Abstract
The plant nursery industry has become an ideal reservoir for Phytophthora species and other soilborne pathogens. In this context, isolation from tissues and soil of ornamental and forest plants from nurseries in four regions of Spain was carried out. A high diversity of Phytophthora species was confirmed. Fourteen Phytophthora phylotypes (P. cactorum, P. cambivora, P. cinnamomi, P. citrophthora, P. crassamura, P. gonapodyides, P. hedraiandra, P. nicotianae, P. niederhauserii, P. palmivora, P. plurivora, P. pseudocryptogea, P. sansomeana, and Phytophthora sp. tropicalis-like 2) were isolated from over 500 plant samples of 22 species in 19 plant genera. Nine species were detected in water sources, two of them (P. bilorbang and P. lacustris) exclusively from water samples. P. crassamura was detected for the first time in Spain. This is the first time P. pseudocryptogea is isolated from Chamaecyparis lawsoniana and Yucca rostrata in Spain.
1. Introduction
Different pests and diseases can affect nursery production, which can turn plants into pathogen vectors [1]. Pathogens affect all plant industry sectors across agriculture, horticulture, forestry, and amenity, and they can have a significant impact on yield, market access, sustainability of production, food security, and product integrity [2]. Fungi is considered the kingdom with the largest number of phytopathogenic species [3]. In addition to this, the kingdom Straminipila embraces other important plant pathogens such as Phytophthora and Pythium [3]. Phytophthora species are responsible for large losses of nursery stock throughout the world [4,5,6,7,8,9,10,11].
Phytophthora is one of the most destructive genera which includes currently over 150 known species and about 100 more that are in the process of being described [11,12,13,14]. Almost all Phytophthora species are ecologically and economically important plant pathogens worldwide, some of them with a broad host range. Phytophthora species possess wide environmental adaptation that ranges from terrestrial to aquatic habitats. Some species, such as Phytophthora infestans (Mont.) de Bary, have been responsible for some of the most important epidemics in history, others such as Phytophthora cinnamomi Rands and Phytophthora ramorum Werres, de Cock and Man in ’t Veld, disrupt and diminish biodiversity in natural ecosystems [15,16,17,18,19,20,21]. Others such as Phytophthora citrophthora (R.E. Smith and E.H. Smith) Leonian, Phytophthora nicotianae Breda de Haan, Phytophthora hedraiandra de Cock and Man in ’t Veld, Phytophthora niederhauserii Z.G. Abad and J.A. Abad and P. ramorum, produce major losses in the nursery industry worldwide [7,11,14,22,23,24,25,26,27].
The inocula of Phytophthora spp., which cause foliar as well as root diseases, can increase from low to high levels within a few days or weeks under favourable conditions [28]. Polycyclic diseases can turn into serious epidemics when environmental conditions favour a rapid production of Phytophthora propagules [5]. The movement of plants and plant products between biogeographical zones due to human activity constitutes the leading pathway for the introduction of pathogens and exotic pests [3,29,30].
Phytophthora in its centre of origin does not necessarily constitute an ecological problem or even noticeable because the binomial pathogen–host has co-evolved [28]. Phytophthora native hosts have developed specific defences which confer some tolerance against the pathogen [11,29,31,32]. Nevertheless, when the pathogen is transferred to a new habitat with favourable conditions, it can likely extend to a wide range of new hosts causing serious ecological and economical losses [33,34]. The arrival of new genotypes, lineages, or exempt mating types into a non-native habitat can pose an additional risk to the ecosystem and possibly drive host range expansion for that species [11,23,29,33,34].
Invasive pathogens have been causing damage to native plant communities, woodlands, and landscapes on a global scale for over a century [29,35]. Nursery trade encourages, unintentionally, the dispersal and establishment of invasive and exotic Phytophthora spp. [3,11,36,37]. Even more, the high specialisation and intensification of nursery production favours the reproduction and hybridisation of invasive species enhancing the dispersion and settlement of these on natural ecosystems [29]. The diversity of the genus has increased rapidly in the last decades due to the appearance of new alien species such as Phytophthora alni subsp. alni Brasier and Kirk, Phytophthora austrocedri Greslebin and Hansen, Phytophthora foliorum Donahoo and Lamour, P. hedraiandra, Phytophthora kernoviae Brasier, Beales and Kirk, Phytophthora lateralis Tucker and Milbrath, P. pinifolia Durán, Gryzenh. and Wingf., Phytophthora pluvialis Reeser, Sutton and Hansen or P. ramorum, which requires routine samplings for their early detection, and due to the numerous surveys on unexplored habitats, such as water reservoirs [34,38].
In Spain, since the first report of P. ramorum in 2002 [39,40], surveys have been carried out in ornamental nurseries, garden centres, public gardens, and forest masses to detect and eradicate this pathogen. These surveys have shown that other species of Phytophthora affect many ornamental plants, posing a risk also to nurseries and natural ecosystems [9,41].
Due to the increasing threat of invasive Phytophthora species, and the high risk of hybridisation, a survey was carried out on producing woody and/or ornamental plant nurseries to investigate the presence of Phytophthora and soilborne fungi present and to exclude the presence of quarantine pathogens. Therefore, the aim of this investigation was to identify Phytophthora species and fungal pathogens in ornamental/forest nurseries in different geographical areas of Spain, which could be a threat to the plant nursery industry and to managed and natural ecosystems.
2. Materials and Methods
2.1. Study Sites
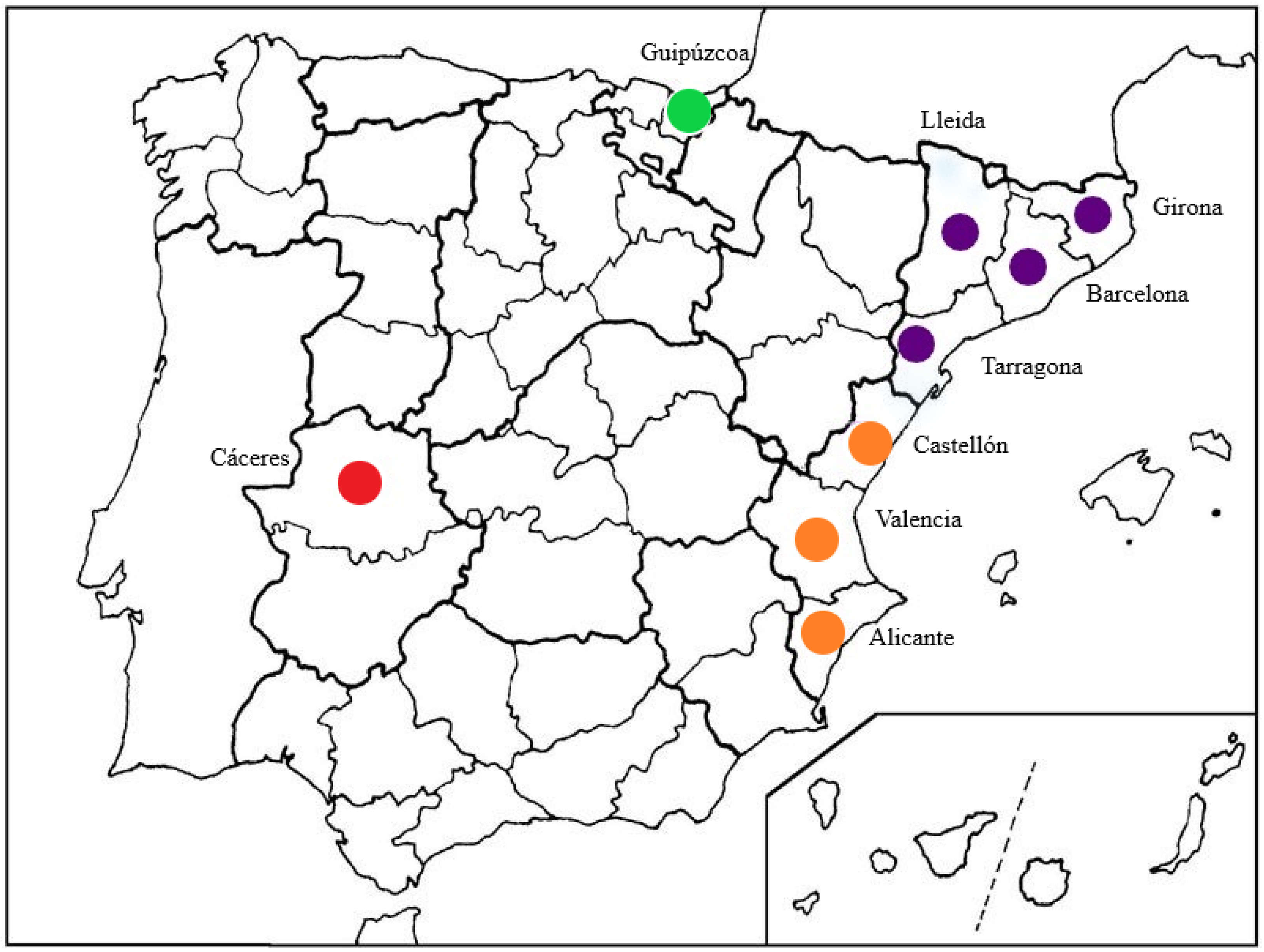
Surveys were conducted in 25 Spanish nurseries located in four geographically different regions during the period 2012–2014 in Catalonia (provinces of Barcelona, Girona, Tarragona, and Lleida), Comunidad Valenciana (provinces of Alicante, Castellón, and Valencia), Extremadura (province of Cáceres), and Basque Country (province of Guipúzcoa) (Figure 1).
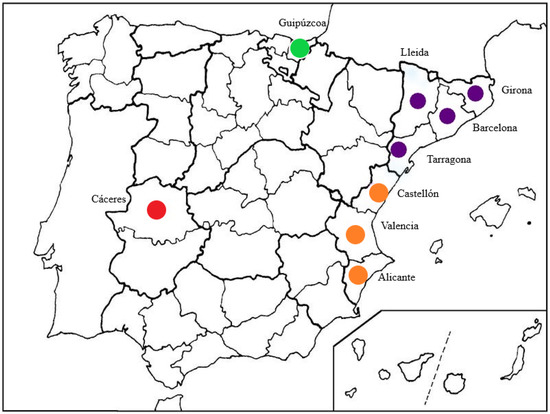
Figure 1.
Spanish provinces in which nursery surveys were conducted: in green Basque Country, in purple Catalonia, in orange Comunidad Valenciana, and in red Extremadura.
In ornamental nurseries, only symptomatic plants were collected, whereas in forest nurseries for habitat restoration non-symptomatic plants were also collected. Foliar symptoms (leaf blotch, blight, chlorosis, defoliation), wilting, dieback, growth reduction, cankers with or without gummosis, rot and presence of dead plants were considered symptoms associated to possible Phytophthora or soilborne pathogen infection (Figure 2). These plants presented in most cases root rot and/or loss of the feeder roots with the presence of necrotic lesions (Figure 2). Plant samples were collected together with their pot media or soil, individually stored in labelled plastic bags, and kept in cold conditions until they were processed in the laboratory at the Instituto Agroforestal Mediterráneo, Universitat Politècnica de València (IAM-UPV). In total, 78 samples were collected from 10 nurseries in Catalonia, 343 samples from 5 nurseries in Comunidad Valenciana, 110 samples from 8 nurseries in Extremadura, and 16 samples from 2 nurseries in Basque Country. Each sample consisted of one plant.

Figure 2.
(A,B,D,G) Dieback type symptoms on Buxus sempervirens, Arbutus unedo, Juniperus horizontalis, and Cupressus macrocarpa. (C) Arbutus unedo with black leaf necrosis that advances along the middle vein from the petiole to the apex. (E) Quercus ilex showing chlorosis and leaf spots. (F): Rot on Yucca rostrata. (H) Araucaria araucana with leaf necrosis. (I) Canker on the stem of a Chamaecyparis lawsoniana.
Thirteen water samples from recirculating irrigation ponds were also collected during the survey in Catalonia and from one nursery located in the Comunidad Valenciana. Ten litres of water were filtered using three cellulose membranes (5.0 µm pore diameter, Millipore Corporation), which were placed in sterile Petri dishes, sealed with parafilm, labelled, and stored in a cooler during transport to the laboratory. Furthermore, while water sampling was being performed, two additional samples consisting of five leaves showing Phytophthora-like spots floating in two of the surveyed water ponds, were collected in Catalonia; these were labelled and transported for processing in the laboratory.
2.2. Isolation from Plant Tissues, Soil, and Water
Plant samples (leaves and/or roots) were separated from substrate media, and the roots were washed and kept for 24 h in tap water that was repeatedly renewed for oxygenation. Samples were superficially disinfected spraying alcohol at 70% for oomycete isolation and disinfected for 1 min in a 1.5% sodium hypochlorite solution and washed twice with sterile distilled water for fungi isolation [25,42]. Small fragments from the lesion edge were plated on semi-selective media for isolation of oomycetes (CMA-PARPB supplemented or not with hymexazol [43]). Plates were incubated at 20 °C in the dark for 3–5 days for fungi and up to 7 days for oomycetes. All the colonies grown on isolation media were transferred to PDA plates and incubated at 20 °C in darkness for 7 days for identification. Pure cultures of all putative Phytophthora isolates were obtained by transferring single hyphal tips to PDA plates.
The soil removed from each plant sample was baited using Granny Smith apples targeting oomycetes species isolation [5]. Four 10 mm-diameter and 1–1.5 cm-deep holes were made on the apple fruit with a cork borer, each one was filled with the soil sample, saturated with distilled water, sealed with adhesive tape, and incubated at room temperature until lesions appeared (4–7 days). Small tissue fragments from lesion edges were plated on CMA-PARPB with and without hymexazol and incubated at 20 °C in darkness. Each colony was transferred to PDA and incubated as described above for plant samples.
Oomycetes isolation from filtered water samples was undertaken also by apple baiting; three longitudinal flap-like cuts (one per membrane filter) were made on a Granny Smith apple. In each flap cut, half of the subsample membrane was placed, sealed with parafilm, and incubated at room temperature until symptoms develop (4–7 days). The re-isolation from the apple was performed from the edge of any lesions that developed after incubation following the protocol described above.
2.3. Identification
Molecular Identification
DNA from Phytophthora and Pythium isolates was extracted from pure cultures grown on PDA by scraping the mycelium and mechanically disrupting it by grinding to a fine powder under liquid nitrogen, using the EZNA Plant Miniprep Kit (Omega Bio-tek, Doraville, GE, USA) following the manufacturer’s instructions.
Nuclear ribosomal DNA ITS amplifications were carried out using the universal primers ITS4 and ITS6 that target conserved regions in the 18S and 28S rDNA genes [44,45]. All PCR reactions were performed using HotBegan™ Taq DNA Polymerase (Canvax Biotech SL, Córdoba, Spain), according to the manufacturer’s instructions, in a PTC 200 thermo-cycler (MJ Research, Waltham, MA, USA) with the following parameters: 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s; and 72 °C for 10 min. Amplified products were purified and sequenced by Macrogen (Amsterdam, The Netherlands).
The isolates were identified to the species level by conducting Basic Local Alignment Search Tool (BLAST) searches with the sequence data on international collection databases (Phytophthora Database, PhyID, and GenBank) and a customised database that included the new Phytophthora species described and segregated from Phytophthora complexes and provisional taxons. An isolate was assigned to a species when the identity was above the 99% cut-off in respect to the ex-type isolates. The ITS sequence did not resolve the identity of 10 isolates. Therefore, for these isolates the mitochondrial cytochrome c oxidase I (COI) region was amplified using the primers OomCoxI-Levup and Fm85mod [46].
The DNA sequences from this study (Table 1), together with those of reference species of each clade retrieved from Genbank, were aligned using the ClustalW algorithm [47] contained within the MEGA X software package [48]. The sequences of the reference isolates were selected from ex-type or well-authenticated Phytophthora species recommended in IDphy: molecular and morphological identification of Phytophthora (https://idtools.org/id/phytophthora/molecular.php (accessed on 15 February 2021).). The alignments were inspected and corrected manually. Incomplete portions at either end of the alignments were excluded prior to analyses.

Table 1.
Phytophthora isolates identified in this study.
Phylogenetic analyses were based on Bayesian inference (BI), maximum likelihood (ML), and maximum parsimony (MP). Bayesian analyses were performed using MrBayes v 3.2.6 on the NGPhylogeny.fr web service [49]. Four simultaneous analyses were run for 100,000 generations, sampling every 10,000, with four Markov chain Monte Carlo (MCMC) chains. The first 25% of saved trees were discarded and posterior probabilities were determined from the remaining trees. The ML analyses were completed with the tool Randomized Axelerated Maximum Likelihood (RAxML) implemented on the T-REX web server (http://www.trex.uqam.ca/ (accessed on 11 July 2022).) [50]. ML tree searches were performed under the generalised time-reversible with gamma correction (GTR + Γ) nucleotide substitution model using 1000 pseudoreplicates. The other parameters were used as default settings. MP analyses were performed in MEGA X [48] with the Tree Bisection and Reconnection (TBR) algorithm, where gaps were treated as missing data. The robustness of the topology was evaluated using 1000 bootstrap replications [51]. Measures for the maximum parsimony such as tree length (TL), consistency index (CI), retention index (RI), and rescaled consistency index (RC) were also calculated.
2.4. Conservation of Phytophthora and Pythium Isolates
Pure cultures obtained by hyphal tipping were maintained in the oomycete culture collection at the IAM-UPV. Each isolate was grown on V-8 Juice Agar and incubated at 20 °C for 7 days in darkness. A total of 15 mycelium plugs (6 mm diameter) from the border of the colony were extracted and placed into a 12 cm3 glass flask which contained 1.5% sterile soil extract solution for long-term conservation at 14 °C. The sterile soil extract solution was prepared mixing 100 g of soil with 900 mL distilled water. The mixture was stirred and allowed to stand for 24 h. Subsequently, 50 mL of the supernatant was taken and added to 950 mL of distilled water to be autoclaved.
Phytophthora isolates were also conserved in tubes with Oat Agar medium (72.5 g L−1 oatmeal agar, Sigma Aldrich, Steinheim, Germany) for long-term storage. A single 6 mm-diameter agar disk was placed in each OA tube, incubated at 25 °C until mycelium growth was observed, and then it was sealed with parafilm for conservation at 14 °C.
3. Results
3.1. Symptomatology
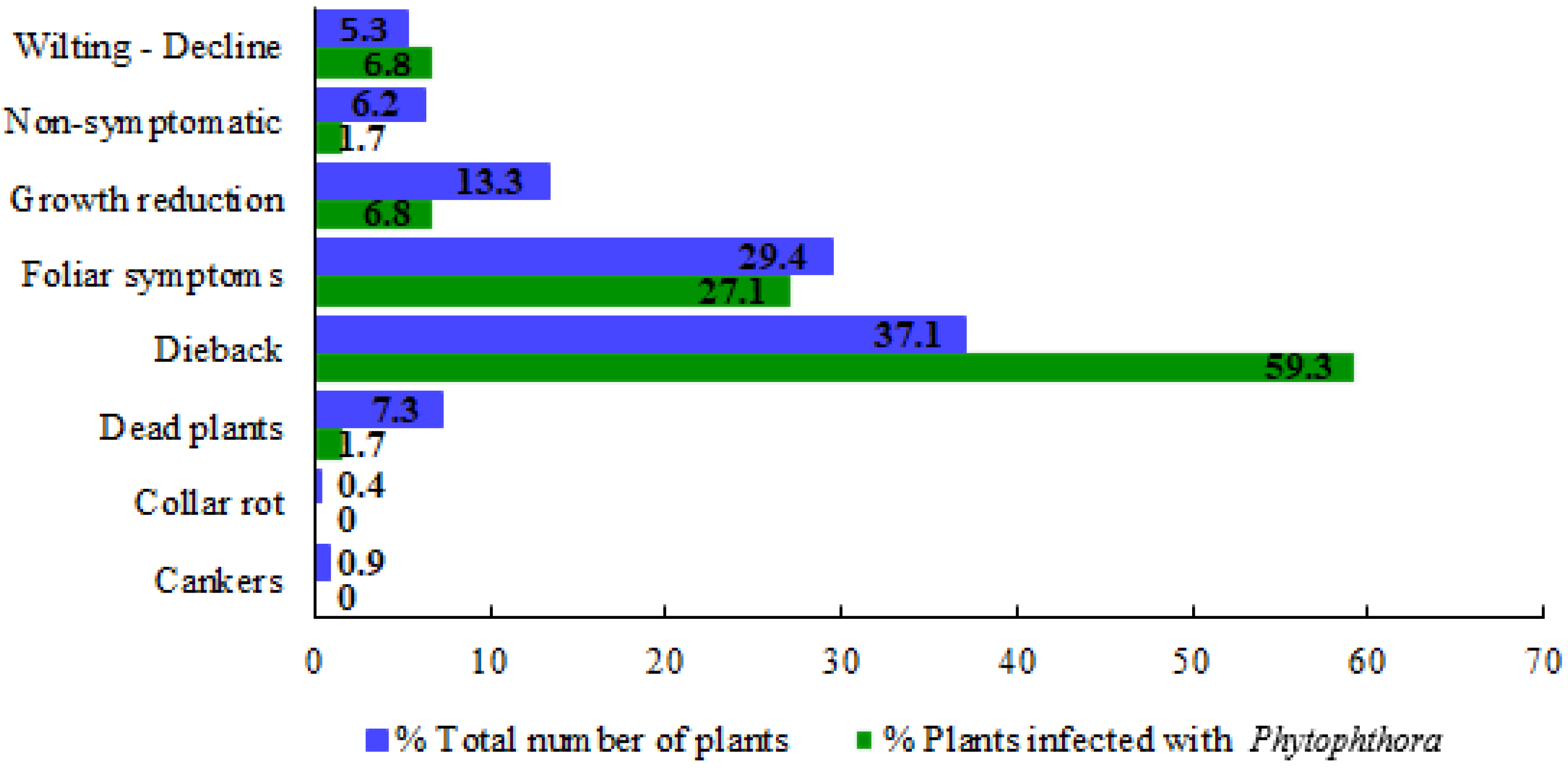
In all nurseries, a broad range of symptoms was observed: cankers (with or without gummosis exudates), collar rot, dead plants, dieback (partial dieback or the whole plant), foliar symptoms (chlorosis, defoliation, leaf spots, irregular shaped blotches in the leaf margins or starting at the leaf apex or petiole, necrotic spots), growth reduction, and wilting (Figure 2). Figure 3 shows the percentage distribution of symptoms observed in the sampled plants collected in the nurseries referred to the total number of plants collected (in blue colour) and to the number of plants on which Phytophthora was isolated (in green colour). The most frequent symptoms among the total number of collected samples were dieback (37.1%), followed by foliar symptoms (29.4%) and growth reduction (13.3%).
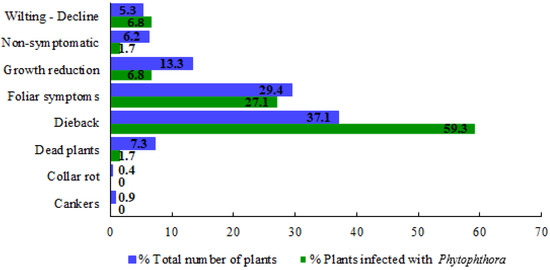
Figure 3.
Symptomatology observed in the sampled nurseries expressed in percentage. Bars in blue colour represent the percentage of samples that present a symptomatology regarding the total number of plants of the survey. Bars in green show the symptomatology associated to Phytophthora expressed in percentage (number of samples regarding to those plants infected by Phytophthora).
A total of 547 samples were collected and oomycetes were identified in 30.7% of the plant samples. The most frequent symptoms observed in samples positive for oomycetes were dieback (43.5%), foliar symptoms (28.6%), and growth reduction (11.3%).
Phytophthora was isolated from 59 plants (Table 2), which means 10.8% of total plant samples collected in this survey. On plants affected by Phytophthora, dieback was the most frequent symptom observed (59.3%), followed by foliar symptoms (27.1%), wilting, and growth reduction (both 6.8%) (Figure 3). In almost all positive Phytophthora plants, the aerial symptomatology corresponded with a damaged root system. Nevertheless, in some plants, the damage was limited to the aerial part, with no visible root symptoms. Furthermore, in only two non-symptomatic plants Phytophthora was isolated.

Table 2.
Phytophthora species isolated from plant tissues, floating leaves from two nursery ponds, and water samples taken from the irrigation system in the surveyed nurseries.
3.2. Phytophthora Species Isolated in the Study
Seventy-one isolates of Phytophthora were recovered from 18 nurseries from the four locations (Table 2). These isolates were obtained from infected tissues (roots) and/or the rhizosphere soil of 547 plant samples belonging to 22 species included in 19 plant genera (Table 2). Thirty-six Phytophthora isolates were isolated from water samples collected in Catalonia region from the irrigation ponds (Table 2).
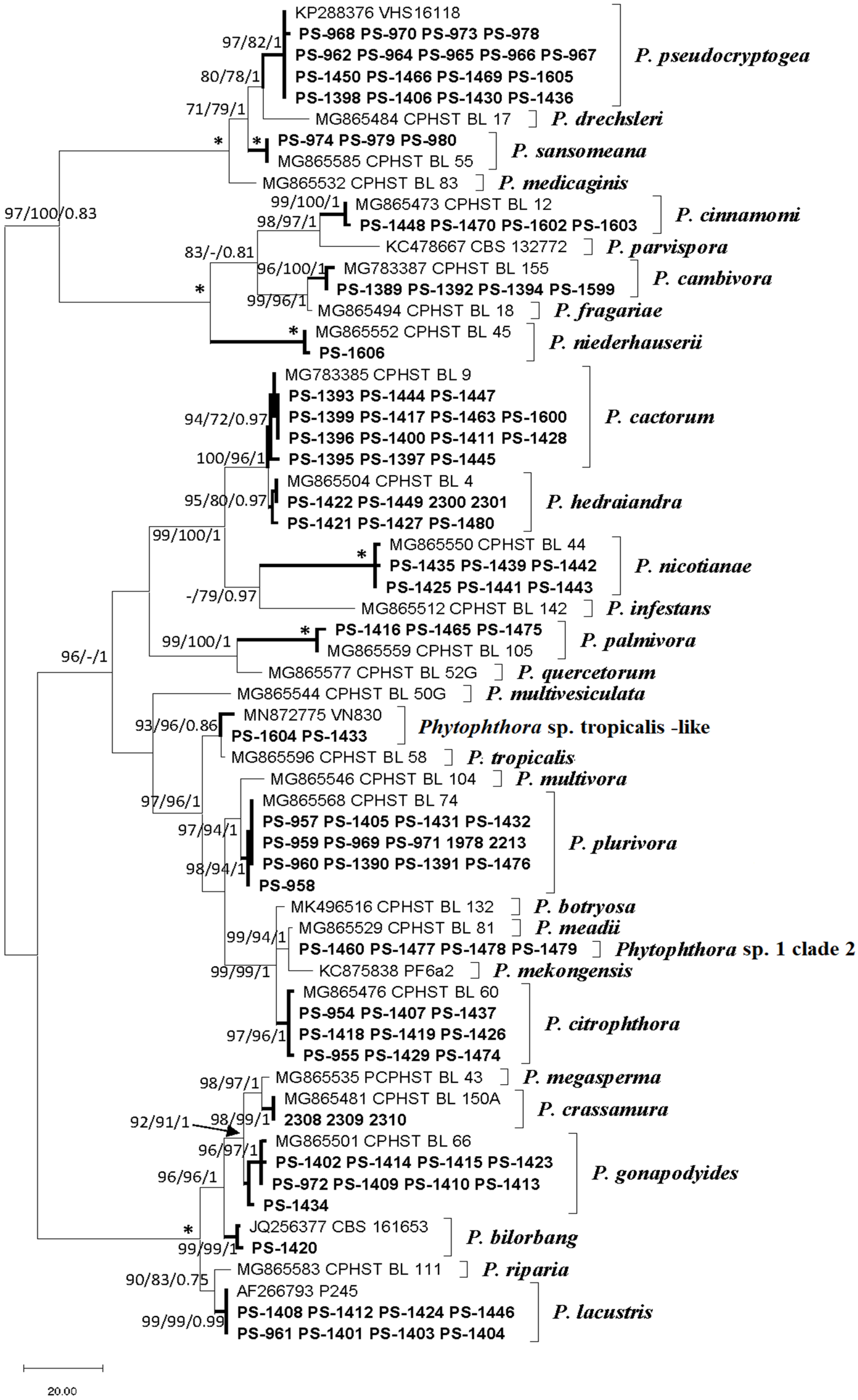
Molecular identification of the isolates revealed the presence of 17 Phytophthora phylotypes (Figure 4). The ITS alignment consisted of 887 positions including gaps. Of these, 568 were constant and 270 were parsimony-informative characters. The heuristic search using MP generated the 10 most parsimonious trees (TL = 603, CI = 0.651, RI = 0.934, RC = 0.608), from which one was selected (Figure 4). The topology of the phylogenetic tree inferred by MP analysis was identical to those obtained by the BI and ML analyses; therefore, only the MP tree is presented with MP and ML bootstrap support values and BI posterior probability scores at the nodes (available on request). Sequences from this study were deposited in Genbank (Table 1).
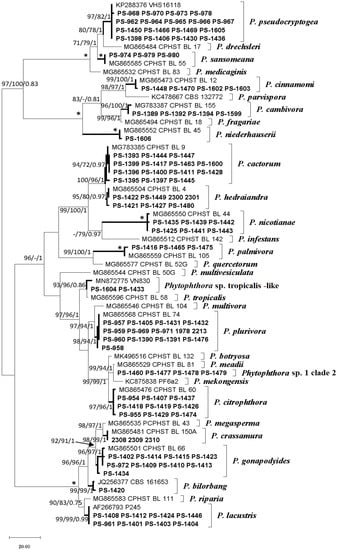
Figure 4.
One of 10 most parsimonious trees resulting from the analysis of the internal transcribed spacer (ITS) sequences from isolates. Support values (Maximum Parsimony bootstrap (MP BS)/Maximum Likelihood bootstrap (ML BS)/Bayesian inference posterior probabilities (BI PP)) are given at the nodes. Bootstrap values less than 70% or posterior probabilities less than 0.7 are indicated with “-”. Branches with an asterisk indicate branch support with MP BS = 100%, ML BS = 100%, and BI PP values = 1.0. The scale bar shows the number of substitutions per site. Species identified in the current study are in bold in the clades. The tree was midpoint rooted.
The species isolated were Phytophthora bilorbang Aghighi and Burgess, Phytophthora cactorum (Lebert and Cohn) J. Schr., Phytophthora cambivora (Petri) Buisman, P. cinnamomi, P. citrophthora, Phytophthora crassamura Scanu, Deidda and Jung, P. hedraiandra, Phytophthora gonapodyides (H.E. Petersen) Buisman, Phytophthora lacustris Brasier, Cacciola, Nechwatal, Jung and Bakonyi, P. nicotianae, P. niederhauserii, Phytophthora palmivora E.J. Butler, Phytophthora plurivora T. Jung and T.I. Burgess, Phytophthora pseudocryptogea Safaiefarahani, Mostowfizadeh, Hardy and Burgess and Phytophthora sansomeana Hansen and Reeser. Two Phytophthora isolates recovered from the roots of Arbutus unedo and Juniperus communis were identified as the informally designated taxon Phytophthora sp. tropicalis-like 2 [52]. There were four Phytophthora isolates that could not be identified to the species level, so they were tentatively named as Phytophthora sp. 1 clade 2.
The ITS of Phytophthora sp. 1 clade 2 was closely related to Phytophthora meadii McRae showing differences in two positions with the ex-type, whereas the COI results placed these isolates close to P. citrophthora. Therefore, these isolates were designated as Phytophthora sp. 1 clade 2.
Among plant and soil samples, P. pseudocryptogea was the species with the highest incidence (21.1%), followed by P. plurivora (15.5%), P. hedraiandra (9.9%), P. citrophthora and P. nicotianae (8.5% each species), P. cactorum, P. cinnamomi, and Phytophthora sp. 1 clade 2 (5.6% each species), P. crassamura and P. sansomeana (4.2% each species), P. gonapodyides, P. palmivora, and Phytophthora sp. tropicalis-like 2 (2.8% each species). Two other species, P. cambivora and P. niederhauserii, had the lowest incidence values (1.4% each species). Some plants were co-infected with more than one Phytophthora species. Mixed infections occurred on Chamaecyparis lawsoniana (P. plurivora–P. psedocryptogea), Citrus sinensis (P. citrophthora–Phytophthora sp. 1 clade 2), Cupressus sempervirens (P. palmivora–P. plurivora), Escallonia sp. (P. citrophthora–P. nicotianae), Juniperus communis (P. gonapodyides–Phytophthora sp. tropicalis-like 2), Pistacia lentiscus (P. nicotianae–P. palmivora), Quercus ilex (P. plurivora–P. pseudocryptogea and P. pseudocryptogea–P. sansomeana), and Rosmarinus officinalis (P. citrophthora–P. nicotianae).
In the aquatic habitats, P. cactorum, P. lacustris, and P. gonapodyides were the most abundant species (26.3%, 21.1%, and 18.4% respectively), followed by P. cambivora, P. citrophthora, and P. plurivora (7.9% each species), P. pseudocryptogea (5.3%). The lowest value of presence in water was 2.6%, shared by P. bilorbang and P. palmivora.
3.3. Pythium and Phytopythium Species Isolated in the Study
In total, 6 Phytopythium species (Pp. chamaehyphon, Pp. helicoides, Pp. litorale, Pp. mercuriale, Pp. montanum, and Pp. vexans) and 11 Pythium species (Py. sterilum, Py. intermedium, Py. attrantheridium, Py. rostratifingens, Py. oopapillum, Py. irregulare, Py. ultimum, Py. undulatum, Py. sylvaticum, Py. Pleroticum, and Py. diclinum) were isolated in the survey.
4. Discussion
This study provides evidence of Phytophthora’s wide spread in ornamental and forest nurseries, since the pathogen was isolated from plant material and water samples in the large majority of surveyed nurseries.
In the surveyed nurseries, the sampled plants showed crown symptoms that could be associated with Phytophthora infection, such as dieback, shoot blight, chlorosis, defoliation, irregular leaf blotches, wilting, and cankers with gummosis. The symptomatology of aerial plant parts was generally associated with root damage such as change in colour, lesions, absence, and/or rot of the feeder roots. This set of observed symptoms agree with the symptomatology described in the literature [9,11,25,27,53,54,55,56]. It should be noted that disease symptoms may be suppressed due to prophylactic fungicide treatments or the natural lag period between root and crown rots and the development of foliar symptoms [26].
Seventy-one Phytophthora isolates from clades 1, 2, 4, 6, 7, and 8 were recovered from 22 species included in 19 plant genera. From some of the plants more than one species of Phytophthora was isolated, revealing mixed infections as in previous nursery surveys [9,10,33,54,57]. Some species were very frequent (P. pseudocryptogea, P. plurivora, P. hedraiandra, P. citrophthora, and P. nicotianae) and others were rare (P. cambivora and P. niederhauserii, as well as the informally designated taxon, Phytophthora sp. tropicalis-like 2).
Four Phytophthora species represented a significant finding for the Spanish nursery sector. This study is the first report of P. crassamura in Spain; Phytophthora crassamura sp. nov. was described by Scanu et al. in Sardinia (Italy) [58] and since then it has been isolated from other hosts in Italy and in California [59,60]. As our isolates were baited from the P. pinea nursery substrate, we cannot state P. pinea as a new P. crassamura host, even the three seedlings showed a highly diminished root system with no secondary feeder roots. This finding suggests that probably P. pinea seedlings are susceptible to P. crassamura. As initially these isolates were misidentified as Phytophthora megasperma Dreschsler, no pathogenicity tests were performed. Phytophthora pseudocryptogea [61] was reported on Quercus ilex in 2018 in different regions of Spain and the present study not only confirms its presence in the nurseries from those regions [62,63] but also it was isolated for the first time in Spain on Chamaecyparis lawsoniana and Yucca rostrata. Moreover, this is the first time P. sansomeana was isolated in Europe and in Q. ilex worldwide. Phytophthora sansomeana was segregated from the P. megasperma complex in 2009 and until now it was only in the United States and in China, from diverse forest and agricultural hosts, such as Douglas-fir nursery seedlings, weeds, and soybean [64,65,66]. Since it is not the first time that the species has been identified in nursery material, its pathogenicity on holm oak should be tested to understand the risk it poses to this fundamental tree species of forest ecosystems and landscapes of Mediterranean Europe. Lastly, two isolates from our study clustered with Phytophthora sp. tropicalis-like 2 described by Jung et al. in 2020 based on ITS blast-assigned identity with the isolate VN830 [52]. This is a provisional first report of Phytophthora sp. tropicalis-like 2 on Arbutus unedo and Juniperus communis.
In previous nursery surveys in Spain, P. cactorum, P. cinnamomi, P. citricola, P. citrophthora, P. cryptogea, Phytophthora drechsleri Tucker, Phytophthora hibernalis Carne, Phytophthora multivora Scott and Jung, P. nicotianae, P. niederhauserii, P. palmivora, P. plurivora, Phytophthora syringae (Kleb.) Kleb., Phytophthora tentaculata Kröber and Marwitz, and P. tropicalis have been reported [9,25,67]. According to Moralejo et al., P. cinnamomi and P. cryptogea (probably P. pseudocryptogea) have escaped from nurseries and are currently spreading in Q. ilex forests, and infect associated shrubs such as Arbutus unedo and Cistus monspeliensis in the lowlands of northern Mallorca [25]. Other studies in nurseries worldwide recovered almost the same species which demonstrates that global nursery trade is the main pathway for Phytophthora dispersion [6,9,11,25,26,27,29,33,53,54,56,57,60,68,69,70,71,72].
In Europe, a very extensive analysis of incidence of Phytophthora spp. was conducted, based on data from 23 countries between 1972 and 2013, in order to study the pathway of Phytophthora from nurseries into natural, semi-natural, and horticultural ecosystems [11]. From nursery plant material, 49 Phytophthora taxa were identified, being P. plurivora, P. cinnamomi, P. cactorum, P. nicotianae, P. ramorum and P. citrophthora the most commonly sampled species, considered all alien pathogens in Europe. From forest and landscape plantings, 56 Phytophthora taxa were recovered, and invasive species with wide host ranges, such as P. plurivora, P. cinnamomi, P. nicotianae, P. cryptogea, and P. cactorum, were the most common. This large-scale study demonstrates that Phytophthora infect nursery stock across Europe and the spread of these pathogens through infested nursery stock into natural ecosystems.
In California, Sims et al. [60] reported P. cactorum as the most frequent species in restoration nurseries but P. hedraiandra, P. multivora, P. occultans, P. crassamura, P. thermophila, and P. pseudocryptogea were also isolated. Rooney-Latham et al. [72] reported P. tentaculata, P. cactorum, P. cryptogea complex, P. cambivora, P. cinnamomi, P. citricola, P. hedraiandra, P. megasperma, P. multivora, P. nicotianae, P. niederhauserii, P. parvispora, P. pini, P. plurivora, and P. riparia in Californian nurseries.
Regarding water surveys, the nine species reported in this study once again agree with Phytophthora spp. recovered from irrigation water, waterways, or riparian ecosystems published in other studies [73,74,75,76,77,78,79,80,81,82]. It is not surprising that as Phytophthora is adapted for aquatic dispersal, multiple Phytophthora spp. have been recovered from waterways or irrigation waters. Indeed, several novel species have been detected in the last decade from water fluxes or riparian ecosystems such as Phytophthora lateralis (clade 8) causing Chamaecyparis lawsoniana decline [83], Phytophthora alni (clade 7) causing Alnus spp. decline [84], and P. ramorum (clade 8) causing sudden oak death on Quercus spp. and Notholithocarpus densiflorus [17]. Detection of Phytophthora taxa belonging to clade 6 has increased in recent years as riparian systems have grown in attention [13,77,85,86]. Phytophthora spp. from clade 6 are thought to be adapted to survive in rivers due to their rapid colonisation of leaves and plant debris [87,88]. Jung et al. consider the possibility that species from clade 6 are probable saprotrophs, as these Phytophthora spp. depend on their ability to rapidly colonise fresh plant material (such as fallen leaves) in order to outcompete other saprotrophic organisms [88]. There is a significant gap in understanding waterborne plant pathogens, particularly in open irrigation systems [78,82,89].
Among other plant pathogens that were also isolated, the most important genera were Pythium and Phytopythium. The percentage of recovered Pythium and Phytopythium species highlights the importance of sanitary measures in the nursery industry. Pythium and Phytopythium are also among the most frequent plant pathogens in nurseries (seed rot and damping-off), Pythium species require free water to complete their cycle but compared with Phytophthora, they have a quicker development and growth. Most Pythium and Phytopythium species used to be considered saprotrophs but nowadays the pathogenicity of some species has been demonstrated [90,91,92,93].
The impact that plant pathogens can have on the plant industry can extend into billions of dollars, but the worst is the environmental risk, which biodiversity, forestry, and agriculture are currently experiencing [11,33,94,95]. Biosecurity needs to be the cornerstone of the global nursery trade to avoid the possibility of Phytophthora spp. spreading to new habitats where they may be exposed to compatible species and potentially form new hybrids [29,96,97].
The exclusion of nursery pathogens from forested areas is a critical issue for forest health [60,98]. Monitoring the pathogen zone, restricting vehicle movement from infested to uninfested areas, cleaning vehicles before entering uninfested areas, preventing infested and uninfested soil mixing, preventing water draining from infested to uninfested areas, and education of public and forestry workers are some of the exclusion measures that should come into full force and effect [60,98].
A high priority should be placed on the production of pathogen-free propagating material by appropriate sanitary practices [99]. The microbial community plays an important role in the protective effect against Oomycetes. Organic soils in the form of compost have long been found to supress a number of Phytophthora and Pythium spp. [99]. Nursery sanitation measures such as the following ought be implemented in all nurseries and garden centres: use of new seedling containers, container media pasteurised; irrigation water Phytophthora-free (sand filters or chlorine interventions); water splash kept off leaves and wetness time minimised; containers kept off the ground; suppressive composts or fungicides avoided; sustained heat treatment to kill resting structures in plant or soil material via composting, solarisation, oven treatment or autoclaving, heating installation in greenhouses, correct aeration between seedling benches and plantations, pH control (a low pH [3.5–4.5] to avoid spore liberation), moderate nitrogen fertilisation, and routine tool disinfection [98].
It has long been known that nursery stock is the most common pathway for the introduction of new Phytophthora species into natural habitats worldwide [11]. Supplying healthy plants should be the fundamental principle of nursery production. Implementing molecular detection through the most recent, effective, and specific assays for Phytophthora [33,62,100] will facilitate early detection and the application of control measures to minimise the risk of spread through plant trade.
5. Conclusions
This study confirms the widespread presence of pathogens in plant nursery stocks and the risk posed by the plants for the planting pathway. Seventeen Phytophthora phylotypes were isolated from tissues and rizosphere soil of 22 plant species in 19 genera and from water samples. The presence of Phytophthora mixed infections is noteworthy. It is also relevant reporting, for the first time, the presence of P. crassamura, P. pseudocryptogea, P. sansomeana, and Phytophthora sp. tropicalis-like 2 in the Spanish nursery industry. The need for preventing Phytophthora dispersion to natural ecosystems must be translated in implementing new policies at the global scale. Good biosecurity practices in nurseries and early detection are critical to mitigate the risk of spread of these pathogens.
Author Contributions
B.M.-S. conducted field sampling, performed experimental work and all data analysis, discussed the results, and wrote the paper. M.L. performed the molecular data analysis. A.P.-S. conducted field sampling and revised the manuscript. P.A.-C. conducted field sampling and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Economía y Competitividad, Spain (grant number AGL2011-30438-C02-01) and Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Spain (grant number EUPHRESCO project 266505-ERA22-CEP-UPV).
Acknowledgments
Our thanks to Tamara Corcobado, Alexandra Puértolas and Alejandro Solla for collecting some of the analyzed samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parke, J.L.; Knaus, B.J.; Fieland, V.J.; Lewis, C.; Grünwald, N.J. Phytophthora community structure analyses in Oregon nurseries inform systems approaches to disease management. Phytopathology 2004, 104, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Hyam, L. Plant biosecurity—The way ahead. Farm Policy J. 2008, 5, 47–57. [Google Scholar]
- Tremblay, É.D.; Duceppe, M.O.; Bérubé, J.A.; Kimoto, T.; Lemieux, C.; Bilodeau, G.J. Screening for exotic forest pathogens to increase survey capacity using metagenomics. Phytopathology 2018, 108, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M.; Jones, R.K. Etiology of Rhododendron dieback caused by four species of Phytophthora. Plant Dis. 1980, 64, 687–691. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996; 562p, ISBN 0-89054-212-0. [Google Scholar]
- Ferguson, A.J.; Jeffers, S.N. Detecting multiple species of Phytophthora in container mixes from ornamental crop nurseries. Plant Dis. 1999, 83, 1129–1136. [Google Scholar] [CrossRef]
- Werres, S.; Marwitz, R.; Man In’t Veld, W.A.; de Cock, A.W.; Bonants, P.J.M.; de Weerdt, M.; Themann, K.; Ilieva, E.; Baayen, R.P. Phytophthora ramorum sp. nov., a new pathogen on Rhododendron and Viburnum. Mycol. Res. 2001, 105, 1155–1165. [Google Scholar] [CrossRef]
- Osterbauer, N.K.; Griesbach, J.A.; Hedberg, J. Surveying for and eradicating Phytophthora ramorum in agricultural commodities. Plant Health Prog. 2004, 5, 8. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; Mora-Sala, B.; León, M.; García-Jiménez, J.; Abad-Campos, P. Enfermedades causadas por Phytophthora en viveros de plantas ornamentales. Bol. Sanid. Veg. Plagas 2012, 38, 143–156. [Google Scholar]
- Panabières, F.; Ali, G.S.; Allagui, M.B.; Dalio, R.J.D.; Gudmestad, N.C.; Kuhn, M.L.; Guharoy, S.; Schena, L.; Zampounis, A. Phytophthora nicotianae diseases worldwide: New knowledge of a long-recognised pathogen. Phytopathol. Mediterr. 2016, 55, 20–40. [Google Scholar]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef]
- Lévesque, C.A. Fifty years of oomycetes—From consolidation to evolutionary and genomic exploration. Fungal Divers. 2011, 50, 35–46. [Google Scholar] [CrossRef]
- Kroon, L.P.N.M.; Brouwer, H.; de Cock, A.W.; Govers, F. The genus Phytophthora anno 2012. Phytopathology 2012, 102, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.N.; Abad, Z.G.; Balci, Y.; Ivors, K. Identification and detection of Phytophthora: Reviewing our progress, identifying our needs. Plant Dis. 2012, 96, 1080–1103. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Robredo, F.; Ferraz, J.F.P. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathol. 1993, 42, 140–145. [Google Scholar] [CrossRef]
- Hüberli, D.; Tommerup, I.C.; Dobrowolski, M.P.; Calver, M.C.; Hardy, G.E.S.J. Phenotypic variation in a clonal lineage of two Phytophthora cinnamomi populations from Western Australia. Mycol. Res. 2001, 105, 1053–1064. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef]
- Brasier, C.M.; Denman, S.; Brown, A.; Webber, J. Sudden Oak Death (Phytophthora ramorum) discovered on trees in Europe. Mycol. Res. 2004, 108, 1107–1110. [Google Scholar] [CrossRef]
- Camilo-Alves, C.S.P.; da Clara, M.I.E.; de Almeida Ribeiro, N.M.C. Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: A review. Eur. J. For. Res. 2013, 132, 411–432. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Leboldus, J.M.; Hamelin, R.C. Ecology and evolution of the Sudden Oak Death pathogen Phytophthora ramorum. Annu. Rev. Phytopathol. 2019, 57, 301–321. [Google Scholar] [CrossRef]
- Serrano, M.S.; Garbelotto, M. Differential response of four Californian native plants to worldwide Phytophthora cinnamomi genotypes: Implications for the modeling of disease spread in California. Eur. J. Plant Pathol. 2020, 156, 851–866. [Google Scholar] [CrossRef]
- Tooley, P.W. Susceptibility of selected Ericaceous ornamental host species in Phytophthora ramorum. Plant Dis. 2004, 88, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Ivors, K.; Garbelotto, M.; Vries, I.D.E.; Ruyter-Spira, C.; Hekkert, B.T.; Rosenzweig, N.; Bonants, P. Microsatellite markers identify three lineages of Phytophthora ramorum in US nurseries, yet single lineages in US forest and European nursery populations. Mol. Ecol. 2006, 15, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.E.L.; Schena, L.; Cacciola, S.O. Tools to detect, identify and monitor Phytophthora species in natural ecosystems. J. Plant Pathol. 2007, 89, 13–28. [Google Scholar]
- Moralejo, E.; Pérez-Sierra, A.M.; Álvarez, L.A.; Belbahri, L.; Lefort, F.; Descals, E. Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathol. 2009, 58, 100–110. [Google Scholar] [CrossRef]
- Leonberger, A.J.; Speers, C.; Ruhl, G.; Creswell, T.; Beckerman, J.L. A survey of Phytophthora spp. in Midwest nurseries, greenhouses, and landscapes. Plant Dis. 2013, 97, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Abad, Z.G.; Abad, J.A.; Cacciola, S.O.; Pane, A.; Faedda, R.; Moralejo, E.; Pérez-Sierra, A.; Abad-Campos, P.; Alvarez-Bernaola, L.A.; Bakonyi, J.; et al. Phytophthora niederhauserii sp. nov., a polyphagous species associated with ornamentals, fruit trees and native plants in 13 countries. Mycologia 2014, 106, 431–447. [Google Scholar] [CrossRef]
- Weste, G. Population dynamics and survival of Phytophthora. In Phytophthora: Its Biology, Taxonomy, Ecology and Pathology; Erwin, D.C., Barnticki-Garcia, S., Tsao, P.H., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1983; pp. 237–257. [Google Scholar]
- Brasier, C.M. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- Eschen, R.; Rigaux, L.; Sukovata, L.; Vettraino, A.M.; Marzano, M.; Grégoire, J.C. Phytosanitary inspection of woody plants for planting at European Union entry points: A practical enquiry. Biol. Invasions 2015, 17, 2403–2413. [Google Scholar] [CrossRef]
- Santini, A.; Ghelardini, L.; de Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2012, 197, 238–250. [Google Scholar] [CrossRef]
- Jung, T.; Horta Jung, M.; Webber, J.F.; Kageyama, K.; Hieno, A.; Masuya, H.; Uematsu, S.; Pérez-Sierra, A.; Harris, A.R.; Forster, J.; et al. The destructive tree pathogen Phytophthora ramorum originates from the laurosilva forests of East Asia. J. Fungi 2021, 7, 226. [Google Scholar] [CrossRef]
- Prigigallo, M.I.; Mosca, S.; Cacciola, S.O.; Cooke, D.E.L.; Schena, L. Molecular analysis of Phytophthora diversity in nursery-grown ornamental and fruit plants. Plant Pathol. 2015, 64, 1308–1319. [Google Scholar] [CrossRef]
- Hulbert, J.M.; Agne, M.C.; Burgess, T.I.; Roets, F.; Wingfield, M.J. Urban environments provide opportunities for early detections of Phytophthora invasions. Biol. Invasions 2017, 19, 3629–3644. [Google Scholar] [CrossRef]
- Peterson, E.; Hansen, E.; Hulbert, J. Source or sink? The role of soil and water borne inoculum in the dispersal of Phytophthora ramorum in Oregon tanoak forests. For. Ecol. Manag. 2014, 322, 48–57. [Google Scholar] [CrossRef]
- Jones, D.R.; Baker, R.H.A. Introductions of non-native plant pathogens into Great Britain, 1970–2004. Plant Pathol. 2007, 56, 891–910. [Google Scholar] [CrossRef]
- Reichard, S.H.; White, P. Horticulture as a pathway of invasive plant introductions in the United States. BioScience 2001, 51, 103–113. [Google Scholar] [CrossRef]
- Harris, A.R.; Webber, J.F. Insights into the potential host range of Phytophthora foliorum. For. Pathol. 2019, 49, e12556. [Google Scholar] [CrossRef]
- Moralejo, E.; Werres, S. First Report of Phytophthora ramorum on Rhododendron sp. in Spain. Plant Dis. 2002, 86, 1052. [Google Scholar] [CrossRef]
- Varela, C.P.; Vázquez, J.P.M.; Casal, O.A. First Report of Phytophthora ramorum on Camellia japonica in Spain. Plant Dis. 2003, 87, 1396. [Google Scholar] [CrossRef]
- Sims, L.L.; Garbelotto, M. Phytophthora species repeatedly introduced in Northern California through restoration projects can spread into adjacent sites. Biol. Invasions 2021, 23, 2173–2190. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.M.; León, M.; Álvarez, L.A.; Alaniz, S.; Berbegal, M.; García-Jiménez, J.; Abad-Campos, P. Outbreak of a new Phytophthora sp. associated with severe decline of almond trees in Eastern Spain. Plant Dis. 2010, 94, 534–541. [Google Scholar] [CrossRef]
- Jeffers, S.N.; Aldwinckle, H.S. Enhancing detection of Phytophthora cactorum in naturally infested soil. Phytopathology 1987, 77, 1475–1482. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, S.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related Oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Robideau, G.P.; de Cock, A.W.A.M.; Coffey, M.D.; Voglmayr, H.; Brouwer, H.; Bala, K.; Chitty, D.W.; Désaulniers, N.; Eggertson, Q.A.; Gachon, C.M.M.; et al. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol. Ecol. Resour. 2011, 11, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Alix, B.; Boubacar, D.A.; Vladimir, M. T-REX: A web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res. 2012, 40, W573–W579. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Jung, T.; Scanu, B.; Brasier, C.M.; Webber, J.; Milenković, I.; Corcobado, T.; Tomšovský, M.; Pánek, m.; Bakonyi, J.; Maia, C.; et al. A survey in natural forest ecosystems of Vietnam reveals high diversity of both new and described Phytophthora taxa including P. ramorum. Forests 2020, 11, 93. [Google Scholar] [CrossRef]
- Cacciola, S.O.; Pane, A.; Polizzi, G. Due specie di Phytophthora agenti di marciume radicale e del colletto del rosmarino. Inf. Fitopatol. 1997, 47, 35–42. [Google Scholar]
- Schwingle, B.W.; Smith, J.A.; Blanchette, R.A. Phytophthora species associated with diseased woody ornamentals in Minnesota nurseries. Plant Dis. 2007, 91, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, N.J.; Goss, E.M.; Press, C.M. Phytophthora ramorum: A pathogen with a remarkably wide host range causing Sudden Oak Death on oaks and ramorum blight on woody ornamentals. Mol. Plant Pathol. 2008, 9, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Bienapfl, J.C.; Balci, Y. Movement of Phytophthora spp. in Maryland’s nursery trade. Plant Dis. 2014, 98, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, S.O.; Polizzi, G. Phytophthora nicotianae e P. palmivora agenti di marciume radicale e del colletto del Pittosporo. Inf. Fitopatol. 1996, 46, 25–29. [Google Scholar]
- Scanu, B.; Linaldeddu, B.T.; Deidda, A.; Jung, T. Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 2015, 10, e0143234. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; La Spada, F.; Pane, A.; Aloi, F.; Evoli, M.; Horta Jung, M.; Scanu, B.; Faedda, R.; Rizza, C.; Puglisi, I. Diversity and Distribution of Phytophthora Species in Protected Natural Areas in Sicily. Forests 2019, 10, 259. [Google Scholar] [CrossRef]
- Sims, L.; Tjosvold, S.; Chambers, D.; Garbelotto, M. Control of Phytophthora species in plant stock for habitat restoration through best management practices. Plant Pathol. 2019, 68, 196–204. [Google Scholar] [CrossRef]
- Safaiefarahani, B.; Mostowfizadeh-Ghalamfarsa, R.; Hardy, G.E.S.J.; Burgess, T.I. Re-evaluation of Phytophthora cryptogea species complex and the description of a new species, Phytophthtora pseudocryptogea sp. nov. Mycol. Prog. 2015, 14, 108. [Google Scholar] [CrossRef]
- Mora-Sala, B.; Berbegal, M.; Abad-Campos, P. The Use of qPCR Reveals a High Frequency of Phytophthora quercina in Two Spanish Holm Oak Areas. Forests 2018, 9, 697. [Google Scholar] [CrossRef]
- Mora-Sala, B.; Abad-Campos, P.; Berbegal, M. Response of Quercus ilex seedlings to Phytophthora spp. root infection in a soil infestation test. Eur. J. Plant Pathol. 2019, 154, 215–225. [Google Scholar] [CrossRef]
- Hansen, E.M.; Wilcox, W.F.; Reeser, P.W.; Sutton, W. Phytophthora rosacearum and P. sansomeana, new species segregated from the Phytophthora megasperma “complex”. Mycologia 2009, 101, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.H.; Gao, F.; Li, G.Y.; Wang, H.; Zheng, X.B.; Wang, Y.C. First report of root rot caused by Phytophthora sansomeana on soybean in China. Plant Dis. 2010, 94, 378. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.F.; Hwang, S.F.; Ahmed, H.U.; Fu, H.; Zhou, Q.; Strelkov, S.E.; Turnbull, G.D. First report of Phytophthora sansomeana causing root rot in field pea in Alberta, Canada. Crop Prot. 2017, 101, 1–4. [Google Scholar] [CrossRef]
- Sanchez, M.E.; Andicoberry, S.; Trapero, A. Pathogenicity of three Phytophthora spp. causing late seedling rot of Quercus ilex ssp. ballota. For. Pathol. 2005, 35, 115–125. [Google Scholar] [CrossRef]
- Donahoo, R.S.; Lamour, K.H. Characterization of Phytophthora species from leaves of nursery woody ornamentals in Tennessee. Hort. Science 2008, 43, 1833–1837. [Google Scholar] [CrossRef]
- Olson, H.A.; Benson, D.M. Characterization of Phytophthora spp. on floriculture crops in North Carolina. Plant Dis. 2011, 95, 1013–1020. [Google Scholar] [CrossRef]
- Prospero, S.; Vercauteren, A.; Heungens, K.; Belbahri, L.; Rigling, D. Phytophthora diversity and the population structure of Phytophthora ramorum in Swiss ornamental nurseries. Plant Pathol. 2013, 62, 1063–1071. [Google Scholar] [CrossRef]
- Prigigallo, M.I.; Abdelfattah, A.; Cacciola, S.O.; Faedda, R.; Sanzani, S.M.; Cooke, D.E.L.; Schena, L. Metabarcoding analysis of Phytophthora diversity using genus-specific primers and 454 pyrosequencing. Phytopathology 2016, 106, 305–313. [Google Scholar] [CrossRef]
- Rooney-Latham, S.; Blomquist, C.L.; Kosta, K.L.; Gou, Y.Y.; Woods, P.W. Phytophthora species are common on nursery stock grown for restoration and revegetation purposes in California. Plant Dis. 2019, 103, 448–455. [Google Scholar] [CrossRef]
- Hwang, J.; Oak, S.W.; Jeffers, S.N. Detecting Phytophthora ramorum and other species of Phytophthora in streams in natural ecosystems using baiting and filtration methods. In Proceedings of the Sudden Oak Death Third Science Symposium, Santa Rosa, CA, USA, 5–9 March 2007; Frankel, S.J., Kliejunas, J.T., Palmieri, K.M., Eds.; US Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2008. [Google Scholar]
- Reeser, P.W.; Sutton, W.; Hansen, E.M.; Remigi, P.; Adams, G.C. Phytophthora species in forest streams in Oregon and Alaska. Mycologia 2011, 103, 22–35. [Google Scholar] [CrossRef]
- Huai, W.X.; Tian, G.; Hansen, E.M.; Zhao, W.X.; Goheen, E.M.; Grünwald, N.J.; Cheng, C. Identification of Phytophthora species baited and isolated from forest soil and streams in northwestern Yunnan province, China. For. Pathol. 2013, 43, 87–103. [Google Scholar] [CrossRef]
- Hüberli, D.; Hardy, G.E.S.J.; White, D.; Williams, N.; Burgess, T.I. Fishing for Phytophthora from Western Australia’s waterways: A distribution and diversity survey. Australas. Plant Pathol. 2013, 42, 251–260. [Google Scholar] [CrossRef]
- Nagel, J.H.; Gryzenhout, M.; Slippers, B.; Wingfield, M.J.; Hardy, G.E.S.J.; Stukely, M.J.C.; Burgess, T.I. Characterization of Phytophthora hybrids from ITS clade 6 associated with riparian ecosystems in South Africa and Australia. Fungal Biol. 2013, 117, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Zappia, R.E.; Hüberli, D.; Hardy, G.E.S.J.; Bayliss, K.L. Fungi and oomycetes in open irrigation systems: Knowledge gaps and biosecurity implications. Plant Pathol. 2014, 63, 961–972. [Google Scholar] [CrossRef]
- Català, S.; Pérez-Sierra, A.; Abad-Campos, P. The use of genus-specific amplicon pyrosequencing to assess Phytophthora species diversity using eDNA from soil and water in northern Spain. PLoS ONE 2015, 10, e0119311. [Google Scholar] [CrossRef]
- Migliorini, D.; Ghelardini, L.; Tondini, E.; Luchi, N.; Santini, A. The potential of symptomless potted plants for carrying invasive soilborne plant pathogens. Divers. Distrib. 2015, 21, 1218–1229. [Google Scholar] [CrossRef]
- Sims, L.L.; Sutton, W.; Reeser, P.; Hansen, E.M. The Phytophthora species assemblage and diversity in riparian alder ecosystems of Western Oregon, USA. Mycologia 2015, 107, 889–902. [Google Scholar] [CrossRef]
- Redondo, M.A.; Boberg, J.; Stenlid, J.; Oliva, J. Contrasting distribution patterns between aquatic and terrestrial Phytophthora species along a climatic gradient are linked to functional traits. ISME J. 2018, 12, 2967–2980. [Google Scholar] [CrossRef]
- Hansen, E.M.; Goheen, D.J.; Jules, E.S.; Ullian, B. Managing Port-Orford-Cedar and the introduced pathogen Phytophthora lateralis. Plant Dis. 2000, 84, 4–14. [Google Scholar] [CrossRef]
- Brasier, C.M.; Kirk, S.A.; Delcan, J.; Cooke, D.E.L.; Jung, T.; Man In’t Veld, W.A. Phytophthora alni sp. nov. and its variants: Designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycol. Res. 2004, 108, 1172–1184. [Google Scholar] [CrossRef]
- Crous, P.W.; Summerell, B.A.; Shivas, R.G.; Burgess, T.I.; de Cock, C.A.; Dreyer, L.L.; Granke, L.L.; Guest, D.I.; Hardy, G.E.S.J.; Hausbeck, M.K.; et al. Fungal planet description sheets: 107–127. Persoonia 2012, 28, 138–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tyler, B.M.; Hong, C. An expanded phylogeny for the genus Phytophthora. IMA Fungus 2017, 8, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Cooke, D.E.L.; Duncan, J.M.; Hansen, E.M. Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides-P. megasperma ITS clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycol. Res. 2003, 107, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.J.; White, D.; Paap, T.; Dunstan, W.A.; Burgess, T.I. Multiple new Phytophthora species from ITS clade 6 associated with natural ecosystems in Australia: Evolutionary and ecological implications. Persoonia 2011, 26, 13–39. [Google Scholar] [CrossRef]
- Guha Roy, S.; Grünwald, N.J. The plant destroyer genus Phytophthora in the 21st century. Rev. Plant Pathol. 2012, 6, 388–412. [Google Scholar]
- Jung, T.; Blaschke, H.; Neumann, P. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. Eur. J. Forest Pathol. 1996, 26, 253–272. [Google Scholar] [CrossRef]
- Romero, M.A.; Sánchez, J.E.; Jiménez, J.J.; Belbahri, L.; Trapero, A.; Lefort, F.; Sánchez, M.E. New Pythium taxa causing root rot in Mediterranean Quercus species in southwest Spain and Portugal. J. Phytopathol. 2007, 115, 289–295. [Google Scholar] [CrossRef]
- Ivors, K.L.; Abad, Z.G.; Benson, D.M. Evaluating the pathogenicity of Pythium vexans isolates from Fraser fir in North Carolina. Plant Health Prog. 2008, 9, 8. [Google Scholar] [CrossRef]
- Weiland, J.E.; Beck, B.R.; Davis, A. Pathogenicity and virulence of Pythium species obtained from forest nursery soils on Douglas-fir seedlings. Plant Dis. 2013, 97, 744–748. [Google Scholar] [CrossRef]
- Yang, X.; Balci, Y.; Brazee, N.J.; Loyd, A.L.; Hong, C.X. A unique species in Phytophthora clade 10, Phytophthora intercalaris sp. nov., recovered from stream and irrigation water in the eastern USA. Int. J. Syst. Evol. Microbiol. 2016, 66, 845–855. [Google Scholar] [CrossRef]
- Li, D.W.; Schultes, N.P.; LaMondia, J.A.; Cowles, R.S. Phytophthora abietivora, a new species isolated from diseased Christmas trees in Connecticut, U.S.A. Plant Dis. 2019, 103, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M. The rise of the hybrid fungi. Nature 2000, 405, 134–135. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.A.; Williams, N.; Hardy, G.E.S.J. Detecting Phytophthora. Crit. Rev. Microbiol. 2009, 35, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, P.; Nicolotti, G. Infectious Forest Diseases; CAB International: Wallingford, UK, 2013; 704p. [Google Scholar]
- Gullino, M.L.; Garibaldi, A. Critical aspects in management of fungal diseases of ornamental plants and directions in research. Phytopathol. Mediterr. 2007, 46, 135–149. [Google Scholar]
- Mora-Sala, B.; Gramaje, D.; Abad-Campos, P.; Berbegal, M. Diversity of Phytophthora species associated with Quercus ilex L. in three Spanish regions evaluated by NGS. Forests 2019, 10, 979. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).