Evaluation of a Most Probable Number Method for Detection and Quantification of Legionella pneumophila
Abstract
:1. Introduction
2. Results
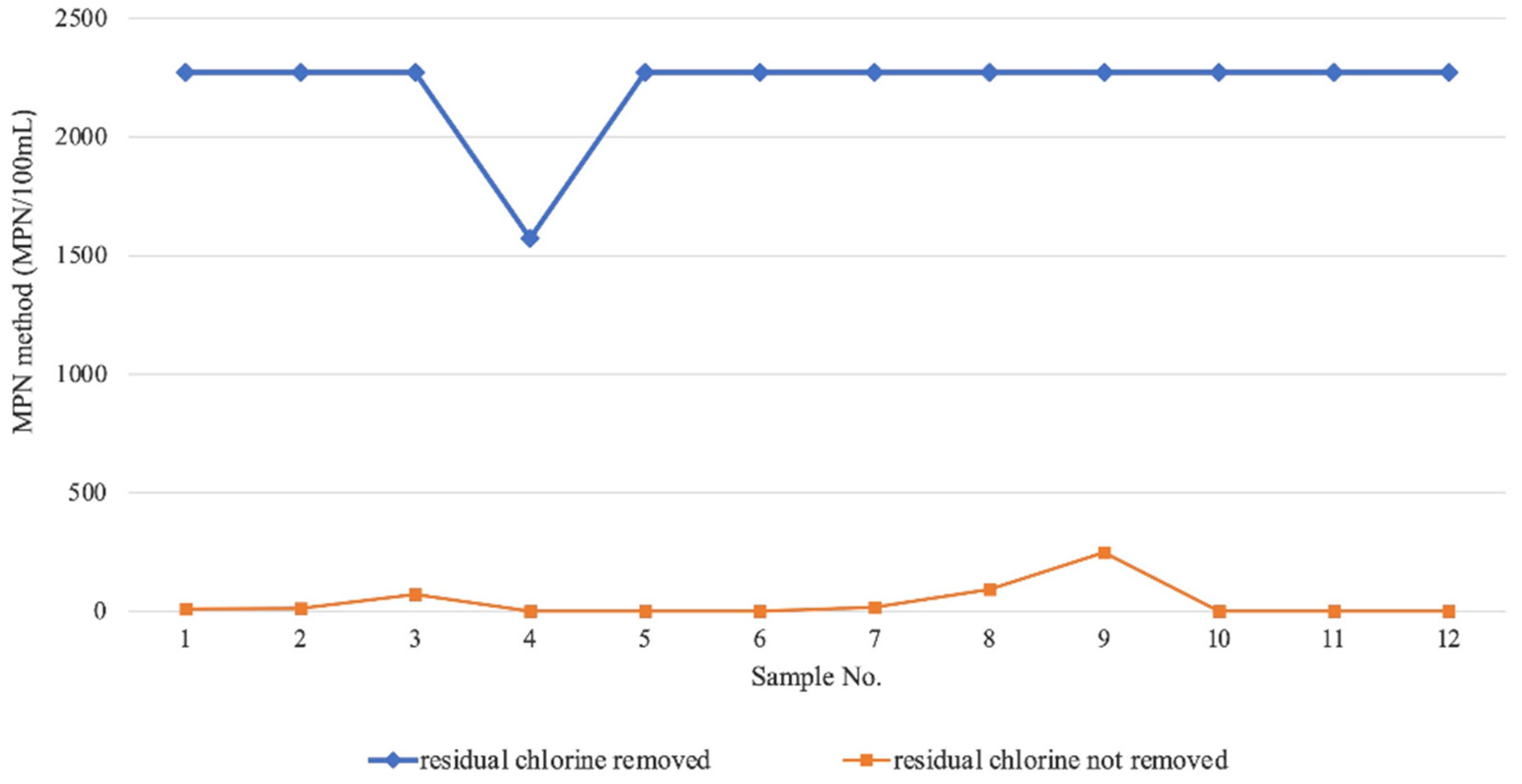
2.1. Analysis of Influencing Factors of Sample Preservation in the MPN Method
2.2. Comparison with Current Detection Methods
2.2.1. Comparison of Qualitative Test Results with GB/T 182014.5-2013
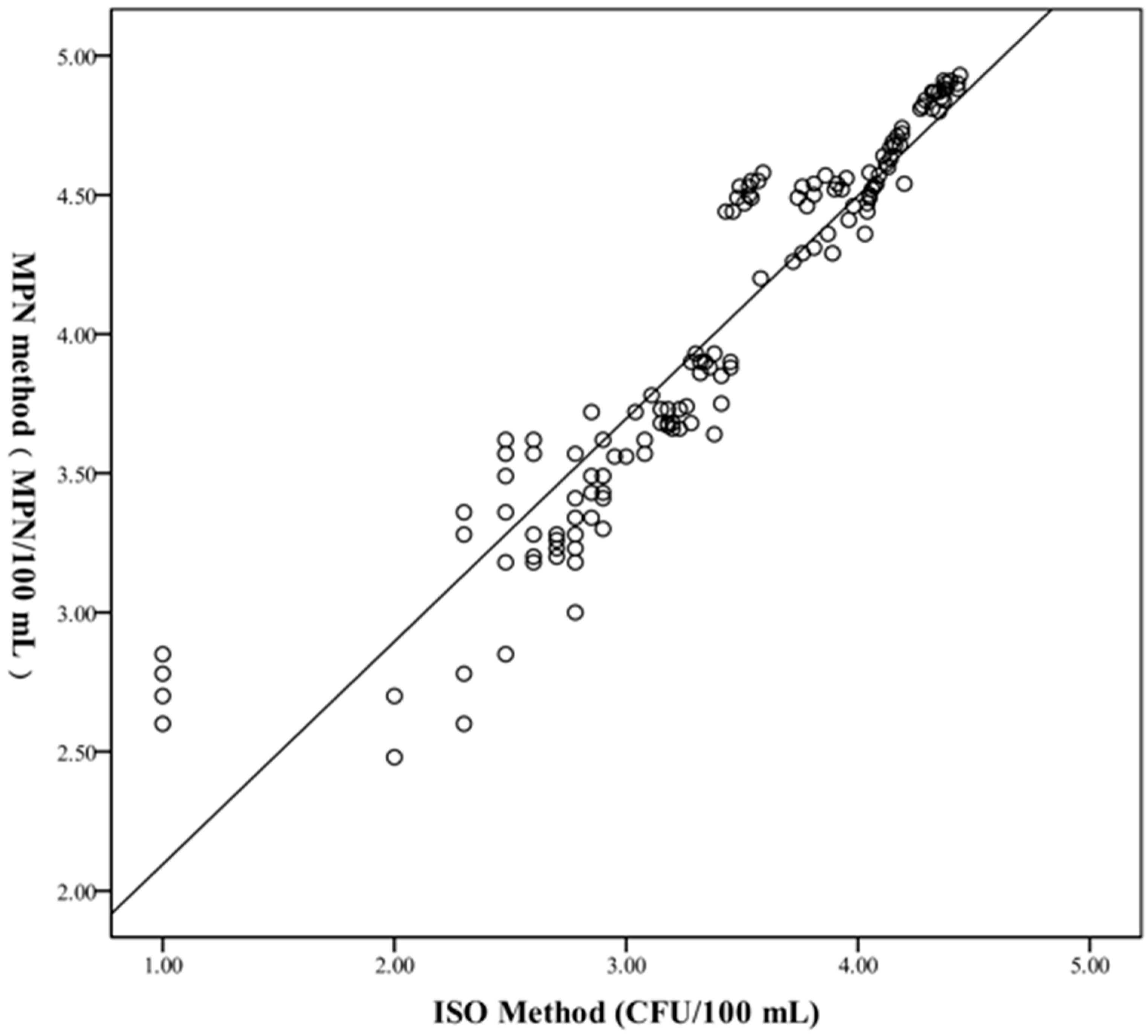
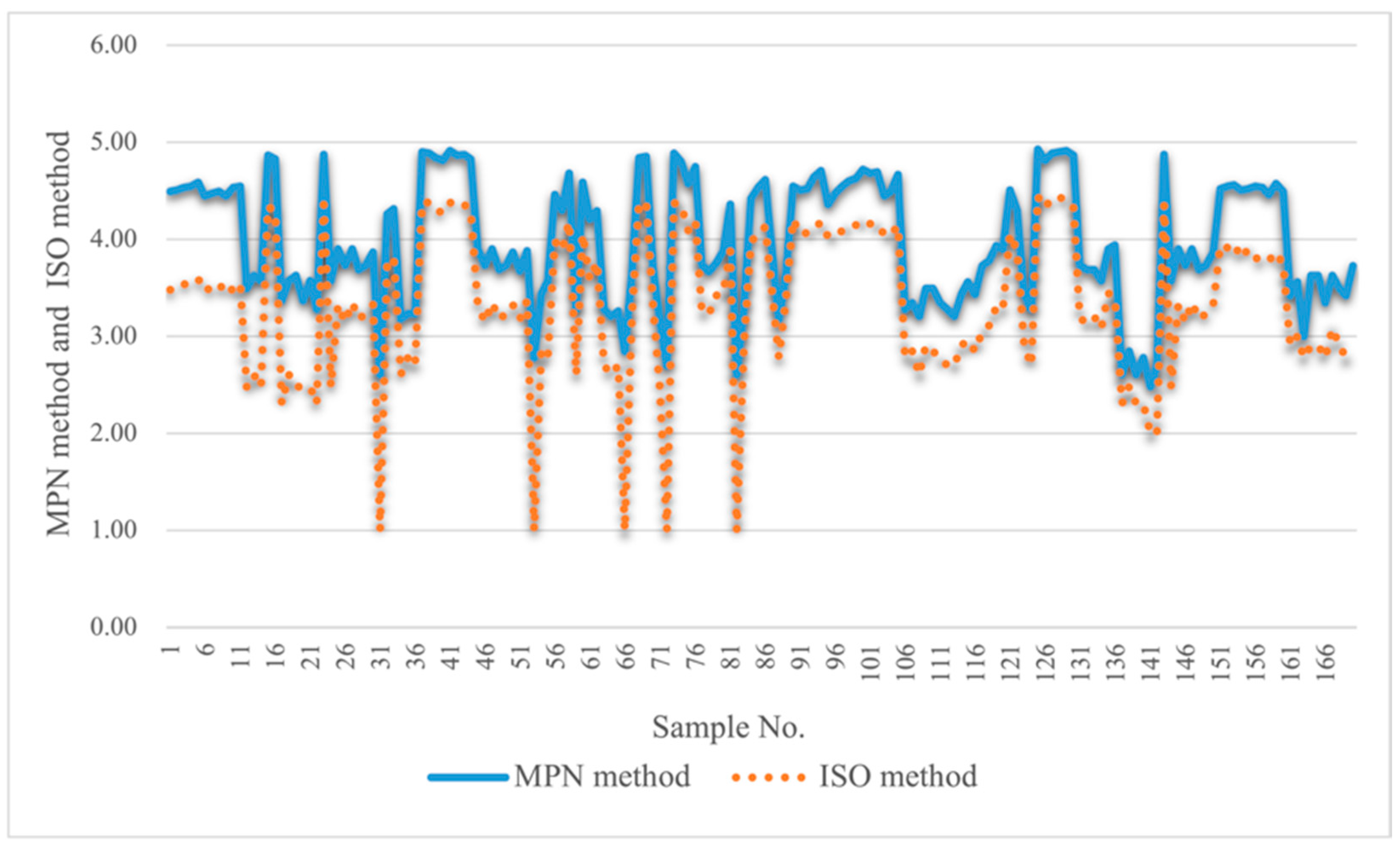
2.2.2. Comparison of Quantitative Test Results with ISO 11731:2017
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. MPN Method
4.3. Confirmatory Test
4.4. ISO 11731:2017
4.5. GB/T 18204.5-2013
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hines, S.A.; Chappie, D.J.; Lordo, R.A.; Miller, B.D.; Janke, R.J.; Lindquist, H.A.; Fox, K.R.; Ernst, H.S.; Taft, S.C. Assessment of relative potential for Legionella species or surrogates inhalation exposure from common water uses. Water Res. 2014, 56, 203–213. [Google Scholar] [CrossRef]
- Hayes-Phillips, D.; Bentham, R.; Ross, K.; Whiley, H. Factors influencing Legionella contamination of domestic household showers. Pathogens 2019, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.Z.; Liao, H.Y.; Luo, L.Z.; Sen, H.S.; Qin, T.; Zhou, H.J.; Li, H.X.; Da Li, C.H.; Chen, J.P. An investigation on the molecular characteristics and intracellular growth ability among environmental and clinical isolates of legionella pneumophila in sichuan province, China. Biomed. Environ. Sci. 2019, 32, 520–530. [Google Scholar] [CrossRef]
- Leoni, E.; Catalani, F.; Marini, S.; Dallolio, L. Legionellosis Associated with Recreational Waters: A Systematic Review of Cases and Outbreaks in Swimming Pools, Spa Pools, and Similar Environments. Int. J. Environ. Res. Public Health 2018, 15, 1612. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Y.; Li, Y.; Wang, X.; Shangguan, Z.H.; Zhou, H.J.; Wu, Y.; Wang, L.; Ren, H.; Hu, Y.; Lin, M.; et al. High Prevalence and Genetic Polymorphisms of Legionella in Natural and Man-Made Aquatic Environments in Wenzhou, China. Int. J. Environ. Res. Public Health 2017, 14, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Gavalda, L.; Garcia-Nunez, M.; Quero, S.; Gutierrez-Milla, C.; Sabria, M. Role of hot water temperature and water system use on Legionella control in a tertiary hospital: An 8-year longitudinal study. Water Res. 2019, 149, 460–466. [Google Scholar] [CrossRef]
- Xiao, G.W.; Chen, G.M.; Xiao, Y.X.; Chen, S.J.; Xie, W.Q.; Ding, J.M. Contamination status of Legionella in partial public places of Meizhou city. J. Trop. Med. 2020, 20, 1637–1640. (In Chinese) [Google Scholar]
- Jiang, L.X.; Zhao, S.H.; Cai, X.; Mu, D.G.; Zhang, X.; Kang, J.; Zhao, L.; Chen, Y. Sequence-based typing of clinical and environmental Legionella pneumophila isolates in Shenyang, China. Enferm. Infecc. Microbiol. Clin. 2020, 39, 383–389. [Google Scholar] [CrossRef]
- Thornley, C.N.; Harte, D.J.; Weir, R.P.; Allen, L.J.; Knightbridge, K.J.; Wood, P.R.T. Legionella longbeachae detected in an industrial cooling tower linked to a legionellosis outbreak, New Zealand, 2015; possible waterborne transmission? Epidemiol. Infect. 2017, 145, 2382–2389. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Duan, G. Legionella Pneumophila Contamination in Water System in Public Places of Chongqing. J. Prev. Med. Inf. 2017, 33, 541–543. (In Chinese) [Google Scholar]
- Crook, B.; Willerton, L.; Smith, D.; Wilson, L.; Poran, V.; Helps, J.; McDermott, P. Legionella risk in evaporative cooling systems and underlying causes of associated breaches in health and safety compliance. Int. J. Hyg. Environ. Health 2020, 224, 113425. [Google Scholar] [CrossRef]
- Nakamura, I.; Amemura-Maekawa, J.; Kura, F.; Kobayashi, T.; Sato, A.; Watanabe, H.; Matsumoto, T. Persistent Legionella contamination of water faucets in a tertiary hospital in Japan. Int. J. Infect. Dis. 2020, 93, 300–304. [Google Scholar] [CrossRef]
- Puri, S.; Boudreaux-Kelly, M.; Walker, J.D.; Clancy, C.J.; Decker, B.K. Clinical Presentation of Community-Acquired Legionella Pneumonia Identified by Universal Testing in an Endemic Area. Int. J. Environ. Res. Public Health 2020, 17, 533. [Google Scholar] [CrossRef] [Green Version]
- Beauté, J.; The European Legionnaires’ Disease Surveillance Network. Legionnaires’ disease in Europe, 2011 to 2015. Euro Surveill. 2017, 22, 30566. [Google Scholar] [CrossRef] [Green Version]
- Garrison, L.E.; Kunz, J.M.; Cooley, L.A.; Moore, M.R.; Lucas, C.; Schrag, S.; Sarisky, J.; Whitney, C.G. Vital signs: Deficiencies in environmental control identified in outbreaks of legionnaires’ disease: North America, 2000–2014. Morb. Mortal. Wkly. Rep. 2016, 65, 576–584. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Legionnaires’ Disease in Europe, 2014; ECDC: Stockholm, Sweden, 2016. [Google Scholar]
- Walker, J.T.; McDermott, P. Confirming the Presence of Legionella pneumophila in Your Water System: A Review of Current Legionella Testing Methods. J. AOAC Int. 2021, 104, 1135–1147. [Google Scholar] [CrossRef]
- Leoni, E.; Legnani, P.P. Comparison of selective procedures for isolation and enumeration of Legionella species from hot water systems. J. Appl. Microbiol. 2001, 90, 27–33. [Google Scholar] [CrossRef]
- Islam, M.A.; Hassen, W.M.; Tayabali, A.F.; Dubowski, J.J. Short Ligand, Cysteine-Modified Warnericin RK Antimicrobial Peptides Favor Highly Sensitive Detection of Legionella pneumophila. ACS Omega 2021, 6, 1299–1308. [Google Scholar] [CrossRef]
- Ahmed, S.; Liwak-Muir, U.; Walker, D.; Zoldowski, A.; Mears, A.; Golovan, S.; Mohr, S.; Lem, P.; Harder, C. Validation and in-field testing of a new on-site qPCR system for quantification of Legionella pneumophila according to ISO/TS 12869:2012 in HVAC cooling towers. J. Water Health 2019, 17, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Boss, R.; Baumgartner, A.; Kroos, S.; Blattner, M.; Fretz, R.; Moor, D. Rapid detection of viable legionella pneumophila in tap water by a qPCR and RT-PCR-based method. J. Appl. Microbiol. 2018, 125, 1216–1225. [Google Scholar] [CrossRef] [Green Version]
- Eble, D.; Gehrig, V.; Schubert-Ullrich, P.; Kppel, R.; Füchslin, H.P. Comparison of the culture method with multiplex PCR for the confirmation of Legionella spp. and Legionella pneumophila. J. Appl. Microbiol. 2021, 131, 2600–2609. [Google Scholar] [CrossRef]
- Sawczyn-Domańska, A. Detection of Legionella spp. and occurrence of virulence genes: Lvh, rtxA and enhC in water samples from artificial water systems. Ann. Agric. Environ. Med. 2021, 28, 617–620. [Google Scholar] [CrossRef]
- ISO/TS 12869. Water Quality—Detection and Quantification of Legionella spp. and/or Legionella pneumophila by Concentration and Genic Amplification by Quantitative Polymerase Chain Reaction (qPCR). 2012. Available online: https://www.iso.org/standard/52079.html (accessed on 1 November 2012).
- Reyneke, B.; Ndlovu, T.; Khan, S.; Khan, W. Comparison of EMA-, PMA- and DNase qPCR for the determination of microbial cell viability. Appl. Microbiol. Biotechnol. 2017, 101, 7371–7383. [Google Scholar] [CrossRef]
- Falzone, L.; Gattuso, G.; Lombardo, C.; Lupo, G.; Salmeri, M. Droplet digital PCR for the detection and monitoring of legionella pneumophila. Int. J. Mol. Med. 2020, 46, 1777–1782. [Google Scholar] [CrossRef]
- Pascale, M.R.; Mazzotta, M.; Salaris, S.; Girolamini, L.; Grottola, A.; Simone, M.L.; Cordovana, M.; Bisognin, F.; Dal Monte, P.; Bucci Sabattini, M.A.; et al. Evaluation of MALDI-TOF Mass spectrometry in diagnostic and environmental surveillance of legionella species: A comparison with Culture and Mip-Gene Sequencing Technique. Front. Microbiol. 2020, 11, 589369. [Google Scholar] [CrossRef]
- Trnková, K.; Kotrbancová, M.; Špaleková, M.; Fulová, M.; Boledovičová, J.; Vesteg, M. MALDI-TOF MS analysis as a useful tool for an identification of Legionella pneumophila, a facultatively pathogenic bacterium interacting with free-living amoebae: A case study from water supply system of hospitals in Bratislava (Slovakia). Exp. Parasitol. 2018, 184, 97–102. [Google Scholar] [CrossRef]
- Díaz-Flores, Á.; Montero, J.C.; Castro, F.J.; Alejandres, E.M.; Bayón, C.; Solís, I.; Fernández-Lafuente, R.; Rodríguez, G. Comparing methods of determining Legionella spp. in complex water matrices. BMC Microbiol. 2015, 15, 91. [Google Scholar] [CrossRef] [Green Version]
- Keserue, H.A.; Cornillie, N.; Ehlert, A.K.; Mills, D.C.; Morger, D.; Piffaretti, A.; Schaffhauser, D.F.; Schwyzer, I.I. Validation of the Legionella pneumophila SG1 DETECT Kit for Quantification of Legionella pneumophila Serogroup 1 Bacteria in Potable Waters, Process Waters, and Surface Waters: AOAC Performance Tested Methodsm 052002. J. AOAC Int. 2021, 104, 776–789. [Google Scholar] [CrossRef]
- Olabarria, G.; Eletxigerra, U.; Rodriguez, I.; Bilbao, A.; Berganza, J.; Merino, S. Highly sensitive and fast Legionella spp. in situ detection based on a loop mediated isothermal amplification technique combined to an electrochemical transduction system. Talanta 2020, 217, 121061. [Google Scholar] [CrossRef]
- Scaturro, M.; Buffoni, M.; Girolamo, A.; Cristino, S.; Ricci, M.L. Performance of Legiolert test vs. ISO 11731 to Confirm Legionella pneumophila Contamination in Potable Water Samples. Pathogens 2020, 9, 690. [Google Scholar] [CrossRef]
- Checa, J.; Carbonell, I.; Manero, N.; Martí, I. Comparative study of Legiolert with ISO 11731-1998 standard method-conclusions from a Public Health Laboratory. J. Microbiol. Methods 2021, 186, 106242. [Google Scholar] [CrossRef] [PubMed]
- Isabelle, B. Comparison of Legiolert and a Conventional Culture Method for Detection of Legionella pneumophila from Cooling Towers in Québec. J. AOAC Int. 2019, 4, 1235–1240. [Google Scholar] [CrossRef]
- Boczek, L.A.; Tang, M.; Formal, C.; Lytle, D.; Ryu, H. Comparison of two culture methods for the enumeration of Legionella pneumophila from potable water samples. J. Water Health 2021, 19, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Sartory, D.P.; Spies, K.; Lange, B.; Schneider, S.; Langer, B. Evaluation of a most probable number method for the enumeration of legionella pneumophila from potable and related water samples. Lett. Appl. Microbiol. 2017, 64, 271–275. [Google Scholar] [CrossRef] [PubMed]
- SCA. The Determination of Legionella Bacteria in Waters and Other Environmental Samples (2020)–Part 2–Culture Methods for Their Detection and Enumeration. 2020, pp. 1–59. Available online: https://www.researchgate.net/publication/346446532_The_determination_of_Legionella_bacteria_in_waters_and_other_environmental_samples_2020_-Part_2_-Culture_Methods_for_their_detection_and_enumeration_Methods_for_the_Examination_of_Waters_and_Associate (accessed on 1 November 2012).
- State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine (AQSIQ). Examination Methods for Public Places―Part 5: Central Air Conditioning Ventilation System GB/T 18204.5-2013. AQSIQ, China. 2013. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=45F2B6F3F56D68AE22080B7E61F91411 (accessed on 31 December 2013).
- ISO 11731:2017; Water Quality—Enumeration of Legionella. ISO: Geneva, Switzerland, 2017.
- Monteiro, S.N.; Robalo, A.M.; Santos, R.J. Evaluation of Legiolert for the Detection of Legionella pneumophila and Comparison with Spread-Plate Culture and qPCR Methods. Curr. Microbiol. 2021, 78, 1792–1797. [Google Scholar] [CrossRef]
- Petrisek, R.; Hall, J. Evaluation of a most probable number method for the enumeration of legionella pneumophila from north american potable and nonpotable water samples. J. Water Health 2018, 16, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Rech, M.M.; Swalla, B.M.; Dobranic, J.K. Evaluation of Legiolert for Quantification of Legionella pneumophila from Non-potable Water. Curr. Microbiol. 2018, 75, 1282–1289. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Baba, M.; Tayama, S. Evaluation of Legiolert for Quantification of Legionella pneumophila from Bath Water Samples. Biocontrol Sci. 2020, 25, 179–182. [Google Scholar] [CrossRef]
- State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine (AQSIQ). Examination Methods for Public Places―Part 6: Technical Specifications of Health Monitoring GB/T 18204.6-2013, AQSIQ, China. 2013. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=E92DD7EEC2A692420E31074E7A09D194 (accessed on 31 December 2013).

| MPN Method | GB/T 18204.5-2013 | Total | χ2-Value | p-Value | |
|---|---|---|---|---|---|
| + | − | ||||
| + | 202 | 113 | 315 | 311.388 | <0.001 |
| − | 9 | 372 | 381 | ||
| Total | 211 | 485 | 696 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, C.; Zhang, Y.; Zhang, Y. Evaluation of a Most Probable Number Method for Detection and Quantification of Legionella pneumophila. Pathogens 2022, 11, 789. https://doi.org/10.3390/pathogens11070789
Niu C, Zhang Y, Zhang Y. Evaluation of a Most Probable Number Method for Detection and Quantification of Legionella pneumophila. Pathogens. 2022; 11(7):789. https://doi.org/10.3390/pathogens11070789
Chicago/Turabian StyleNiu, Chunyan, Yajie Zhang, and Yong Zhang. 2022. "Evaluation of a Most Probable Number Method for Detection and Quantification of Legionella pneumophila" Pathogens 11, no. 7: 789. https://doi.org/10.3390/pathogens11070789






