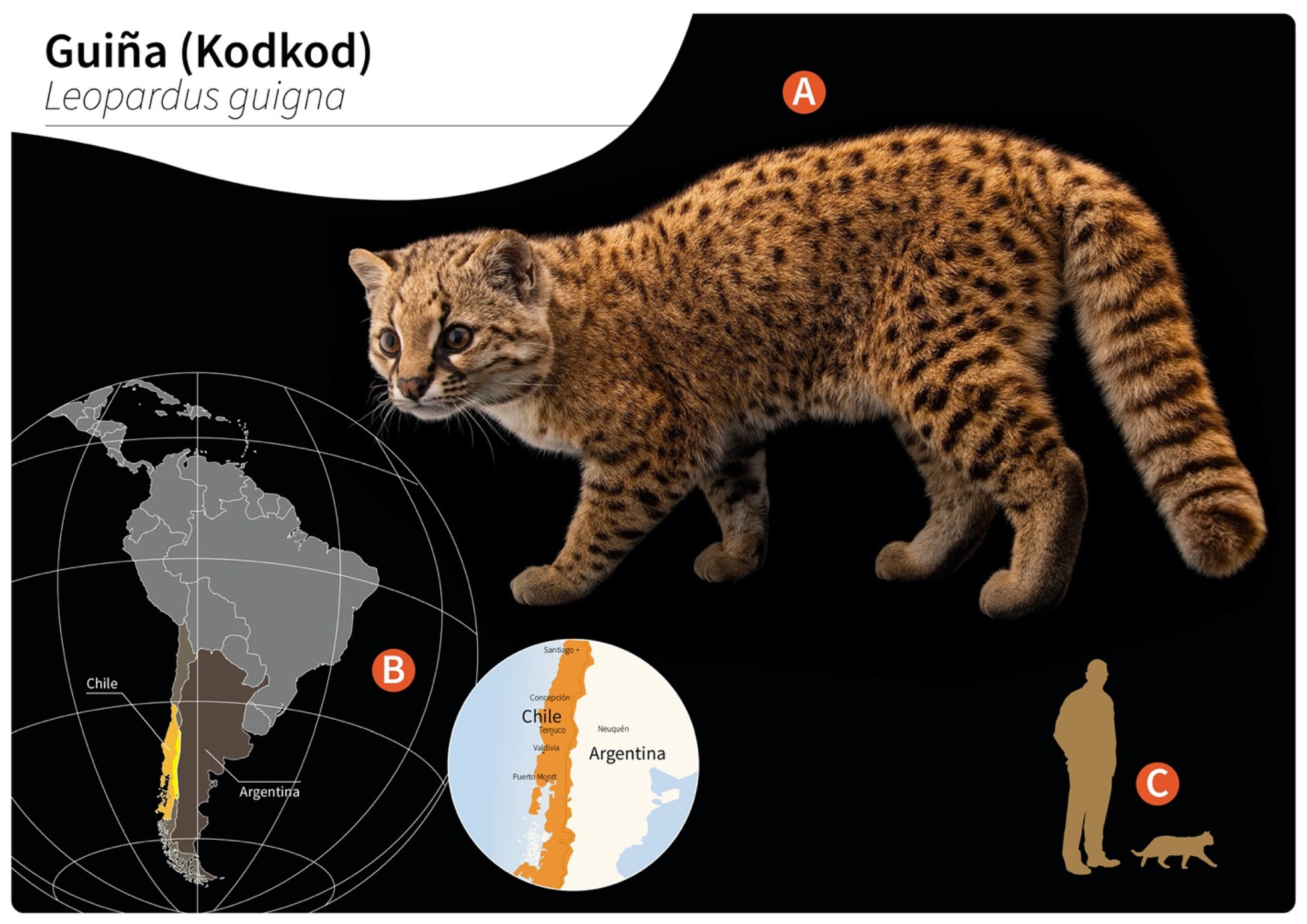
Gurltia paralysans: A Neglected Angio-Neurotropic Parasite of Domestic Cats (Felis catus) and Free-Ranging Wild Felids (Leopardus spp.) in South America
Abstract
:1. Introduction
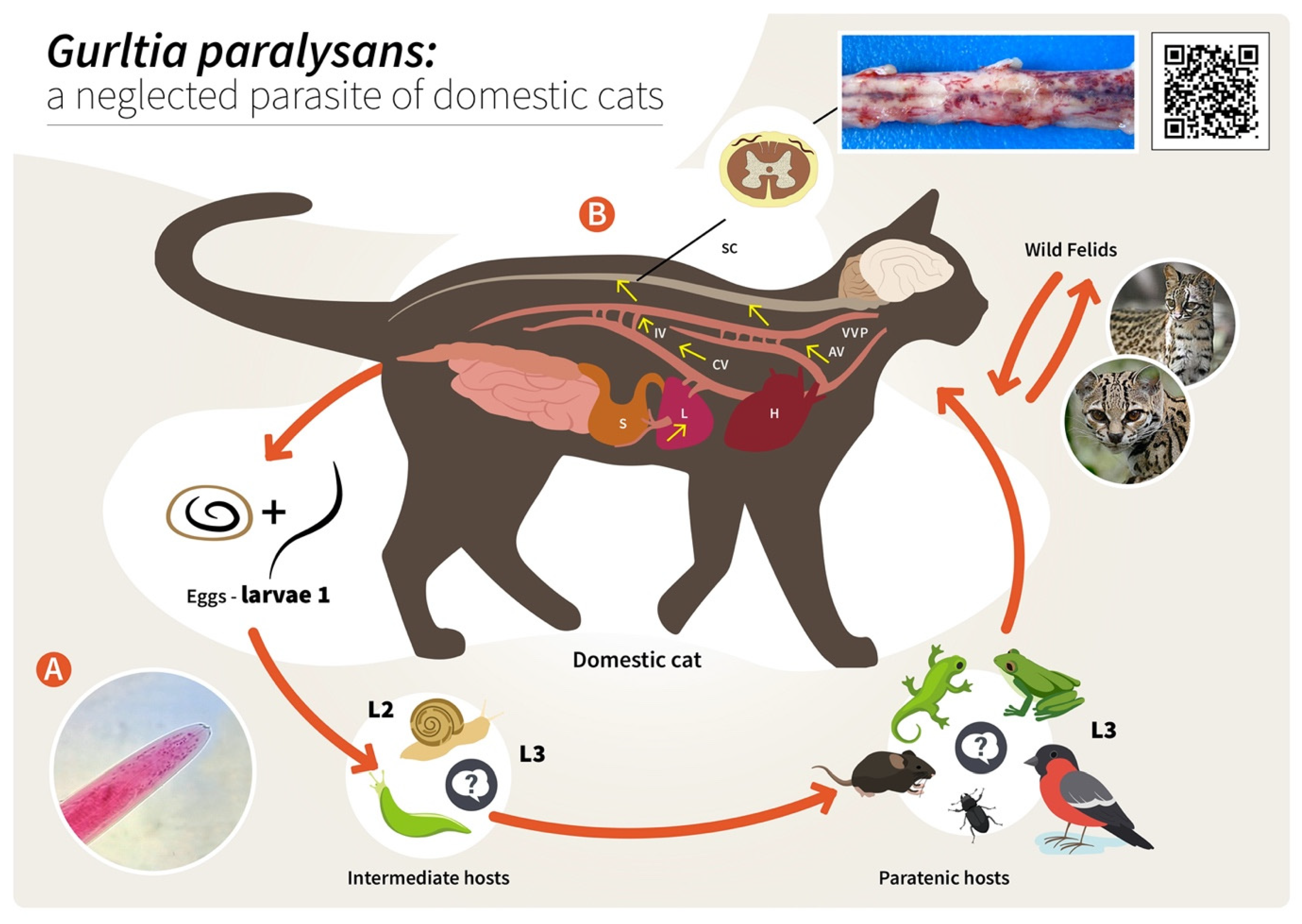
2. Hypothetical Life Cycle of Gurltia paralysans (Angiostrongylidae)
3. Epizootiology and Environmental Factors Associated with the Presence of Feline Gurltiosis
4. Diagnosis
5. Clinical Signs
6. Post Mortem Spinal Cord Examination
7. Proposed Gurltia paralysans-Induced Endothelium and Polymorphonuclear Neutrophil (PMN) Activation, Resulting in Vascular Pathogenesis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gómez, M.; Moroni, M.; Muñoz, P.; Taubert, A.; Hermosilla, C.; Hirzmann, J.; Rojas, L. Gurltia paralysans: A neglected parasite of domestic cats. Austral J. Vet. Sci. 2021, 52, 33–45. [Google Scholar] [CrossRef]
- Wolffhugel, K. Paraplegia cruralis felis causada por Gurltia paralysans nov. gen., n. sp. Rev. Chil. Hist. Nat. 1933, 37, 190–192. [Google Scholar]
- Wolffhugel, K. Paraplegia cruralis parasitaria felis durch Gurltia paralysans nov. gen., nov. sp. (Nematoda). 2. Infektkr. Haustiere 1934, 46, 28–47. [Google Scholar]
- Dazzi, C.; Santos, A.; Machado, T.; Ataíde, M.; Rodríguez, R.; Muller, A.; Sepúlveda, P.; Costa da Motta, A. First case report of nematode parasitic myelopathy in a wild feline in Brazil. Braz. J. Vet. Parasitol. 2020, 29, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, M.; García, C.; Maldonado, I.; Pantchev, N.; Taubert, A.; Hermosilla, C.; Moroni, M.; Muñoz, P.; Durán, A.; Mieres, M.; et al. Intra vitam diagnosis of neglected Gurltia paralysans infections in domestic cats (Felis catus) by a commercial serology test for canine angiostrongylosis and insights into clinical and histopathological findings-Four case reports. Pathogens 2020, 9, 921. [Google Scholar] [CrossRef]
- Barrios, N.; Gómez, M.; Zanelli, M.; Rojas-Barón, L.; Sepúlveda-García, P.; Alabí, A.; Adasme, M.; Múller, A.; Rosenfeld, C.; González-Lagos, C.; et al. A Molecular Survey on Neglected Gurltia paralysans and Aelurostrongylus abstrusus Infections in Domestic Cats (Felis catus) from Southern Chile. Pathogens 2021, 10, 1195. [Google Scholar] [CrossRef]
- Uribe, M.; López-Osorio, S.; Chaparro-Gutiérrez, J.J. The Neglected Angio-Neurotrophic Parasite Gurltia paralysans (Nematoda: Angiostrongylidae): Northernmost South American Distribution, Current Knowledge, and Future Perspectives. Pathogens 2021, 10, 1601. [Google Scholar] [CrossRef]
- Acuña-Olea, F.; Sacristán, I.; Aguilar, E.; García, S.; López, M.J.; Oyarzún-Ruiz, P.; Brito, J.L.; Fredes, F.; Napolitano, C. Gastrointestinal and cardiorespiratory endoparasites in the wild felid guigna (Leopardus guigna) in Chile: Richness increases with latitude and first records for the host species. Int. J. Parasitol. Parasites Wildl. 2020, 13, 13–21. [Google Scholar] [CrossRef]
- Grob, D.; Conejeros, I.; López-Osorio, S.; Velásquez, Z.D.; Segeritz, L.; Gärtner, U.; Schaper, R.; Hermosilla, C.; Taubert, A. Canine Angiostrongylus vasorum-Induced Early Innate Immune Reactions Based on NETs Formation and Canine Vascular Endothelial Cell Activation In Vitro. Biology 2021, 10, 427. [Google Scholar] [CrossRef]
- Moroni, M.; Muñoz, P.; Mieres, M.; Gómez, M.; Vera, F. Severe spinal cord thrombophlebitis and meningoyelitis by Gurltia paralysans in a cat: A case report. Vet. Rec. Case Rep. 2016, 4, 1. [Google Scholar] [CrossRef]
- Gómez, M.; Mieres, M.; Moroni, M.; Mora, A.; Barrios, N.; Simeone, C.; Lindsey, D. Meningomyelitis due to nematode infection in four cats. Vet. Parasitol. 2010, 170, 327–330. [Google Scholar] [CrossRef]
- Moroni, M.; Muñoz, P.; Gómez, M.; Mieres, M.; Rojas, M.; Lillo, C.; Aguirre, F.; Acosta-Jamett, G.; Kaiser, M.; Lindsay, D. Gurltia paralysans (Wolffhugel, 1933): Description of adults and additional case reports of numerological diseases in three domestic cats from Southern Chile. Vet. Parasitol. 2012, 184, 377–380. [Google Scholar] [CrossRef]
- Hartung, S.; Weyrich, A.; Moroni, M.; Gómez, M.; Herden, C. Histological and Immunohistochemical Characterization of Vascular Alterations in Meninges of Cats Infected with Gurltia paralysans. Pathogens 2022, 11, 88. [Google Scholar] [CrossRef]
- Alzate, G.; Aranzazu, D.; Alzate, A.; Chaparro, J. Domestic cat paraplegia compatible with Gurltia paralysans nematode. First cases reported in Colombia. Rev. Colomb. Cienc. Pecu. 2011, 24, 663–669. [Google Scholar]
- Guerrero, I.; Paludi, A.; Saumell, L. Primera Descripción en Argentina de Gurltia paralysans en un Felino Doméstico. DVM Thesis, Universidad Del Centro De La Prov. Buenos Aires, Tandil, Argentina, 2011. [Google Scholar]
- Rivero, R.; Matto, C.; Adrien, M.; Nan, F.; Bell, T.; Gardiner, C. Parasite meningomyelitis in cats in Uruguay. Rev. Bras. Parasitol. Vet. 2011, 20, 259–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mieres, M.; Gómez, M.; Lillo, C.; Rojas, M.; Moroni, M.; Muñoz, P.; Acosta-Jamett, G.; Wiegand, R. Clinical, imaging and pathological characteristics of Gurltia paralysans myelopathy in domestic cats from Chile. Vet. Radiol. Ultrasound. 2013, 53, 1–8. [Google Scholar]
- Togni, M.; Panziera, W.; Souza, T.; Oliveira, J.; Mazzanti, A.; Barros, C.; Fighera, R. Epidemiological, clinical and pathological aspects of Gurltia paralysans infections in cats. Pesqui. Veterinária Bras. 2013, 33, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Bono, M.; Orcellet, V.; Marengo, R.; Bosio, A.; Junkers, E.; Plaza, D.; Marini, M.; Sánchez, A.; Rubio, M.; Candiotti, V. A description of three cases of parasitic meningomyelitis in felines of the province of Santa Fé, Argentina. Parasitaria 2016, 74, 1–4. [Google Scholar]
- Udiz-Rodríguez, R.; García-Livia, K.; Valladares-Salmerón, M.; Dorta-Almenar, M.; Martin-Carrillo, N.; Martin-Alonso, A.; Isquierdo-Rodríguez, E.; Feliu, C.; Valladares, B.; Foronda, P. First ocular report of Gurltia paralysans (Wolffhugel, 1933) in cat. Vet. Parasitol. 2018, 255, 74–77. [Google Scholar] [CrossRef]
- Melo Neto, G.; da Silva, L.; Alves, R.; Olinda, R.; Dantas, A.; Torres, M. Infeccao por Gurltia paralysans em gatos domésticos no Estado de Pernambuco, Brasil. Acta Scientiae Veterinariae 2019, 47, 418. [Google Scholar]
- Oliveira, B. Politraumatismo em Gato-Maracajá (Leopardus wiedii) com Infeccao por Gurltia paralysans; Semana do Conhecimiento UPF: Passo Fundo, Italy, 2015; pp. 1–3. [Google Scholar]
- Peña, G. Hallazgos Clínicos, Hematológicos, Bioquímicos y de Lavado Broncoalveolar en 8 Gatos Domésticos (Felis catus) con Paraparesis/Plejia Producida por Gurltia paralysans. DVM Thesis, Universidad Austral De Chile, Valdivia, Chile, 2014. [Google Scholar]
- Morgan, E.R.; Modry, D.; Paredes-Esquivel, C.; Foronda, P.; Traversa, D. Angiostrongylosis in Animals and Humans in Europe. Pathogens 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, L.; Cardona, A.; Taubert, A.; Hermosilla, C.; Ruiz, A. Autochthonous Angiostrongylus cantonensis, Angiostrongylus vasorum and Aelurostrongylus abstrusus infections in native terrestrial gastropods from the Macaronesian Archipelago of Spain. Parasitol. Res. 2021, 120, 2671–2680. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-García, P.; Gómez, M.; Moroni, M.; Muñoz, P.; Muller, A. Evaluation of terrestrial gastropods intermediate hosts of Gutlia paralysans in southern Chile. Braz. J. Vet. Parasitol. 2021, 30, 1. [Google Scholar]
- Gómez, M.; Freeman, L. Revisión del plexo venoso vertebral en el perro (Review of the vertebral venous plexus in the dog). Int. J. Morphol. 2003, 21, 237–244. [Google Scholar]
- Katchanov, J.; Nawa, Y. Helminthic invasion of the central nervous system: Many roads lead to Rome. Parasitol. Int. 2010, 59, 491–496. [Google Scholar] [CrossRef]
- Shahlaie, K.; Hawk, M.; Hu, B.; Theis, J.; Kim, K. Parasitic central nervous system infections: Echinococcus and Schistosoma. Rev. Neurol. Dis. 2005, 2, 76–85. [Google Scholar]
- Paz, J.; Valente, M.; Casella, E.B.; Marques-Dias, M.J. Spinal cord schistosomiasis in children: Analysis of seven cases. Arq Neuropisiquiatr. 2002, 60, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, N. Descripción Histopatológica de Lesiones Encefálicas en Gatos Domésticos Infectados con Gurltia paralysans. DVM Thesis, Universidad Austral De Chile, Valdivia, Chile, 2017. [Google Scholar]
- Handeland, K.; Gibbons, L.; Skorping, A. Aspects of the life cycle and pathogenesis of Elaphostrongylus cervi in red deer (Cervus elaphus). J. Parasitol. 2000, 86, 1061–1066. [Google Scholar] [CrossRef]
- Handeland, K. Cerebrospinal nematodiasis in a moose in Norway. J. Wildl. Dis. 2002, 38, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Hemmingsen, W.; Halvorsen, O.; Skorping, A. Migration of adult Elaphostrongylus rangiferi (Nematoda: Protostrongylidae) from the spinal subdural space to the muscles of reindeer (Rangifer tarandus). J. Parasitol. 1993, 79, 728–732. [Google Scholar] [CrossRef]
- Lankester, M. Understanding the impact of meningeal worm, Parelaphostrongylus tenuis, on moose populations. Alces J. Devoted Biol. Manag. Moose 2010, 46, 53–70. [Google Scholar]
- Reinstein, S.; Lucio-Forster, A.; Bowman, D.; Eberhard, M.; Hoberg, E.; Pot, S.; Miller, P. Surgical extraction of an intraocular infection of Parelaphostrongylus tenuis in a horse. J. Am. Vet. Med. Assoc. 2010, 15, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Modrý, D.; Fecková, B.; Putnová, B.; Manalo, S.; Otranto, D. Alternative pathway in Angiostrongylus cantonensis (Metastrongyloidea: Angiostrongyloidea) transmission. Parasitology 2021, 148, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ferdushy, T.; Hasan, M. Survival of first stage larvae (L1) of Angiostrongylus vasorum under various conditions of temperature and humidity. Parasitol Res. 2010, 107, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Hirzmann, J.; Rodríguez, E.; Moroni, M.; Taubert, A.; Gibbons, L.; Hermosilla, C.; Gómez, M. Redescription and first molecular characterization of the little-known feline neutropic nematode Gurtia paralysans (Nematoda: Metastrongyloidea). Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 119–125. [Google Scholar]
- López-Contreras, F.; Rojas-Barón, L.; Gómez, M.; Morera, F.; Sepúlveda, P.; Moroni, M.; Muñoz, P.; Acosta-Jammett, G.; Mieres, M.; Hirzmann, J.; et al. Molecular Detection of Gurltia paralysans by Semi-Nested PCR in Cerebrospinal Fluid and Serum Samples from Domestic Cats (Felis catus). Animals 2020, 10, 1169. [Google Scholar] [CrossRef]
- Rinaldi, L.; Cortese, L.; Meomartino, L.; Pagano, T.B.; Pepe, P.; Cringoli, G.; Papparella, S. Angiostrongylus vasorum: Epidemiological, clinical and histopathological insights. BMC Vet. Res. 2014, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Vienenkötter, J.; Hermosilla, C.; Taubert, A.; Herden, C.; Gómez, M.; Muñoz, P.; Moroni, M. Spinal cord lesions in a cat infected with Gurltia paralysans. J. Comp. Pathol. 2015, 152, 80. [Google Scholar] [CrossRef]
- Jara, C. Caracterización de la Respuesta Leucocitaria y Glial en Gatos con Meningomielitis por Gurltia paralysans. DVM Thesis, Universidad Austral De Chile, Valdivia, Chile, 2018. [Google Scholar]
- Verscheure, A. Hallazgos Histológicos en Hígado, Pulmón y Riñón de Gatos Domésticos con Paraparesia o Paraplejia por Gurltia paralysans. DVM Thesis, Universidad Austral De Chile, Valdivia, Chile, 2014. [Google Scholar]
- Cook, S.; Priestnall, S.L.; Blake, D.; Meeson, R.L. Angiostrongylus vasorum Causing Severe Granulomatous Hepatitis with Concurrent Multiple Acquired PSS. J. Am. Anim. Hosp. Assoc. 2015, 51, 320–324. [Google Scholar] [CrossRef]
- Silva, L.M.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond Phagocytosis: Phagocyte-Derived Extracellular Traps Act Efficiently against Protozoan Parasites In Vitro and In Vivo. Mediators Inflamm. 2016, 2016, 5898074. [Google Scholar] [CrossRef] [Green Version]
- Villagra-Blanco, R.; Silva, L.M.R.; Conejeros, I.; Taubert, A.; Hermosilla, C. Pinniped- and Cetacean-Derived ETosis Contributes to Combating Emerging Apicomplexan Parasites (Toxoplasma gondii, Neospora caninum) Circulating in Marine Environments. Biology 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermosilla, C.; Caro, T.M.; Silva, L.M.; Ruiz, A.; Taubert, A. The intriguing host innate immune response: Novel anti-parasitic defence by neutrophil extracellular traps. Parasitology 2014, 141, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Grob, D.; Conejeros, I.; Velásquez, Z.D.; Preußer, C.; Gärtner, U.; Alarcón, P.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Trypanosoma brucei brucei Induces Polymorphonuclear Neutrophil Activation and Neutrophil Extracellular Traps Release. Front. Immunol. 2020, 11, 559561. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Machado-Ribeiro da Silva, L.; Rentería-Solis, Z.; Taubert, A.; Hermosilla, C. Neutrophil extracellular traps in the intestinal mucosa of Eimeria-infected animals. Asian Pac. J. Trop. Biomed. 2016, 6, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Caro, T.; Conejeros, I.; Zhou, E.; Pikhovych, A.; Gartner, U.; Hermosilla, C.; Kulke, D.; Taubert, A. Dirofilaria immitis microfilariae and third-stage larvae induce canine NETosis resulting in different types of neutrophil extracellular traps. Front. Immunol. 2018, 9, 968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Conejeros, I.; Velasquez, Z.D.; Muñoz-Caro, T.; Gartner, U.; Hermosilla, C.; Taubert, A. Simultaneous and positively correlated NET formation and autophagy in Besnoitia besnoiti tachyzoite-exposed bovine polymorphonuclear neutrophils. Front. Immunol. 2019, 10, 1131. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Muñoz-Caro, T.; Gartner, U.; Mejer, H.; Schaper, R.; Hermosilla, C.; Taubert, A. Gastropod-derived haemocyte extracellular traps entrap metastrongyloid larval stages of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Troglostrongylus brevior. Parasit. Vectors. 2017, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Taubert, A.; Zahner, H.; Hermosilla, C. Dynamics of transcription of immunomodulatory genes in endothelial cells infected with different coccidian parasites. Vet. Parasitol. 2006, 142, 214–222. [Google Scholar] [CrossRef]
- Maksimov, P.; Hermosilla, C.; Kleinertz, S.; Hirzmann, J.; Taubert, A. Besnoitia besnoiti infections activate primary bovine endothelial cells and promote PMN adhesion and NET formation under physiological flow condition. Parasitol. Res. 2016, 115, 1991–2001. [Google Scholar] [CrossRef]
- Conejeros, I.; Velásquez, Z.D.; Grob, D.; Zhou, E.; Salecker, H.; Hermosilla, C.; Taubert, A. Histone H2A and Bovine Neutrophil Extracellular Traps Induce Damage of Besnoitia besnoiti-Infected Host Endothelial Cells but Fail to Affect Total Parasite Proliferation. Biology 2019, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Neumann, A.; Brogden, G.; von Köckritz-Blickwede, M. Extracellular Traps: An Ancient Weapon of Multiple Kingdoms. Biology 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnyder, M.; Fahrion, A.; Riond, B.; Ossent, P.; Webster, P.; Kranjc, A.; Glaus, T.; Deplazes, P. Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol. Res. 2010, 107, 1471–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaus, T.; Schnyder, M.; Dennler, M.; Tschuor, F.; Wenger, M.; Sieber-Ruckstuhl, N. Natürliche Angiostrongylus vasorum Infektion: Charakterisierung des Krankheitsbildes bei drei Hunden mit pulmonärer Hypertonie [Natural infection with Angiostrongylus vasorum: Characterisation of 3 dogs with pulmonary hypertension]. Schweiz. Arch. Tierheilkd. 2010, 152, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, P.S.; Lai, S.C. Matrix metalloproteinase-9 leads to blood-brain barrier leakage in mice with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. Acta. Trop. 2014, 140, 141–150. [Google Scholar] [CrossRef]
- Löf, A.; Müller, J.P.; Brehm, M.A. A biophysical view on von Willebrand factor activation. J. Cell. Physiol. 2018, 233, 799–810. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Z.; Long, Q.; Huang, J.; Hong, T.; Liu, W.; Lin, J. Insights Into Immunothrombosis: The Interplay Among Neutrophil Extracellular Trap, von Willebrand Factor, and ADAMTS13. Front. Immunol. 2020, 11, 610696. [Google Scholar] [CrossRef]
- Whitley, N.T.; Corzo-Menendez, N.; Carmichael, N.G.; McGarry, J.W. Cerebral and conjunctival haemorrhages associated with von Willebrand factor deficiency and canine angiostrongylosis. J. Small Anim. Pract. 2005, 46, 75–78. [Google Scholar] [CrossRef]
- Adamantos, S.; Waters, S.; Boag, A. Coagulation status in dogs with naturally occurring Angiostrongylus vasorum infection. J. Small Anim. Pract. 2015, 56, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Gillis-Germitsch, N.; Kockmann, T.; Asmis, L.M.; Tritten, L.; Schnyder, M. The Angiostrongylus vasorum Excretory/Secretory and Surface Proteome Contains Putative Modulators of the Host Coagulation. Front. Cell Infect. Microbiol. 2021, 11, 753320. [Google Scholar] [CrossRef]
- Bourque, A.C.; Conboy, G.; Miller, L.M.; Whitney, H. Pathological findings in dogs naturally infected with Angiostrongylus vasorum in Newfoundland and Labrador, Canada. J. Vet. Diagn. Investig. 2008, 20, 11–20. [Google Scholar] [CrossRef] [Green Version]



| Age | Location | Number of Cases | Clinical Presentation | Diagnosis | Reference |
|---|---|---|---|---|---|
| 1–3 y | Chile (Los Ríos/Los Lagos regions) | 3 | Paraparesis (ambulatory) PL ataxia PL muscle atrophy Anal/urinary incontinence | Post mortem (histopathology) | [12] |
| 6–8 m | Colombia (Antioquia municipality) | 6 | Paraparesis (ambulatory) PL ataxia Spinal pain PL muscle atrophy Anal/urinary incontinence Decrease superficial/deep pain in PL | Post mortem (histopathology, Myelo) | [14] |
| 2 y | Argentina (Buenos Aires province) | 1 | Paraparesis (non-ambulatory) PL muscle atrophy Increase spinal reflexes in PL Decrease superficial/deep pain in PL | Post mortem (histopathology) | [15] |
| NA | Uruguay (Fray Bentos) | 2 | Paraparesis (ambulatory) Paraplegia PL ataxia | Post mortem (histopathology) | [16] |
| 1–3 y | Chile | 3 | Paraparesis (ambulatory) PL ataxia PL muscle atrophy Anal/urinary incontinence Spinal hyperaesthesia PL trembling Increase spinal reflexes in PL Paraplegia | Post mortem (histopathology, specimens extracted from SSE) | [11] |
| 8 m–10 y | Chile | 9 | Paraparesis (ambulatory) Paraparesis (non-ambulatory) Paraplegia PL ataxia Increase spinal reflexes in PL Spinal hyperaesthesia Anal/urinary incontinence | Post mortem (histopathology, specimens extracted from SSE, Myelo, CT, MRI | [17] |
| NA | Brazil (Río Grande do Sul) | 4 | Paraparesis (ambulatory) PL muscle atrophy Vesical atony Tail atony | Post mortem (histopathology) | [18] |
| NA | Argentina (Santa Fé) | 3 | Paraparesis (ambulatory) Paraplegia Decrease spinal reflexes in PL Decrease superficial pain in PL Skin lesions in the metatarsal region | Post mortem (histopathology) | [19] |
| 8 m | Chile (Ancud, Los Lagos regions) | 1 | Paraparesis (ambulatory) Anal/urinary incontinence Tail atony | Myelo-CT, CSF (mononuclear pleocytosis), post mortem (histopathology) | [10] |
| 2 y | Spain (Tenerife) | 1 | Uveitis in left eye | Specimen extracted from anterior chamber of the eye, PCR | [20] |
| NA | Brazil (Pernambuco) | 11 | Paraparesis (ambulatory) PL ataxia, PL muscle atrophy Skin lesions in metatarsal and phalangeal regions | Post mortem (histopathology) | [21] |
| 36 m | Chile | 10 | Paraparesis (ambulatory) Paraplegia PL ataxia Anal/urinary incontinence | Post mortem (histopathology), IDEXX (Angio Detect), specimens extracted from SSE | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Barón, L.; Taubert, A.; Hermosilla, C.; Gómez, M.; Moroni, M.; Muñoz, P. Gurltia paralysans: A Neglected Angio-Neurotropic Parasite of Domestic Cats (Felis catus) and Free-Ranging Wild Felids (Leopardus spp.) in South America. Pathogens 2022, 11, 792. https://doi.org/10.3390/pathogens11070792
Rojas-Barón L, Taubert A, Hermosilla C, Gómez M, Moroni M, Muñoz P. Gurltia paralysans: A Neglected Angio-Neurotropic Parasite of Domestic Cats (Felis catus) and Free-Ranging Wild Felids (Leopardus spp.) in South America. Pathogens. 2022; 11(7):792. https://doi.org/10.3390/pathogens11070792
Chicago/Turabian StyleRojas-Barón, Lisbeth, Anja Taubert, Carlos Hermosilla, Marcelo Gómez, Manuel Moroni, and Pamela Muñoz. 2022. "Gurltia paralysans: A Neglected Angio-Neurotropic Parasite of Domestic Cats (Felis catus) and Free-Ranging Wild Felids (Leopardus spp.) in South America" Pathogens 11, no. 7: 792. https://doi.org/10.3390/pathogens11070792
APA StyleRojas-Barón, L., Taubert, A., Hermosilla, C., Gómez, M., Moroni, M., & Muñoz, P. (2022). Gurltia paralysans: A Neglected Angio-Neurotropic Parasite of Domestic Cats (Felis catus) and Free-Ranging Wild Felids (Leopardus spp.) in South America. Pathogens, 11(7), 792. https://doi.org/10.3390/pathogens11070792








