Rabies Virus Seroprevalence among Dogs in Limpopo National Park and the Phylogenetic Analyses of Rabies Viruses in Mozambique
Abstract
:1. Introduction
2. Material and Methods
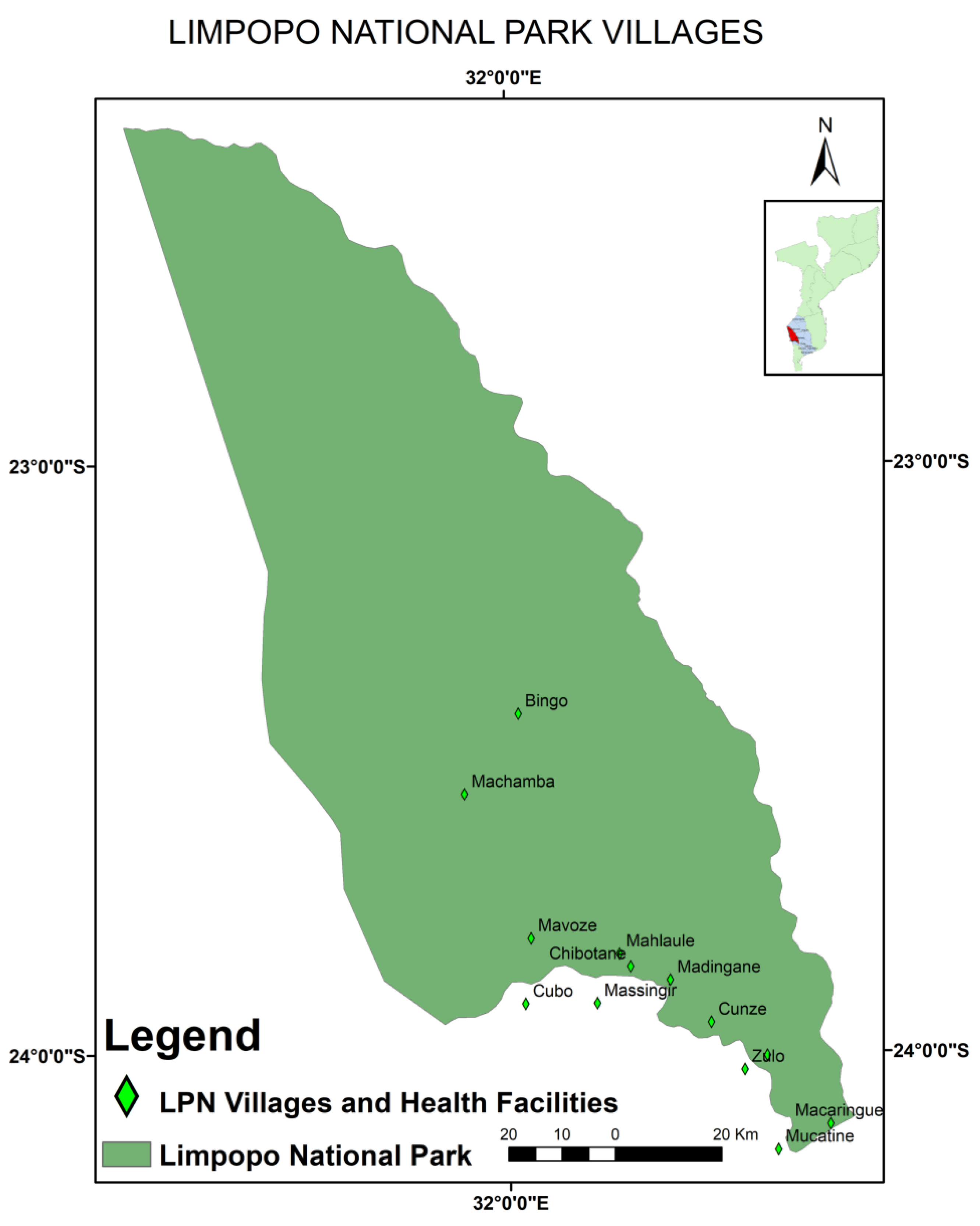
2.1. Study Area and Study Design
2.2. Sampling and Source of the Samples
2.3. Viruses for Molecular Characterisation
2.4. Specimen Processing
2.4.1. Blood Processing and ELISA
2.4.2. Lyssavirus Rabies Detection and Sequencing
2.4.3. Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Seroprevalence of Rabies Virus
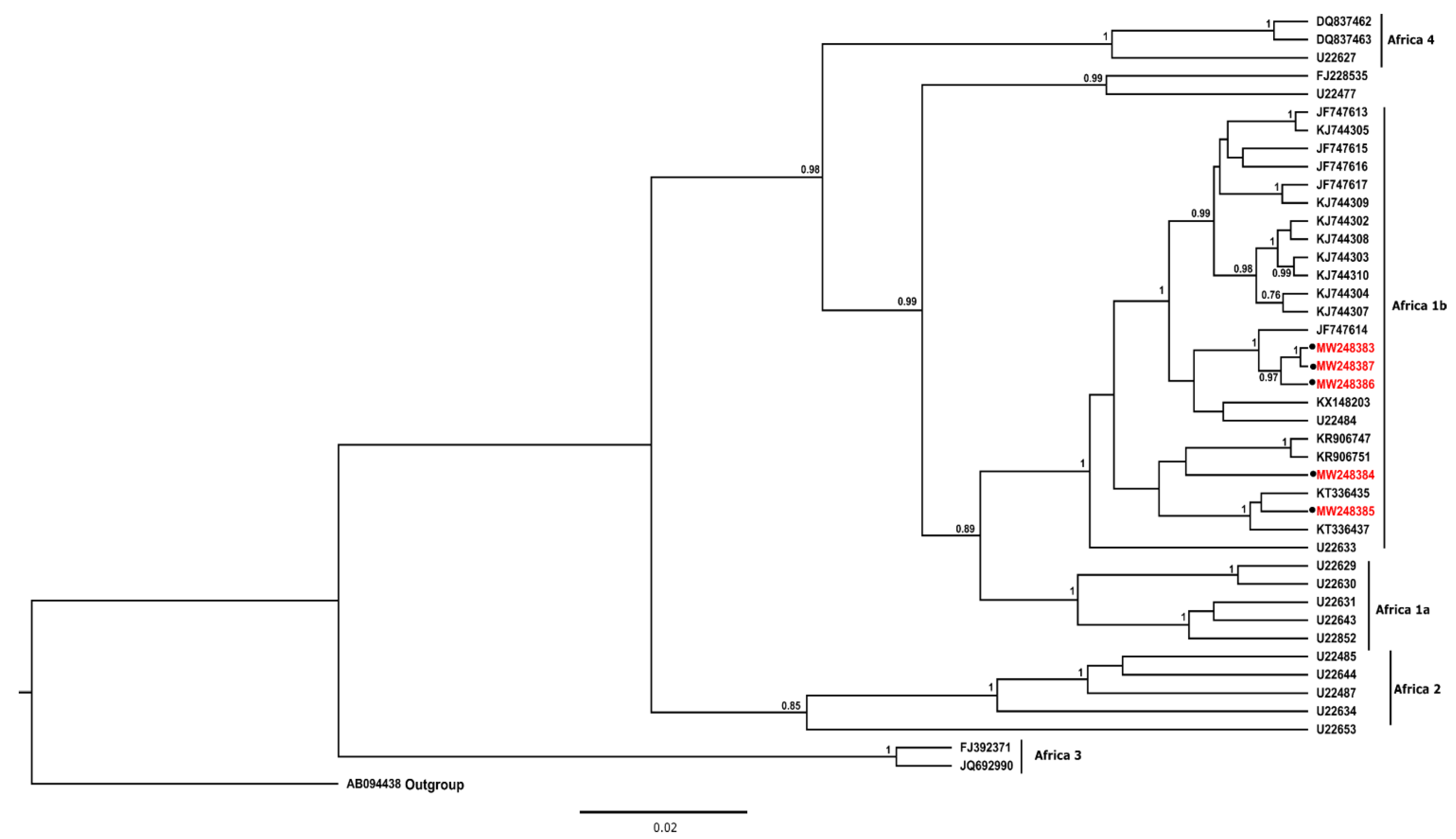
3.2. Phylogenetic Analysis
4. Discussion and Conclusions
5. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICTV. Rhabdoviridae. Available online: https://ictv.global/report/chapter/rhabdoviridae/rhabdoviridae/lyssavirus (accessed on 24 July 2022).
- Singh, M.P.; Goyal, K.; Majumdar, M.; Ratho, R.K. Prevalence of rabies antibodies in street and household dogs in Chandigarh, India. Trop. Anim. Health Prod. 2011, 43, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Horton, D.L.; Freuling, C.; Müller, T.; Fooks, A.R. Control and prevention of canine rabies: The need for building laboratory-based surveillance capacity. Antivir. Res. 2013, 98, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Dodet, B.; Tejiokem, M.C.; Aguemon, A.R.; Bourhy, H. Human rabies deaths in Africa: Breaking the cycle of indifference. Int. Health 2015, 7, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Travassos Dias, M.P.R.; Rodrigues, F. Rabies in Mozambique. Country Report. In Proceedings of the SEARG Meeting, Ezulwini, Swaziland, 12–15 May 2003; pp. 39–41. [Google Scholar]
- Nel, L.H. Discrepancies in data reporting for rabies, Africa. Emerg. Infect. Dis. 2013, 19, 529–533. [Google Scholar] [CrossRef]
- Salomao, C.; Nacima, A.; Cuamba, L.; Gujral, L.; Amiel, O.; Baltazar, C.; Cliff, J.; Gudo, E.S. Epidemiology, clinical features and risk factors for human rabies and animal bites during an outbreak of rabies in Maputo and Matola cities, Mozambique, 2014: Implications for public health interventions for rabies control. PLoS Negl. Trop. Dis. 2017, 11, e0005787. [Google Scholar] [CrossRef]
- Jemberu, W.T.; Molla, W.; Almaw, G.; Alemu, S. Incidence of rabies in humans and domestic animals and people’s awareness in North Gondar Zone, Ethiopia. PLoS Negl. Trop. Dis. 2013, 7, e2216. [Google Scholar] [CrossRef]
- Taylor, L.H.; Hampson, K.; Fahrion, A.; Abela-Ridder, B.; Nel, L.H. Difficulties in estimating the human burden of canine rabies. Acta Trop. 2017, 165, 133–140. [Google Scholar] [CrossRef]
- Government of Mozambique. Strategy for the Control of Rabies (2010–2014). In Proceedings of the Council of Ministers, 42nd Session, Maputo, Mozambique, 23 November 2010; pp. 1–19. [Google Scholar]
- Ministry of Health; Ministry of Agriculture and Rural Development. National Strategy Plan for the Control of Rabies in Mozambique (2020–2024); Government of the Republic of Mozambique: Maputo, Mozambique, 2019; pp. 1–23.
- National Livestock Development Directorate. Mozambique Relevant Country Data; Report No.: In Timeframe (e.g., annual reports, total over the past 2 years); Ministry of Agriculture and Rural Development: Maputo, Mozambique, 2019.
- Bilaide, S.F. Retrospective Study of Animal and Human Rabies Occurences in Mozambique [Monograph]; University Eduardo Mondlane: Maputo, Mozambique, 2019. [Google Scholar]
- Ministry of Agriculture and Food Security. National Agriculture Investment Plan 2014–2018. (Comprehensive Africa Agriculture Development Programme); Ministry of Agriculture and Food Security: Maputo, Mozambique, 2014; pp. 1–100.
- Coetzer, A.; Anahory, I.; Dias, P.T.; Sabeta, C.T.; Scott, T.P.; Markotter, W.; Nel, L.H. Enhanced diagnosis of rabies and molecular evidence for the transboundary spread of the disease in Mozambique. J. S. Afr. Vet. Assoc. 2017, 88, e1–e9. [Google Scholar] [CrossRef]
- CDC. One Health Zoonotic Disease Prioritization for Multisectoral Engagement in Mozambique; Workshop Summary Centers for Disease Control and Prevention, Maputo: 2019. pp. 1–18. Available online: https://stacks.cdc.gov/view/cdc/82183 (accessed on 10 May 2020).
- OIE. Rabies (infection with rabies virus) and other lyssaviruses. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; World Organisation for Animal Health: Paris, France, 2018; pp. 578–609. [Google Scholar]
- WHO. Mozambique. Rabies Country Profile. World Health Organization, 2013. Available online: https://www.who.int/rabies/epidemiology/Rabies_CP_Mozambique_09_2013.pdf (accessed on 15 February 2018).
- WHO. WHO Expert Consultation on Rabies: Third Report; WHO Technical Report Series 2018; World Health Organization: Geneva, Switzerland, 2018; p. 1012. [Google Scholar]
- Cambule, A.H.; Smaling, E.M.A. Assessment of Soil Organic Carbon Stocks in the Limpolo National Park: From Legacy Data to Digital Soil Mapping; University of Twente: Enschede, The Netherlands, 2013. [Google Scholar]
- Stalmans, M.; Gertenbach, W.P.D.; Carvalho-Serfontein, F. Plant communities and landscapes of the Parque Nacional do Limpopo, Moçambique. Koedoe 2004, 47, 61–81. [Google Scholar] [CrossRef]
- Mapatse, M.; Sabeta, C.; Fafetine, J.; Abernethy, D. Knowledge, attitudes, practices (KAP) and control of rabies among community households and health practitioners at the human-wildlife interface in Limpopo National Park, Massingir District, Mozambique. PLoS Negl. Trop. Dis. 2022, 16, e0010202. [Google Scholar] [CrossRef] [PubMed]
- Haro-Alvarez, P.; Lopez-Valencia, G.; Tinoco-Gracia, L.; Renteria-Evangelista, T.; Medina-Basulto, G. Seroprevalence and traceback of animals suspected of carrying Ehrlichia canis, in dogs attended in veterinary clinics in Mexicali, Baja California, Mexico. J. Anim. Vet. Adv. 2007, 6, 850–854. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Publishing Company: Oxford, UK, 2007. [Google Scholar]
- Cochran, W.G. Sampling Techniques; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Markotter, W.; Kuzmin, I.; Rupprecht, C.E.; Randles, J.; Sabeta, C.T.; Wandeler, A.I.; Nel, L.H. Isolation of Lagos Bat Virus from Water Mongoose. Emerg. Infect. Dis. 2006, 12, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, D.; Bourhy, H.; Tordo, N. PCR technique as an alternative method for diagnosis and molecular epidemiology of rabies virus. Mol. Cell. Probes 1991, 5, 229–240. [Google Scholar] [CrossRef]
- Coertse, J.; Weyer, J.; Nel, L.H.; Markotter, W. Improved PCR Methods for Detection of African Rabies and Rabies-Related Lyssaviruses. J. Clin. Microbiol. 2010, 48, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; Maio, N.D.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Zinsstag, J.; Dürr, S.; Penny, M.A.; Mindekem, R.; Roth, F.; Gonzalez, S.M.; Naissengar, S.; Hattendorf, J. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proc. Natl. Acad. Sci. USA 2009, 106, 14996–15001. [Google Scholar] [CrossRef]
- Yousaf, M.Z.; Qasim, M.; Zia, S.; Ashfaq, U.A.; Khan, S. Rabies molecular virology, diagnosis, prevention and treatment. Virol. J. 2012, 9, 50. [Google Scholar] [CrossRef]
- Fehlner-Gardiner, C. Rabies control in North America-past, present and future. Rev. Sci. Tech. 2018, 37, 421–437. [Google Scholar] [CrossRef]
- Clavijo, A.; Vilas, V.J.D.R.; Mayen, F.L.; Yadon, Z.E.; Beloto, A.J.; Vigilato, M.A.N.; Schneider, M.C.; Cosivi, O. Gains and future road map for the elimination of dog-transmitted rabies in the Americas. Am. J. Trop. Med. Hyg. 2013, 89, 1040. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.C.; Aguilera, X.P.; Barbosa da Silva Junior, J.; Ault, S.K.; Najera, P.; Martinez, J.; Requejo, R.; Nicholls, R.S.; Yadon, Z.; Silva, J.C.; et al. Elimination of Neglected Diseases in Latin America and the Caribbean: A Mapping of Selected Diseases. PLoS Negl. Trop. Dis. 2011, 5, e964. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Bessell, P.R.; Conan, A.; Polak, P.; Sabeta, C.T.; Reininghaus, B.; Knobel, D.L. Spatiotemporal epidemiology of rabies at an interface between domestic dogs and wildlife in South Africa. Sci. Rep. 2018, 8, 10864. [Google Scholar] [CrossRef]
- Nel, L.; Jacobs, J.; Jaftha, J.; Meredith, C. Natural spillover of a distinctly Canidae-associated biotype of rabies virus into an expanded wildlife host range in southern Africa. Virus Genes 1997, 15, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Osofsky, S.A.; Cumming, D.H.M.; Kock, M.D. Transboundary management of natural resources and the importance of a ‘One Health’ approach. In State of the Wild 2008–2009: A Global Portrait of Wildlife, Wildlands, and Oceans; Fearn, E., Woods, W., Eds.; Island Press: Washington, DC, USA, 2008; pp. 89–98. [Google Scholar]
- Simone, E. Evaluation of the Level of Protection Against Rabies in Dogs and the Awareness of Dog Owners Regarding the Disease in Manica Province, Mozambique [MSc Thesis in Preventive Veterinary Medicine]; University Eduardo Mondlane: Maputo, Mozambique, 2016. [Google Scholar]
- Moore, S.M.; Gilbert, A.; Vos, A.; Freuling, C.M.; Ellis, C.; Kliemt, J.; Müller, T. Rabies virus antibodies from oral vaccination as a correlate of protection against lethal infection in wildlife. Trop. Med. Infect. Dis. 2017, 2, 31. [Google Scholar] [CrossRef]
- Kitala, P.; McDermott, J.; Kyule, M.; Gathuma, J.; Perry, B.; Wandeler, A. Dog ecology and demography information to support the planning of rabies control in Machakos District, Kenya. Acta Trop. 2001, 78, 217–230. [Google Scholar] [CrossRef]
- Rossouw, L.; Boshoff, C.; Sabeta, C.; Kotzé, J. A preliminary investigation of exposure to rabies virus in selected wildlife in the Kruger National Park, South Africa. KOEDOE-Afr. Prot. Area Conserv. Sci. 2021, 63, 1651. [Google Scholar] [CrossRef]
- Eze, U.U.; Ngoepe, E.C.; Anene, B.M.; Ezeokonkwo, R.C.; Nwosuh, C.; Sabeta, C.T. Detection of lyssavirus antigen and antibody levels among apparently healthy and suspected rabid dogs in South-Eastern Nigeria. BMC Res. Notes 2018, 11, 920. [Google Scholar] [CrossRef]
- Carey, A.B.; McLean, R.G. The ecology of rabies: Evidence of co-adaptation. J. Appl. Ecol. 1983, 20, 777–800. [Google Scholar] [CrossRef]
- Dodds, W.J.; Larson, L.J.; Christine, K.L.; Schultz, R.D. Duration of immunity after rabies vaccination in dogs: The Rabies Challenge Fund research study. Can. J. Vet. Res. 2020, 84, 153–158. [Google Scholar]
- Ludrik, S.; Sherikar, A.T.; Zende, R.J.; Paturkar, A.M.; Waskar, V.S.; Kshirsagar, D.D. Detection of rabies virus antibodies in vaccinated and unvaccinated stray dogs in Mumbai city. J. Vet. Public Health 2009, 7, 71–73. [Google Scholar]
- Wosu, L.O.; Anyanwu, H.N. Seroepidemiological survey of rabies virus antibodies in nonvaccinated dogs in Nsukka Environs, Nigeria. J. Vet. Med. Ser. B 1990, 37, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Arega, S.; Conan, A.; Sabeta, C.T.; Crafford, J.E.; Wentzel, J.; Reininghaus, B.; Biggs, L.; Leisewitz, A.L.; Quan, M.; Toka, F.; et al. Rabies vaccination of 6-week-old puppies born to immunized mothers: A randomized controlled trial in a high-mortality population of owned, free-roaming dogs. Trop. Med. Infect. Dis. 2020, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, A.I.; Oluwayelu, D.O.; Ohore, O.G.; Cadmus, S.I.B. Lack of protection against rabies in neighbourhood dogs in some peri-urban and rural areas of Ogun and Oyo states, Nigeria. Afr. J. Med. Med. Sci. 2014, 43, 157–162. [Google Scholar]
- Berndtsson, L.T.; Nyman, A.K.; Rivera, E.; Klingeborn, B. Factors associated with the success of rabies vaccination of dogs in Sweden. Acta Vet. Scand. 2011, 53, 22. [Google Scholar] [CrossRef] [Green Version]
- HogenEsch, H.; Thompson, S.; Dunham, A.; Ceddia, M.; Hayek, M. Effect of age on immune parameters and the immune response of dogs to vaccines: A cross-sectional study. Vet. Immunol. Immunopathol. 2004, 97, 77–85. [Google Scholar] [CrossRef]
- Mapatse, M. Public Health Awareness and Seroprevalence of Rabies in Dogs in Limpopo National Park, and the Phylogeny of Rabies Virus in Mozambique. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2021. [Google Scholar]
- Gazi, A.; Seyyal, A. Detection of neutralising antibody titration in vaccinated owned and Stray dogs against rabies virus. J. Fac. Vet. Med. Istanbul Univ. 2011, 37, 97–106. [Google Scholar]
- Mansfield, K.L.; Sayers, R.; Fooks, A.R.; Burr, P.D.; Snodgrass, D. Factors affecting the serological response of dogs and cats to rabies vaccination. Vet. Rec. 2004, 154, 423–426. [Google Scholar] [CrossRef]
- van Sittert, S.J.; Raath, J.; Akol, G.W.; Miyen, J.M.; Mlahlwa, B.; Sabeta, C.T. Rabies in the Eastern Cape Province of South Africa--where are we going wrong? J. S. Afr. Vet. Assoc. 2010, 81, 207–215. [Google Scholar] [CrossRef]
- Kissi, B.; Tordo, N.; Bourhy, H. Genetic polymorphism in the rabies virus nucleoprotein gene. Virology 1995, 209, 526–537. [Google Scholar] [CrossRef]
- Swanepoel, R.; Barnard, B.J.; Meredith, C.D.; Bishop, G.C.; Brückner, G.K.; Foggin, C.M.; Hübschle, O.J. Rabies in southern Africa. Onderstepoort J. Vet. Res. 1993, 60, 325–346. [Google Scholar] [PubMed]
- Dias, P.T.; Novoa, A.M.; Cliff, J.L. Rabies in Mozambique. In Rabies in the Tropics; Kuwert, E., Merieux, C., Koprowski, H., Bögel, K., Eds.; Springer: Berlim, Germany, 1985; pp. 406–414. [Google Scholar]
- Sabeta, C.; Ukamaka, E.; Mapatse, M. Limitations of Diagnostic Tests Using Rabies as an Example. EC Vet. Sci. 2021, 6, 60–63. [Google Scholar]
- Sabeta, C.T.; Weyer, J.; Geertsma, P.; Mohale, D.; Miyen, J.; Blumberg, L.H.; Leman, P.A.; Paweska, J.T.; Geertsma, P.; Walters, J. Emergence of Rabies in the Gauteng Province, South Africa: 2010–2011. J. S. Afr. Vet. Assoc. 2013, 84, E1–E5. [Google Scholar] [CrossRef] [PubMed]
- Ngoepe, E.; Chirima, J.G.; Mohale, D.; Mogano, K.; Suzuki, T.; Makita, K.; Sabeta, C.T. Rabies outbreak in black-backed jackals (Canis mesomelas), South Africa, 2016. Epidemiol. Infect. 2022, 150, e137. [Google Scholar] [CrossRef]
- Sabeta, C.T.; Shumba, W.; Mohale, D.K.; Miyen, J.M.; Wandeler, A.I.; Nel, L.H. Mongoose rabies and the African civet in Zimbabwe. Vet. Rec. 2008, 163, 580. [Google Scholar] [CrossRef]
- Zulu, G.C.; Sabeta, C.T.; Nel, L.H. Molecular epidemiology of rabies: Focus on domestic dogs (Canis familiaris) and black-backed jackals (Canis mesomelas) from northern South Africa. Virus Res. 2009, 140, 71–78. [Google Scholar] [CrossRef]

| Variables | Frequency (%) |
|---|---|
| Dog age groups | |
| <1 Year | 164 (39.2) |
| 1–2 Years | 65 (15.6) |
| >2 Years | 189 (45.2) |
| Sex of the dogs | |
| Male | 280 (66.9) |
| Female | 138 (33.1) |
| History of dog vaccination | |
| Unvaccinated | 335 (80.1) |
| Vaccinated | 83 (19.9) |
| Antibody Level | |
| PB < 40% (Negative) | 373 (89.2) |
| PB ≥ 40% (Positive) | 45 (10.8) |
| Variables | Frequency (%) | ||
|---|---|---|---|
| Age of dogs | Unvaccinated | Vaccinated | Total |
| <1 Year | 141 (42.1) | 22 (26.5) | 163 |
| 1–2 Years | 54 (16.1) | 12 (14.5) | 66 |
| >2 Years | 140 (41.8) | 49 (59.1) | 189 |
| Sex of dogs | Unvaccinated | Vaccinated | Total |
| Male | 216 (77.1) | 64 (22.9) | 280 |
| Female | 119 (86.2) | 19 (13.8) | 138 |
| Vaccination status according to PB | Negative | Positive | Total |
| Unvaccinated | 306 (91.3) | 29 (8.7) | 335 |
| Vaccinated | 67 (80.7) | 16 (19.3) | 83 |
| Variables | Frequency (%) | Total | p-Value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Group age | ||||
| <1 Year | 156 (95.7) | 7 (4.3) | 163 (100) | 0.001 |
| 1–2 Years | 61 (92.4) | 5 (7.6) | 66 (100) | 0.001 |
| >2 Years | 156 (82.5) | 33 (17.5) | 189 (100) | 0.059 |
| Total | 373 (89.2) | 45 (10.8) | 418 (100) | |
| Sex | ||||
| Female | 124 (89.9) | 14 (10.1) | 138 (100) | 0.774 |
| Male | 249 (88.9) | 31(11.1) | 280 (100) | |
| Total | 373 (89.2) | 18 (10.8) | 418 (100) | |
| Lab Reference | Animal | Collection Site | DFAT | RT-PCR | qRT-PCR | Gene Copies/µL |
|---|---|---|---|---|---|---|
| 496/18 | Canine | Maputo | Positive | Negative | Positive | 640 |
| 597/18 | Canine | Sofala | Positive | Negative | Positive | 789 |
| 501/18 | Canine | Gaza | Positive | Negative | Positive | 708 |
| MW248383 | Canine | Gaza | Positive | Positive | Positive | 6.4 × 106 |
| 124/18 | Canine | Maputo | Positive | Positive | Positive | 1 × 107 |
| 393/18 | Feline | Sofala | Positive | Positive | Positive | 2.5 × 106 |
| 468/17 | Canine | Gaza | Positive | Positive | Positive | 8.8 × 106 |
| 368/18 | Canine | Nampula | Positive | Positive | Positive | 1 × 107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapatse, M.; Ngoepe, E.; Abernethy, D.; Fafetine, J.M.; Anahory, I.; Sabeta, C. Rabies Virus Seroprevalence among Dogs in Limpopo National Park and the Phylogenetic Analyses of Rabies Viruses in Mozambique. Pathogens 2022, 11, 1043. https://doi.org/10.3390/pathogens11091043
Mapatse M, Ngoepe E, Abernethy D, Fafetine JM, Anahory I, Sabeta C. Rabies Virus Seroprevalence among Dogs in Limpopo National Park and the Phylogenetic Analyses of Rabies Viruses in Mozambique. Pathogens. 2022; 11(9):1043. https://doi.org/10.3390/pathogens11091043
Chicago/Turabian StyleMapatse, Milton, Ernest Ngoepe, Darrell Abernethy, José Manuel Fafetine, Iolanda Anahory, and Claude Sabeta. 2022. "Rabies Virus Seroprevalence among Dogs in Limpopo National Park and the Phylogenetic Analyses of Rabies Viruses in Mozambique" Pathogens 11, no. 9: 1043. https://doi.org/10.3390/pathogens11091043
APA StyleMapatse, M., Ngoepe, E., Abernethy, D., Fafetine, J. M., Anahory, I., & Sabeta, C. (2022). Rabies Virus Seroprevalence among Dogs in Limpopo National Park and the Phylogenetic Analyses of Rabies Viruses in Mozambique. Pathogens, 11(9), 1043. https://doi.org/10.3390/pathogens11091043










