Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates Selection
2.2. Enterococcal Isolation
2.3. Antibiotic Susceptibility Test
2.4. Enterococcal Biofilm Formation
2.4.1. Using Congo Red Agar (CRA)
2.4.2. Using Microtiter Plates (MTP)
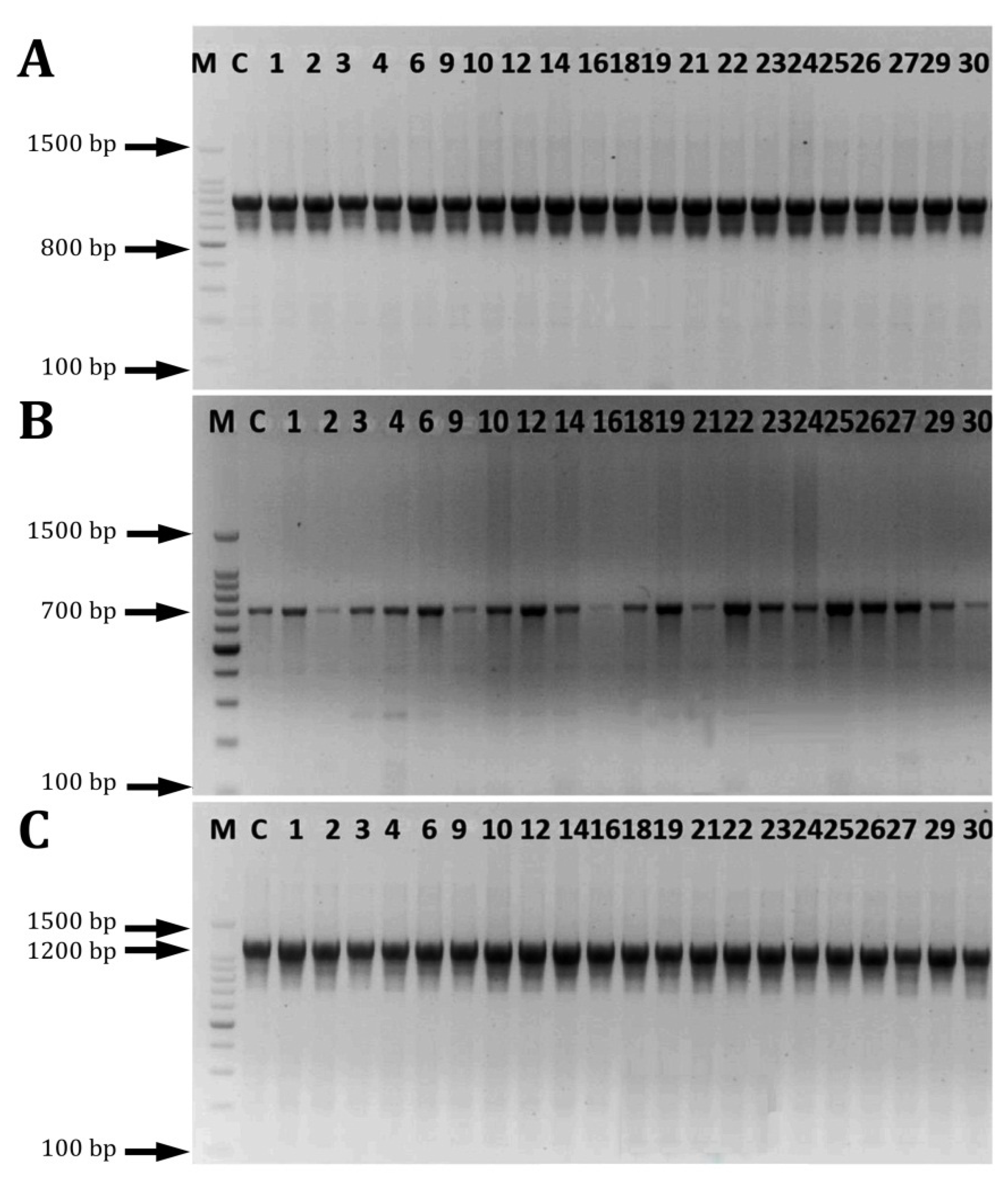
2.5. PCR Amplification and Phylogenetic Analysis
2.6. Detection of Biofilm-Associated Genes
2.7. Statistical Analysis
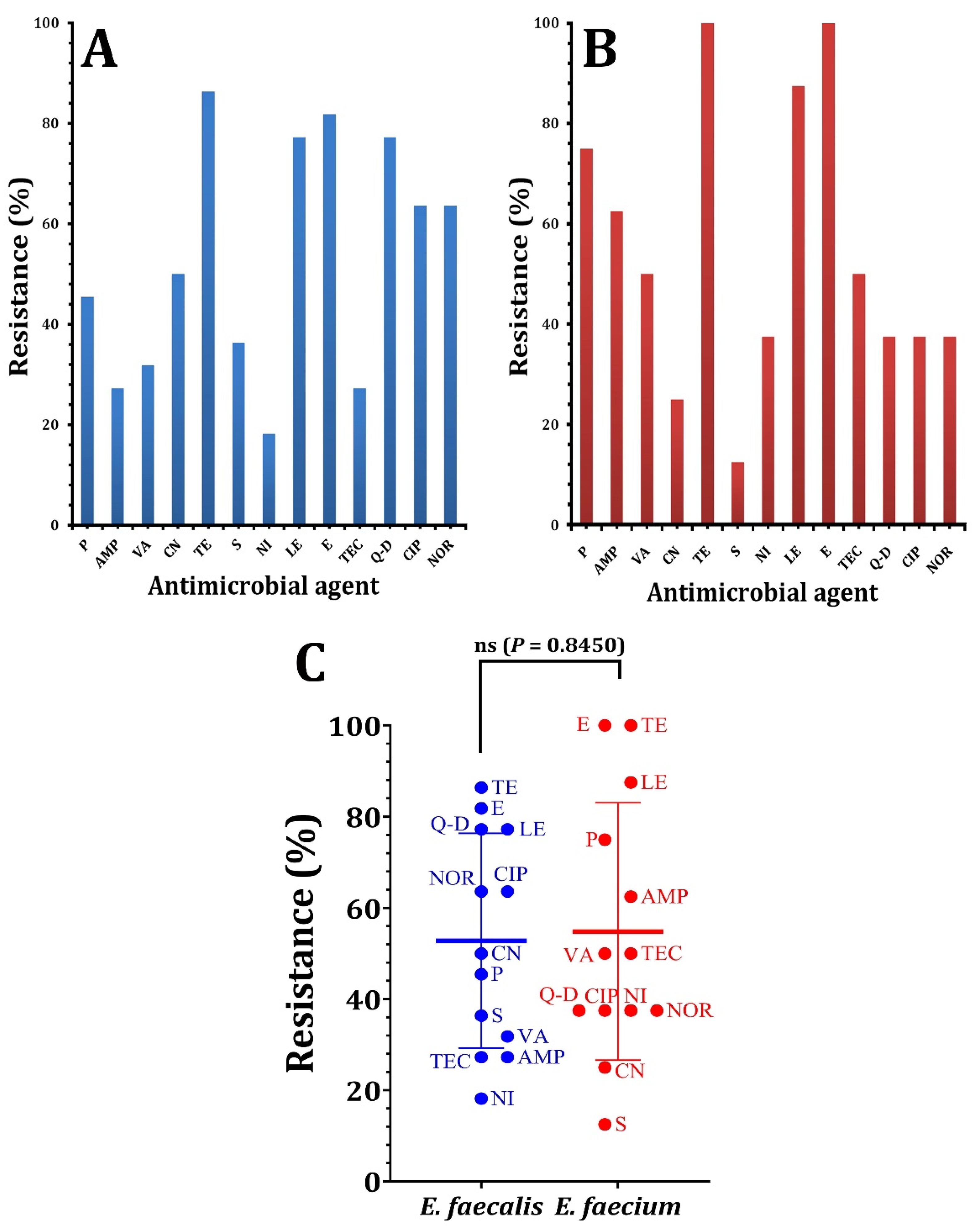
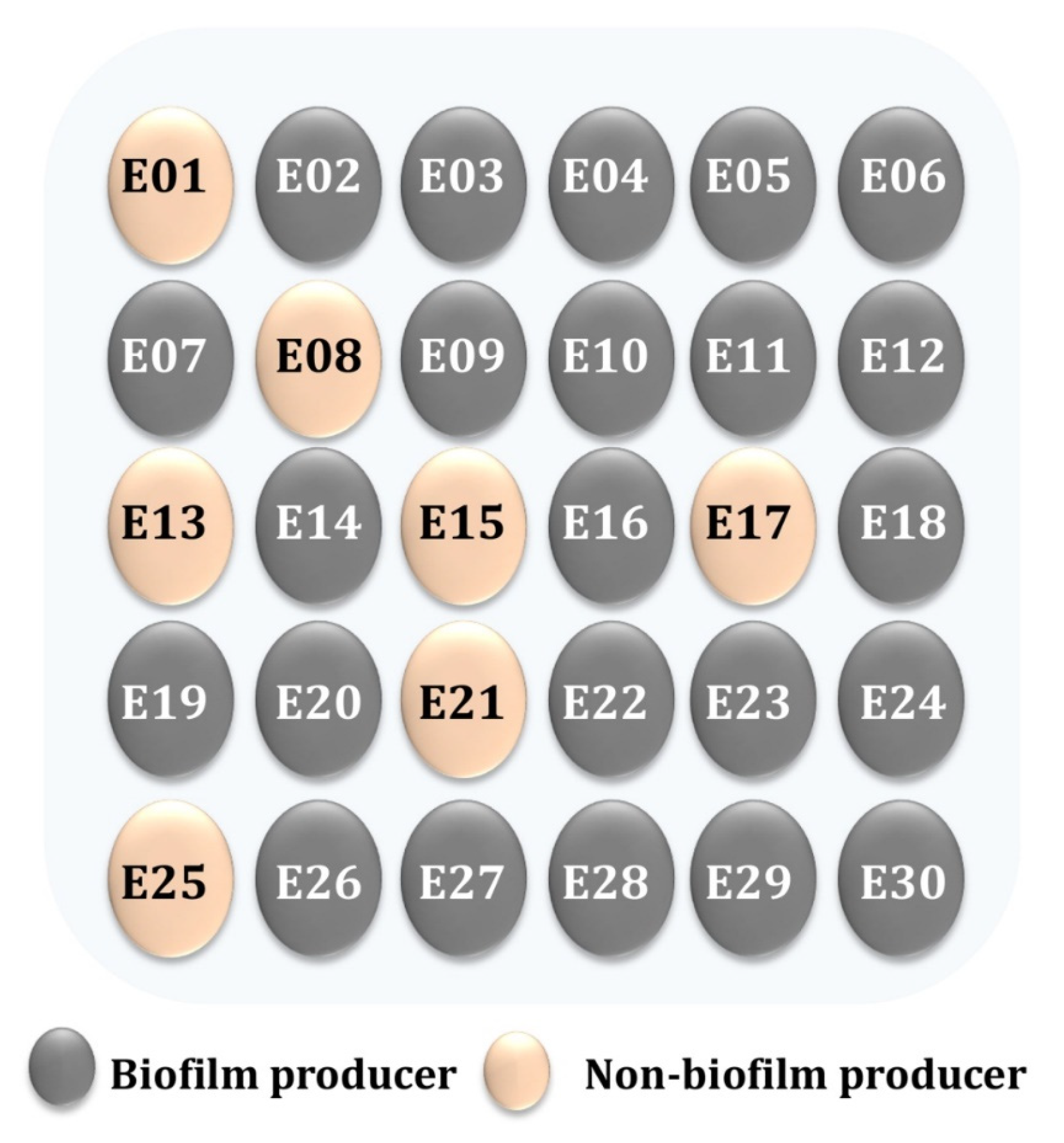
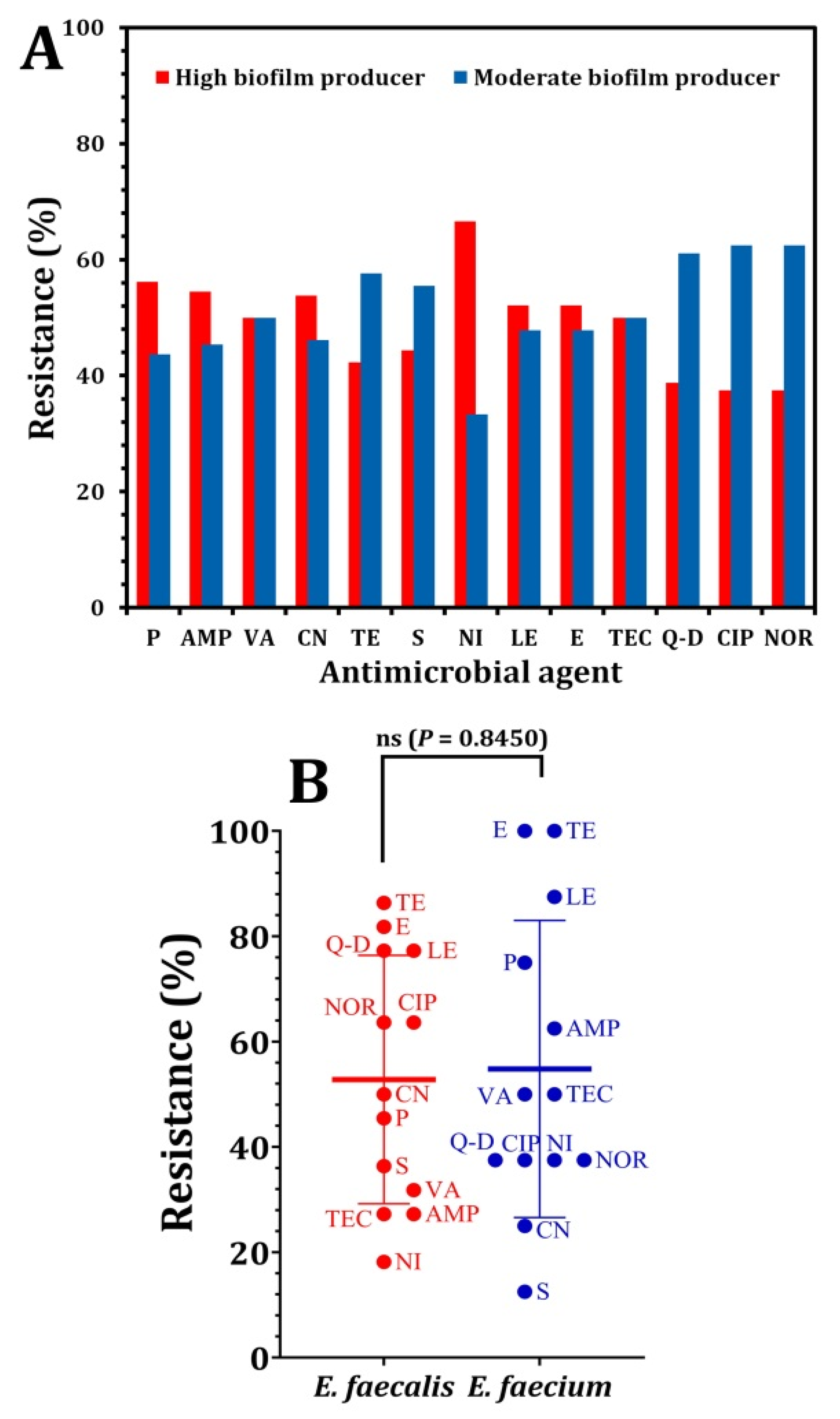
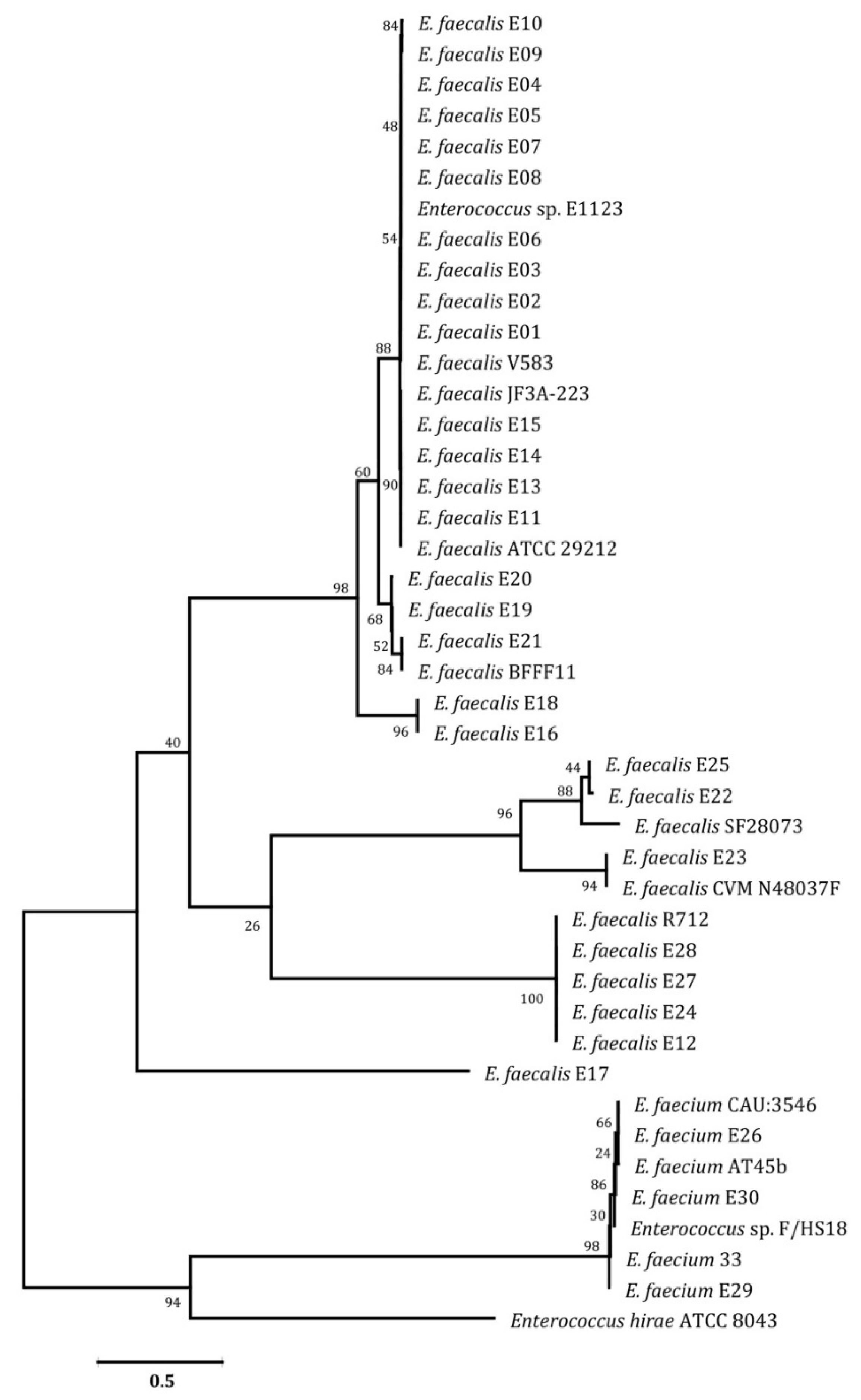
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Brien, V.P.; Hannan, T.J.; Nielsen, H.V.; Hultgren, S.J. Drug and vaccine development for the treatment and prevention of urinary tract infections. Microbiol. Spectr. 2016, 4, 589–646. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Das, A. Molecular identification of multi drug resistant bacteria from urinary tract infected urine samples. Microb. Pathog. 2016, 98, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Grużewska, A.; Szweda, P.; Wicha, J.; Parulska, U. Antibiotic resistance of uropathogens isolated from patients hospitalized in district hospital in central Poland in 2020. Antibiotics 2021, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The many faces of Enterococcus spp. commensal, probiotic and opportunistic pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. Cranberry polyphenols and prevention against urinary tract infections: Relevant considerations. Molecules 2020, 25, 3523. [Google Scholar]
- Bazaid, A.S.; Saeed, A.; Alrashidi, A.; Alrashidi, A.; Alshaghdali, K.A.; Hammam, S.; Alreshidi, T.; Alshammary, M.; Alarfaj, A.; Thallab, R.; et al. Antimicrobial surveillance for bacterial uropathogens in Ha’il, Saudi Arabia: A Five-year multicenter retrospective study. Infect. Drug Resist. 2021, 16, 1455–1465. [Google Scholar] [CrossRef]
- Ali, S.S.; Sonbol, F.I.; Sun, J.; Hussein, M.A.; Hafez, A.-E.E.; Abdelkarim, E.A.; Kornaros, M.; Ali, A.; Azab, M. Molecular characterization of virulence and drug resistance genes-producing Escherichia coli isolated from chicken meat: Metal oxide nanoparticles as novel antibacterial agents. Microb. Pathog. 2020, 143, 104164. [Google Scholar] [CrossRef]
- Ali, S.S.; Moawad, M.S.; Hussein, M.A.; Azab, M.; Abdelkarim, E.A.; Badr, A.; Sun, J.; Khalil, M. Efficacy of metal oxide nanoparticles as novel antimicrobial agents against multi-drug and multi-virulent Staphylococcus aureus isolates from retail raw chicken meat and giblets. Int. J. Food Microbiol. 2021, 344, 109116. [Google Scholar] [CrossRef]
- Ali, S.S.; Shaaban, M.T.; Abomohra, A.E.-F.; El-Safity, K. Macroalgal activity against multiple drug resistant Aeromonas hydrophila: A novel treatment study towards enhancement of fish growth performance. Microb. Pathog. 2016, 101, 89–95. [Google Scholar] [CrossRef]
- Ali, S.S.; Morsy, R.; El-Zawawy, N.A.; Fareed, M.F.; Bedaiwy, M.Y. Synthesized zinc peroxide nanoparticles (ZnO2-NPs): A novel antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn wounds. Int. J. Nanomed. 2017, 12, 6059. [Google Scholar] [CrossRef]
- Samani, R.J.; Tajbakhsh, E.; Momtaz, H.; Samani, M.K. Prevalence of virulence genes and antibiotic resistance pattern in Enterococcus faecalis isolated from urinary tract infection in Shahrekord, Iran. Rep. Biochem. Mol. Biol. 2021, 10, 50–59. [Google Scholar] [CrossRef]
- Saraswathy, M.P. Multidrug resistant Enterococci isolated from urine samples at a tertiary care hospital. Indian J. Microbiol. Res. 2015, 2, 214–219. [Google Scholar] [CrossRef]
- Soto, S.M. Importance of biofilms in urinary tract infections: New therapeutic approaches. Adv. Biol. 2014, 2014, 543974. [Google Scholar] [CrossRef]
- Hashem, Y.A.; Abdelrahman, K.A.; Aziz, R.K. Phenotype-genotype correlations and distribution of key virulence factors in Enterococcus faecalis isolated from patients with urinary tract infections. Infect. Drug Resist. 2021, 14, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. Iscience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Fallah, F.; Yousefi, M.; Pourmand, M.R.; Hashemi, A.; Alam, A.N.; Afshar, D. Phenotypic and genotypic study of biofilm formation in Enterococci isolated from urinary tract infections. Microb. Pathog. 2017, 108, 85–90. [Google Scholar] [CrossRef]
- Kafil, H.S.; Mobarez, A.M. Assessment of biofilm formation by Enterococci isolates from urinary tract infections with different virulence profiles. J. King Saud Univ.-Sci. 2015, 27, 312–317. [Google Scholar] [CrossRef]
- Kayaoglu, G.; Ørstavik, D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit. Rev. Oral Biol. Med. 2004, 15, 308–320. [Google Scholar] [CrossRef]
- Moniri, R.; Ghasemi, A.; Moosavi, S.G.A.; Dastehgoli, K.; Rezaei, M. Virulence Gene’s relationship with biofilm formation and detection of aac (6′)/aph (2′′) in Enterococcus faecalis isolated from patients with urinary tract infection. Jundishapur J. Microbiol. 2013, 6, e94137. [Google Scholar] [CrossRef]
- Heidari, H.; Emaneini, M.; Dabiri, H.; Jabalameli, F. Virulence factors, antimicrobial resistance pattern and molecular analysis of Enterococcal strains isolated from burn patients. Microb. Pathog. 2016, 90, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Declaration of Helsinki History Website. In Ethical Principles For Medical Research; Declaration of Helsinki; The JAMA Network: Helsinki, Finland, 2015.
- CLSI. Performance Standards for Antimicrobial Susceptibility Tests, 13th ed.; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; Sonbol, F.I. Investigation of biofilm formation on contact eye lenses caused by methicillin resistant Staphylococcus aureus. Niger. J. Clin. Pr. 2014, 17, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Bonaventura, G.; Djukic, S.; Irkovic, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Sun, J.; Wu, J.; Huizi, L. Screening and construction of a novel microbial consortium SSA-6 enriched from the gut symbionts of wood-feeding termite, Coptotermes formosanus and its biomass-based biorefineries. Fuel 2019, 236, 1128–1145. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7 molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Hashem, Y.A.; Amin, H.M.; Essam, T.M.; Yassin, A.S.; Aziz, R.K. Biofilm formation in enterococci: Genotype-phenotype correlations and inhibition by vancomycin. Sci. Rep. 2017, 7, 5733. [Google Scholar] [CrossRef]
- Vankerckhoven, V.; Van Autgaerden, T.; Vael, C.; Lammens, C.; Chapelle, S.; Rossi, R.; Jabes, D.; Goossens, H. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J. Clin. Microbiol. 2004, 42, 4473–4479. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Sievert, D.M. Antimicrobial resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Chen, H.; Wu, W.; Ni, M.; Liu, Y.; Zhang, J.; Xia, F.; Wang, H. Linezolid-resistant clinical isolates of Enterococci and Staphylococcus cohnii from a multicentre study in China: Molecular epidemiology and resistance mechanisms. Int. J. Antimicrob. Agents 2013, 42, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.W. Aminoglycoside resistance in enterococci. Clin. Infect. Dis. 2000, 31, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Maschieto, A.; Martinez, R.; Palazzo, I.C.V.; Darini, A.L.D.C. Antimicrobial resistance of Enterococcus sp. isolated from the intestinal tract of patients from a university hospital in Brazil. Memórias do Instituto Oswaldo Cruz 2004, 99, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, S.; Veloo, Y.; Thahir, S.S.A.; Shaharudin, R. Resistance towards critically important antimicrobials among Enterococcus faecalis and E. faecium in poultry farm environments in Selangor, Malaysia. Antibiotics 2022, 11, 1118. [Google Scholar] [CrossRef]
- Wei, L.; Wu, Q.; Zhang, J.; Guo, W.; Chen, M.; Xue, L.; Wang, J.; Ma, L. Prevalence and genetic diversity of Enterococcus faecalis isolates from mineral water and spring water in China. Front. Microbiol. 2017, 8, 1109. [Google Scholar] [CrossRef]
- Ferede, Z.T.; Tullu, K.D.; Derese, S.G.; Yeshanew, A.G. Prevalence and antimicrobial susceptibility pattern of Enterococcus species isolated from different clinical samples at Black Lion Specialized Teaching Hospital, Addis Ababa, Ethiopia. BMC Res. Notes 2018, 11, 793. [Google Scholar] [CrossRef]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial biofilms in urinary tract infections and prostatitis: Etiology, pathogenicity, and combating strategies. Pathogens 2016, 30, 65. [Google Scholar] [CrossRef]
- Baldassarri, L.; Cecchini, R.; Bertuccini, L.; Ammendolia, M.G.; Iosi, F.; Arciola, C.R.; Orefici, G. Enterococcus spp. produces slime and survives in rat peritoneal macrophages. Med. Microbiol. Immunol. 2001, 190, 113–120. [Google Scholar] [CrossRef]
- Duprè, I.; Zanetti, S.; Schito, A.M.; Fadda, G.; Sechi, L.A. Incidence of virulence determinants in clinical Enterococcus faecium and Enterococcus faecalis isolates collected in Sardinia (Italy). J. Med. Microbiol. 2003, 52, 491–498. [Google Scholar] [CrossRef]
- Mohamed, J.A.; Huang, D.B. Biofilm formation by enterococci. J. Med. Microbiol. 2007, 56, 15811588. [Google Scholar] [CrossRef]
- Lasa, I. Towards the identification of the common features of bacterial biofilm development. Int. Microbiol. 2006, 9, 21–28. [Google Scholar] [PubMed]
- Mathur, T.; Singhal, S.; Khan, S.; Upadhyay, D.J.; Fatma, T.; Rattan, A. Detection of biofilm formation among the clinical isolates of Staphylococci: An evaluation of three different screening methods. Indian J. Med. Microbiol. 2006, 24, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Detection and antibiotic susceptibility pattern of biofilm producing gram-positive and gram-negative bacteria isolated from a tertiary care hospital of Pakistan. Malays J. Microbiol. 2011, 7, 57–60. [Google Scholar] [CrossRef][Green Version]
- Akhter, J.; Ahmed, S.; Anwar, S. Antimicrobial susceptibility patterns of Enterococcus species isolated from urinary tract infections. Bangladesh J. Med Microbiol. 2014, 8, 16–20. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, A.; Pal, N.K.; Sarkar, S.; Gupta, M.S. Antibiotic resistance pattern of Enterococci isolates from nosocomial infections in a tertiary care hospital in Eastern India. J. Nat. Sci. Biol. Med. 2015, 6, 394. [Google Scholar] [CrossRef]
- Sindhanai, V.; Avanthiga, S.S.; Chander, V.S. Antibiotic susceptibility pattern of biofilm forming and biofilm non forming Enterococci species. OSR J. Dent. Med. Sci. 2016, 15, 33–37. [Google Scholar]
- Janda, J.M.; Abbott, S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007, 45, 27612764. [Google Scholar] [CrossRef]
- Clarridge, J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef]
- Shankar, V.; Baghdayan, A.S.; Huycke, M.M.; Lindahl, G.; Gilmore, M.S. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 1999, 67, 193–200. [Google Scholar] [CrossRef]
- Zecconi, A.; Scali, F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol. Lett. 2013, 150, 12–22. [Google Scholar] [CrossRef]
- Soares, R.O.; Fedi, A.C.; Reiter, K.C.; Caierão, J.; d’Azevedo, P.A. Correlation between biofilm formation and gelE, esp, and agg genes in Enterococcus spp. clinical isolates. Virulence 2014, 5, 634–637. [Google Scholar] [CrossRef] [PubMed]


| Pattern Code | Isolate Code | # Antimicrobial Resistance | No. of Antimicrobial Classes |
|---|---|---|---|
| A | E05 | TE V, CIP VII, NOR VII, Q-D IX | 3 |
| E29 | P I, AMP I, TE V, LE VII | ||
| B | E03 | VA II, TEC II, TE V, LE VII, CIP VII, NOR VII, E VIII | 4 |
| E11 | P I, AMP I, TE V, LE VII, E VIII | ||
| E13 | CN III, LE VII, CIP VII, NOR VII, E VIII, Q-D IX | ||
| E19 | TE V, LE VII, CIP VII, NOR VII, E VIII, Q-D IX | ||
| E23 | P I, AMP I, S III, LE VII, E VIII | ||
| C | E01 | P I, AMP I, VA II, TEC II, TE V, LE VII, CIP VII, NOR VII, E VIII | 5 |
| E02 | CN III, S III, TE V, CIP VII, NOR VII, E VIII, Q-D IX | ||
| E04, E09 | P I, CN III, S III, TE V, CIP VII, NOR VII, LE VII, Q-D IX | ||
| E06 | CN III, S III, TE V, LE VII, CIP VII, NOR VII, E VIII, Q-D | ||
| E08 | AMP I, TE V, LE VII, E VIII, Q-D IX | ||
| E10 | P I, AMP I, VA II, TEC II, TE V, NIVI, LE VII | ||
| E12, E16 | CN III, TE V, LE VII, CIP VII, NOR VII, E VIII, Q-D IX | ||
| E15 | P I, AMP I, CN III, TE V, LE VII, E VIII | ||
| E17 | P I, AMP I, VA II,TEC II,TE V, CIP VII, NOR VII,E VIII | ||
| E20 | VA II, TEC II, TE V, CIP VII, NOR VII, E VIII, Q-D IX | ||
| E26 | VA II, TEC II, TE V, NI VI, LE VII, E VIII | ||
| E30 | P I, CN III, TE V, LE VII, Q-D IX | ||
| D | E22 | P I, CN III, TE V, LE VII, E VIII, Q-D IX | 6 |
| E27 | P I, AMP I, VA II, TEC II, CN III, TE V, LE VII, E VIII | ||
| E28 | P I, AMP I, VA II, TEC II, S III,TE V, LE VII, CIP VII, NOR VII, E VIII | ||
| E | E18 | P I, CN III, S III, TE V, LE VII, CIP VII, NOR VII, E VIII, Q-D IX | 7 |
| E21 | P I, VA II, TEC II, CN III, S III, TE V, LE VII, E VIII, Q-D IX | ||
| E14 | P I, AMP I, VA II, TEC II S III, TE V, NI VI, LE VII, CIP VII, NOR VII, E VIII |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections. Pathogens 2023, 12, 34. https://doi.org/10.3390/pathogens12010034
Khalil MA, Alorabi JA, Al-Otaibi LM, Ali SS, Elsilk SE. Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections. Pathogens. 2023; 12(1):34. https://doi.org/10.3390/pathogens12010034
Chicago/Turabian StyleKhalil, Maha A., Jamal A. Alorabi, Lamya M. Al-Otaibi, Sameh S. Ali, and Sobhy E. Elsilk. 2023. "Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections" Pathogens 12, no. 1: 34. https://doi.org/10.3390/pathogens12010034
APA StyleKhalil, M. A., Alorabi, J. A., Al-Otaibi, L. M., Ali, S. S., & Elsilk, S. E. (2023). Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections. Pathogens, 12(1), 34. https://doi.org/10.3390/pathogens12010034






