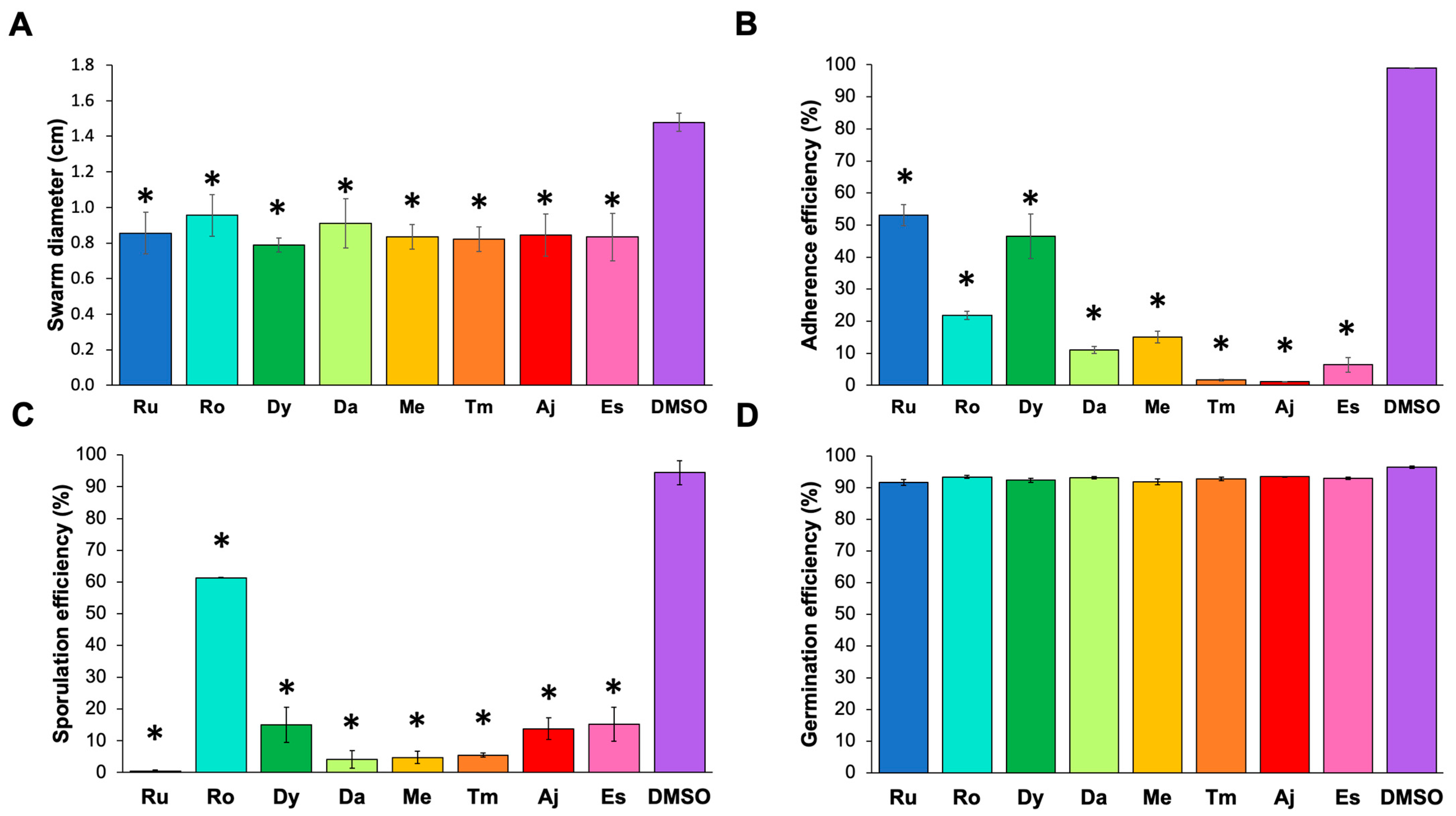
2.5. Effect of the Extracts on Virulence Factors
3. Discussion
4. Materials and Methods
4.1. Plant Extracts
4.2. Bacterial Strains
4.3. Antibacterial Activity
4.4. Phytochemical Analysis of the Extracts
4.5. Evaluation of the Synergistic Effects among the Extracts
4.6. Action Mechanism of the Extracts
4.7. Motility Assays
4.8. Adherence Assay
4.9. Sporulation Assays
4.10. Germination Assays
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global Antibiotic Consumption 2000 to 2010: An Analysis of National Pharmaceutical Sales Data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Petrosillo, N.; Granata, G.; Cataldo, M.A. Novel Antimicrobials for the Treatment of Clostridium difficile Infection. Front. Med. 2018, 5, 96. [Google Scholar]
- Palma-Tenango, M.; Miguel-Chávez, R.S.; Soto-Hernández, R.M. Aromatic and Medicinal Plants in Mexico. In Aromatic and Medicinal Plants—Back to Nature; El-Shemy, H.A., Ed.; InTechOpen: London, UK, 2017. [Google Scholar]
- Muñetón Pérez, P. Plantas medicinales: Un complemento vital para la salud de los mexicanos. Entrevista con el Mtro. Erick Estrada Lugo. Rev. Digit. Univ. 2009, 10, 9. [Google Scholar]
- Sharma, A.; del Flores-Vallejo, R.C.; Cardoso-Taketa, A.; Villarreal, M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017, 208, 264–329. [Google Scholar] [CrossRef]
- Lucía, C.P.A.; Jacqueline, B.R.; Alberto, B.R.L.; David, B.A.; Beatriz, R.A. Actualized inventory of medicinal plants used in traditional medicine in Oaxaca, Mexico. J. Ethnobiol. Ethnomed. 2021, 17, 7. [Google Scholar]
- Roshan, N.; Riley, T.V.; Knight, D.R.; Hammer, K.A. Effect of natural products on the production and activity of Clostridium difficile toxins in vitro. Sci. Rep. 2018, 8, 15735. [Google Scholar] [CrossRef] [Green Version]
- Roshan, N.; Riley, T.; Knight, D.; Steer, J.; Hammer, K. Natural products show diverse mechanisms of action against Clostridium difficile. J. Appl. Microbiol. 2019, 126, 468–479. [Google Scholar] [CrossRef]
- Roshan, N.; Riley, T.; Hammer, K. Antimicrobial activity of natural products against Clostridium difficile in vitro. J. Appl. Microbiol. 2017, 123, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Roshan, N.; Riley, T.V.; Hammer, K.A. Effects of natural products on several stages of the spore cycle of Clostridium difficile in vitro. J. Appl. Microbiol. 2018, 125, 710–723. [Google Scholar] [CrossRef]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [Green Version]
- Atmani, A.; Sekhri, L.; Khaled, B. A Comparative Study of the Antibacterial Activity of Juncus Maritumus Asch & Buschen; Its Synergic Effect with Some Standard Antibiotics and Some Other Medicinal Plants. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 6–13. [Google Scholar]
- Dzotam, J.K.; Kuete, V. Antibacterial and Antibiotic-Modifying Activity of Methanol Extracts from Six Cameroonian Food Plants against Multidrug-Resistant Enteric Bacteria. BioMed Res. Int. 2017, 2017, 1583510. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Hu, Y.-Y.; Chen, W.-X. Antibacterial mechanism and activities of black pepper chloroform extract. J. Food Sci. Technol. 2015, 52, 8196–8203. [Google Scholar] [CrossRef] [Green Version]
- Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther. Adv. Infect. Dis. 2015, 3, 23–42. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhang, Y.; Zhao, Q. New Components of the Lignin Biosynthetic Metabolon. Trends Plant Sci. 2018, 23, 557–559. [Google Scholar] [CrossRef]
- Ahmed, S.; Griffin, T.S.; Kraner, D.; Schaffner, M.K.; Sharma, D.; Hazel, M.; Leitch, A.R.; Orians, C.M.; Han, W.; Stepp, J.R.; et al. Environmental Factors Variably Impact Tea Secondary Metabolites in the Context of Climate Change. Front. Plant Sci. 2019, 10, 939. [Google Scholar] [CrossRef] [Green Version]
- Jakabová, S.; Vincze, L.; Farkas, Á.; Kilár, F.; Boros, B.; Felinger, A. Determination of tropane alkaloids atropine and scopolamine by liquid chromatography–mass spectrometry in plant organs of Datura species. J. Chromatogr. A 2012, 1232, 295–301. [Google Scholar] [CrossRef]
- Singh, L.; Singh, O. Datura stramonium:: An overview of its phytochemistry and pharmacognosy. Res. J. Pharmacogn. Phytochem. 2013, 5, 143–148. [Google Scholar]
- Eftekhar, F.; Yousefzadi, M.; Tafakori, V. Antimicrobial activity of Datura innoxia and Datura stramonium. Fitoterapia 2005, 76, 118–120. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.; Wang, W.; Bai, F.; Zhu, J.; Li, J.; Li, X.; Xu, Y.; Sun, T.; He, Y. Antimicrobial effect and membrane-active mechanism of tea polyphenols against Serratia marcescens. World J. Microbiol. Biotechnol. 2014, 30, 451–460. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Hong, J.; Yoon, Y.; Choi, K.-H. Evaluation on Antimicrobial Activity of Psoraleae semen Extract Controlling the Growth of Gram-Positive Bacteria. Korean J. Food Sci. Anim. Resour. 2017, 37, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Pinto, N.C.; Silva, J.B.; Menegati, L.M.; Guedes, M.C.M.; Marques, L.B.; Da Silva, T.P.; Melo, R.; De Souza-Fagundes, E.M.; Salvador, M.; Scio, E.; et al. Cytotoxicity and bacterial membrane destabilization induced by Annona squamosa L. extracts. An. Acad. Bras. Ciênc. 2017, 89, 2053–2073. [Google Scholar] [CrossRef]
- Sukumar, M.R.; König, B. Pomegranate extract specifically inhibits Clostridium difficile growth and toxin production without disturbing the beneficial bacteria in vitro. Infect. Drug Resist. 2018, 11, 2357–2362. [Google Scholar] [CrossRef] [Green Version]
- Arullappan, S.; Zakaria, Z.; Basri, D.F. Preliminary screening of antibacterial activity using crude extracts of Hibiscus rosa sinensis. Trop. Life Sci. Res. 2009, 20, 109–118. [Google Scholar]
- Savithramma, N.; Linga Rao, M.; Suhrulatha, D. Screening of Selected Medicinal Plants for Secondary Metabolites. Middle East J. Sci. Res. 2011, 8, 579–584. [Google Scholar]
- Ahmad, I.; Rissyelly, R.; Kurniawan, A.; Munim, A. Screening of Extraction Method for Alkaloid Enrichment of Peperomia pellucida (L.) Kunth. Asian J. Pharm. Clin. Res. 2017, 10, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Camorlinga, M.; Sanchez-Rojas, M.; Torres, J.; Romo-Castillo, M. Phenotypic characterization of non-toxigenic Clostridioides difficile strains isolated from patients in Mexico. Front. Microbiol. 2019, 10, 84. [Google Scholar] [CrossRef]

| Plant Extract | Common Name | Parts Used | Yield (%) | Extract Concentration (mg/mL) | MIC (mg/mL) | Phytochemical Analysis |
|---|---|---|---|---|---|---|
| Ruta graveolens (Ru) | Rue | Aerial | 8.3 | 5.01 | 0.5 | Alkaloid, sterol, Terpenoid, and polyphenols |
| Rosmarinus officinalis (Ro) | Rosemary | Leaves | 10.4 | 13 | 0.13 | Alkaloid, sterol, and polyphenols |
| Dysphania ambrosioides (Dy) | Epazote, Mexican Tea, Jesuit’s tea | Leaves | 12.3 | 39.5 | 3.9 | Alkaloid and terpenoid |
| Datura ferox (Da) | Toloache, Long Spined thorn apple, Angel’s trumpets | Leaves | 10.7 | 17.3 | 0.85 | Alkaloid, Terpenoid, and polyphenol |
| Mentha piperita (Me) | Mint | Leaves | 7.8 | 9.3 | 0.46 | Polyphenol |
| Thymus vulgaris (Tm) | Thyme | Leaves | 8.2 | 9.8 | 0.49 | Alkaloid, Terpenoid, and polyphenol |
| Artemisia absinthium (Aj) | Ajenjo, wormwood | Aerial | 3 | 4.8 | 0.48 | Sterol and Terpenoid |
| Artemisia ludoviciana (Es) | Estafiate, Silver, Louisiana wormwood | Leaves | 8.1 | 9.7 | 0.48 | Alkaloid and polyphenol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Alva, J.E.; Espinoza-Simón, E.; Bayona-Pérez, Y.; Ruiz-Pérez, N.C.; Ochoa, S.A.; Xicohtencatl-Cortes, J.; Torres, J.; Romo-Castillo, M. In Vitro Analysis of Extracts of Plant Used in Mexican Traditional Medicine, Which Are Useful to Combat Clostridioides difficile Infection. Pathogens 2022, 11, 774. https://doi.org/10.3390/pathogens11070774
Martínez-Alva JE, Espinoza-Simón E, Bayona-Pérez Y, Ruiz-Pérez NC, Ochoa SA, Xicohtencatl-Cortes J, Torres J, Romo-Castillo M. In Vitro Analysis of Extracts of Plant Used in Mexican Traditional Medicine, Which Are Useful to Combat Clostridioides difficile Infection. Pathogens. 2022; 11(7):774. https://doi.org/10.3390/pathogens11070774
Chicago/Turabian StyleMartínez-Alva, Jacqueline E., Emilio Espinoza-Simón, Yuli Bayona-Pérez, Nancy C. Ruiz-Pérez, Sara A. Ochoa, Juan Xicohtencatl-Cortes, Javier Torres, and Mariana Romo-Castillo. 2022. "In Vitro Analysis of Extracts of Plant Used in Mexican Traditional Medicine, Which Are Useful to Combat Clostridioides difficile Infection" Pathogens 11, no. 7: 774. https://doi.org/10.3390/pathogens11070774






