The Use of Natural Methods to Control Foodborne Biofilms
Abstract
:1. Introduction
2. Bacteria, Viruses, and Fungi to Control Biofilms
3. Phytochemicals and Essential Oils
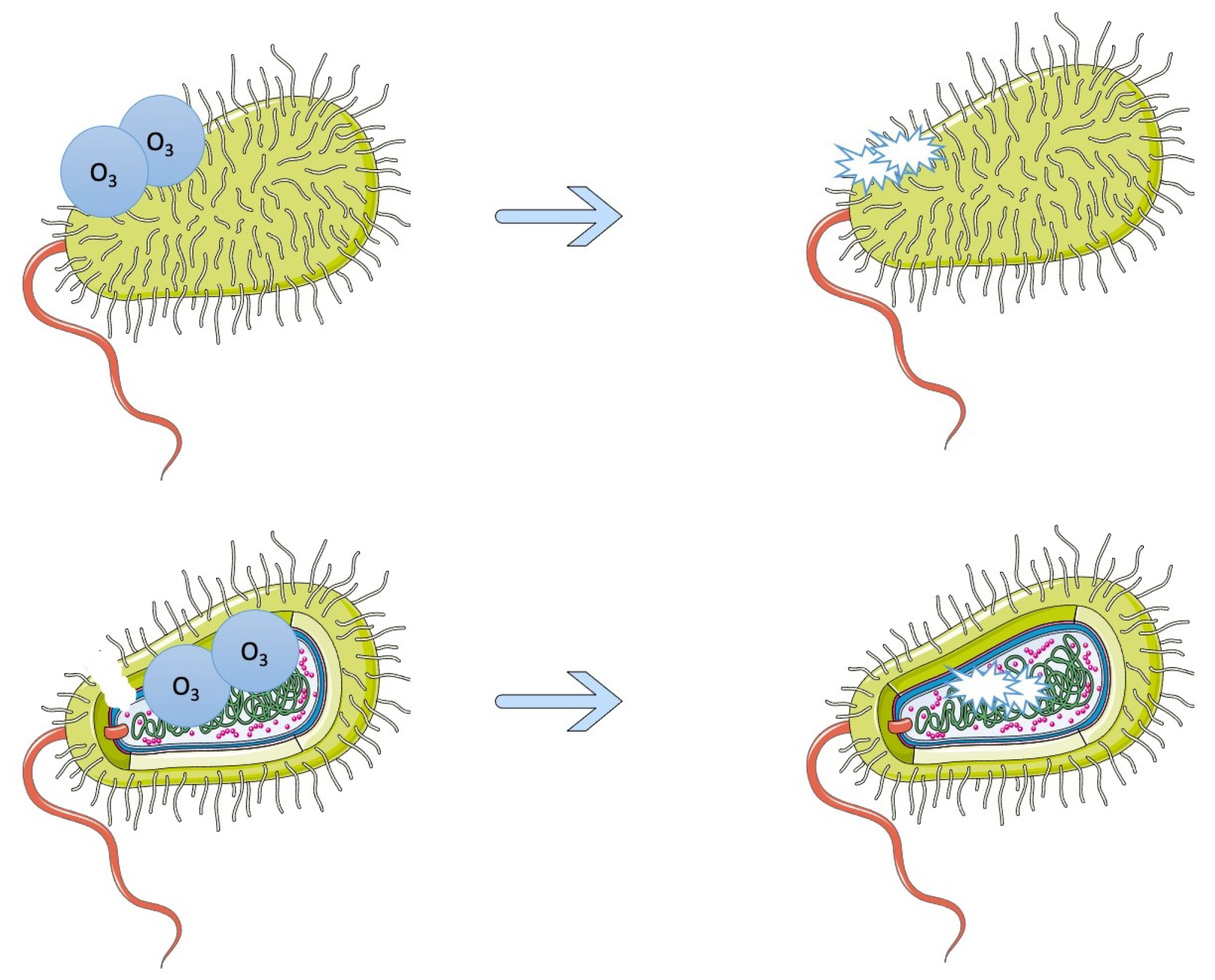
4. Gaseous and Aqueous Control, as Well as Photocatalysis
5. Enzymatic Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aryal, M.; Muriana, P.M. Efficacy of Commercial Sanitizers Used in Food Processing Facilities for Inactivation of Listeria Monocytogenes, E. Coli O157:H7, and Salmonella Biofilms. Foods 2019, 8, 639. [Google Scholar] [CrossRef] [Green Version]
- Iñiguez-Moreno, M.; Gutiérrez-Lomelí, M.; Avila-Novoa, M.G. Kinetics of Biofilm Formation by Pathogenic and Spoilage Microorganisms under Conditions That Mimic the Poultry, Meat, and Egg Processing Industries. Int. J. Food Microbiol. 2019, 303, 32–41. [Google Scholar] [CrossRef]
- Rather, M.; Gupta, K.; Bardhan, P.; Borah, M.; Sarkar, A.; Eldiehy, K.; Bhuyan, S.; Mandal, M. Microbial Biofilm: A Matter of Grave Concern for Human Health and Food Industry. J. Basic Microbiol. 2021, 61, 678. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naïtali, M.; Briandet, R. Biofilm-Associated Persistence of Food-Borne Pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Oxaran, V.; Dittmann, K.K.; Lee, S.H.I.; Chaul, L.T.; Fernandes de Oliveira, C.A.; Corassin, C.H.; Alves, V.F.; De Martinis, E.C.P.; Gram, L. Behavior of Foodborne Pathogens Listeria Monocytogenes and Staphylococcus Aureus in Mixed-Species Biofilms Exposed to Biocides. Appl. Environ. Microbiol. 2018, 84, e02038-18. [Google Scholar] [CrossRef] [Green Version]
- Gavrilova, E.; Anisimova, E.; Gabdelkhadieva, A.; Nikitina, E.; Vafina, A.; Yarullina, D.; Bogachev, M.; Kayumov, A. Newly Isolated Lactic Acid Bacteria from Silage Targeting Biofilms of Foodborne Pathogens during Milk Fermentation. BMC Microbiol. 2019, 19, 248. [Google Scholar] [CrossRef]
- Kim, N.-N.; Kim, W.J.; Kang, S.-S. Anti-Biofilm Effect of Crude Bacteriocin Derived from Lactobacillus Brevis DF01 on Escherichia Coli and Salmonella Typhimurium. Food Control 2019, 98, 274–280. [Google Scholar] [CrossRef]
- Kang, T.-K.; Kim, W.-J. Characterization of an Amylase-Sensitive Bacteriocin DF01 Produced by Lactobacillus Brevis DF01 Isolated from Dongchimi, Korean Fermented Vegetable. Korean J. Food Sci. Anim. Resour. 2010, 30, 795–803. [Google Scholar] [CrossRef]
- Tatsaporn, T.; Kornkanok, K. Using Potential Lactic Acid Bacteria Biofilms and Their Compounds to Control Biofilms of Foodborne Pathogens. Biotechnol. Rep. 2020, 26, e00477. [Google Scholar] [CrossRef]
- Bindiya, E.S.; Tina, K.J.; Sasidharan, R.S.; Bhat, S.G. BaCf3: Highly Thermostable Bacteriocin from Bacillus Amyloliquefaciens BTSS3 Antagonistic on Food-Borne Pathogens. 3 Biotech 2019, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Shabbir, S.; Noor, T.; Imran, M. Antimicrobial and Antibiofilm Potential of Bacteriocin Loaded Nano-Vesicles Functionalized with Rhamnolipids against Foodborne Pathogens. LWT 2019, 116, 108583. [Google Scholar] [CrossRef]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined Antimicrobial Effect of Essential Oils and Bacteriocins against Foodborne Pathogens and Food Spoilage Bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.-D. Reduction of Escherichia coli O157:H7 in Biofilms Using Bacteriophage BPECO 19. J. Food Sci. 2017, 82, 1433–1442. [Google Scholar] [CrossRef]
- Garvey, M. Bacteriophages and Food Production: Biocontrol and Bio-Preservation Options for Food Safety. Antibiotics 2022, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Meaden, S.; Koskella, B. Exploring the Risks of Phage Application in the Environment. Front Microbiol. 2013, 4, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Guenther, S.; Herzig, O.; Fieseler, L.; Klumpp, J.; Loessner, M.J. Biocontrol of Salmonella Typhimurium in RTE Foods with the Virulent Bacteriophage FO1-E2. Int. J. Food Microbiol. 2012, 154, 66–72. [Google Scholar] [CrossRef]
- Kang, H.-W.; Kim, J.-W.; Jung, T.-S.; Woo, G.-J. Wksl3, a New Biocontrol Agent for Salmonella Enterica Serovars Enteritidis and Typhimurium in Foods: Characterization, Application, Sequence Analysis, and Oral Acute Toxicity Study. Appl. Env. Microbiol. 2013, 79, 1956–1968. [Google Scholar] [CrossRef] [Green Version]
- Endersen, L.; Coffey, A. The Use of Bacteriophages for Food Safety. Curr. Opin. Food Sci. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Islam, M.S.; Zhou, Y.; Liang, L.; Nime, I.; Liu, K.; Yan, T.; Wang, X.; Li, J. Application of a Phage Cocktail for Control of Salmonella in Foods and Reducing Biofilms. Viruses 2019, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, F.; Segall, A.M. A Century of Phage Lessons. Nature 2015, 528, 46–47. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Ding, Y.; Huang, C.; Wang, J.; Wang, J.; Wang, X. Genomic Characterization of a Novel Bacteriophage STP55 Revealed Its Prominent Capacity in Disrupting the Dual-Species Biofilm Formed by Salmonella Typhimurium and Escherichia Coli O157: H7 Strains. Arch. Microbiol. 2022, 204, 597. [Google Scholar] [CrossRef] [PubMed]
- Yew, C.-H.T.; Gurumoorthy, N.; Nordin, F.; Tye, G.J.; Zaman, W.S.W.K.; Tan, J.J.; Ng, M.H. Integrase Deficient Lentiviral Vector: Prospects for Safe Clinical Applications. PeerJ 2022, 10, e13704. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage Biocontrol Applications in Food Production and Processing. Curr. Issues Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef]
- Gdoura-Ben Amor, M.; Culot, A.; Techer, C.; AlReshidi, M.; Adnan, M.; Jan, S.; Baron, F.; Grosset, N.; Snoussi, M.; Gdoura, R.; et al. Isolation, Partial Characterization and Application of Bacteriophages in Eradicating Biofilm Formation by Bacillus Cereus on Stainless Steel Surfaces in Food Processing Facilities. Pathogens 2022, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.H.; Han, S.; Choi, M.; Kim, B.-H.; Park, S.H.; Ha, S.-D. Biofilm Eradication Ability of Phage Cocktail against Listeria Monocytogenes Biofilms Formed on Food Contact Materials and Effect on Virulence-Related Genes and Biofilm Structure. Food Res. Int. 2022, 157, 111367. [Google Scholar] [CrossRef]
- Cepko, L.C.S.; Garling, E.E.; Dinsdale, M.J.; Scott, W.P.; Bandy, L.; Nice, T.; Faber-Hammond, J.; Mellies, J.L. Myoviridae Phage PDX Kills Enteroaggregative Escherichia Coli without Human Microbiome Dysbiosis. J. Med. Microbiol. 2020, 69, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Kapila, Y.L. Oral Health’s Inextricable Connection to Systemic Health: Special Populations Bring to Bear Multimodal Relationships and Factors Connecting Periodontal Disease to Systemic Diseases and Conditions. Periodontology 2021, 87, 11–16. [Google Scholar] [CrossRef]
- Esposito, A.M.; Esposito, M.M.; Ptashnik, A. Phylogenetic Diversity of Animal Oral and Gastrointestinal Viromes Useful in Surveillance of Zoonoses. Microorganisms 2022, 10, 1815. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Zhang, Y.; Lei, G.; Xu, K.; Zhao, N.; Lu, J.; Meng, F.; Yu, L.; Yan, J.; et al. Integrated Gut Virome and Bacteriome Dynamics in COVID-19 Patients. Gut Microbes 2021, 13, 1887722. [Google Scholar] [CrossRef]
- Vunduk, J.; Wan-Mohtar, W.A.A.Q.I.; Mohamad, S.A.; Abd Halim, N.H.; Mohd Dzomir, A.Z.; Žižak, Ž.; Klaus, A. Polysaccharides of Pleurotus Flabellatus Strain Mynuk Produced by Submerged Fermentation as a Promising Novel Tool against Adhesion and Biofilm Formation of Foodborne Pathogens. LWT 2019, 112, 108221. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Vunduk, J.; Todorovic, N.; Jakovljevic, D.; Zizak, Z.; Pavlovic, V.; Levic, S.; Niksic, M.; Van Griensven, L.J.L.D. Biological Potential of Extracts of the Wild Edible Basidiomycete Mushroom Grifola Frondosa. Food Res. Int. 2015, 67, 272–283. [Google Scholar] [CrossRef]
- Fasciana, T.; Gargano, M.L.; Serra, N.; Galia, E.; Arrigo, I.; Tricoli, M.R.; Diquattro, O.; Graceffa, G.; Vieni, S.; Venturella, G.; et al. Potential Activity of Albino Grifola Frondosa Mushroom Extract against Biofilm of Meticillin-Resistant Staphylococcus Aureus. J. Fungi 2021, 7, 551. [Google Scholar] [CrossRef] [PubMed]
- Aqueous Extracts of Wild Mushrooms Show Antimicrobial and Antiadhesion Activities against Bacteria and Fungi—Klančnik—2017—Phytotherapy Research—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ptr.5934 (accessed on 28 October 2022).
- Schillaci, D.; Arizza, V.; Gargano, M.L.; Venturella, G. Antibacterial Activity of Mediterranean Oyster Mushrooms, Species of Genus Pleurotus (Higher Basidiomycetes). IJM 2013, 15, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Kollakalnaduvil Raghavan, R.M.; Ali Pannippara, M.; Kesav, S.; Mathew, A.; Bhat, G.S.; Hatha AA, M.; Kk, E. MFAP9: Characterization of an Extracellular Thermostable Antibacterial Peptide from Marine Fungus with Biofilm Eradication Potential. J. Pharm. Biomed. Anal. 2021, 194, 113808. [Google Scholar] [CrossRef]
- Green Synthesized Silver Nanoparticles by Marine Endophytic Fungus Penicillium Polonicum and Its Antibacterial Efficacy against Biofilm Forming, Multidrug-Resistant Acinetobacter Baumanii—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0882401017315127 (accessed on 28 October 2022).
- Rahman, M.R.T.; Lou, Z.; Yu, F.; Wang, P.; Wang, H. Anti-Quorum Sensing and Anti-Biofilm Activity of Amomum Tsaoko (Amommum Tsao-Ko Crevost et Lemarie) on Foodborne Pathogens. Saudi J. Biol. Sci. 2017, 24, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Hammer, B.K.; Bassler, B.L. Quorum Sensing Controls Biofilm Formation in Vibrio Cholerae. Mol. Microbiol. 2003, 50, 101–104. [Google Scholar] [CrossRef]
- Inés Molina, R.D.; Campos-Silva, R.; Díaz, M.A.; Macedo, A.J.; Blázquez, M.A.; Alberto, M.R.; Arena, M.E. Laurel Extracts Inhibit Quorum Sensing, Virulence Factors and Biofilm of Foodborne Pathogens. LWT 2020, 134, 109899. [Google Scholar] [CrossRef]
- Ramos, C.; Teixeira, B.; Batista, I.; Matos, O.; Serrano, C.; Neng, N.R.; Nogueira, J.M.F.; Nunes, M.L.; Marques, A. Antioxidant and Antibacterial Activity of Essential Oil and Extracts of Bay Laurel Laurus Nobilis Linnaeus (Lauraceae) from Portugal. Nat. Prod. Res. 2012, 26, 518–529. [Google Scholar] [CrossRef]
- Yilmaz, E.S.; Timur, M.; Aslim, B. Antimicrobial, Antioxidant Activity of the Essential Oil of Bay Laurel from Hatay, Turkey. J. Essent. Oil Bear. Plants 2013, 16, 108–116. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Papapostolou, M.; Nenadis, N.; Mantzouridou, F.T.; Tsimidou, M.Z. Bay Laurel (Laurus Nobilis L.) Essential Oil as a Food Preservative Source: Chemistry, Quality Control, Activity Assessment, and Applications to Olive Industry Products. Foods 2022, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Somrani, M.; Inglés, M.-C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect against Listeria Monocytogenes. Foods 2020, 9, E567. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The Role of Diallyl Sulfides and Dipropyl Sulfides in the In Vitro Antimicrobial Activity of the Essential Oil of Garlic, Allium Sativum L., and Leek, Allium Porrum L. Phytother. Res. 2013, 27, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Ross, Z.M.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.V.; Maslin, D.J. Antimicrobial Properties of Garlic Oil against Human Enteric Bacteria: Evaluation of Methodologies and Comparisons with Garlic Oil Sulfides and Garlic Powder. Appl. Env. Microbiol. 2001, 67, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, H.; Shen, S.; Xu, J.; Lin, S.; Yuan, Y.; Jones, G.S. Synergistic Interactions of Cinnamaldehyde in Combination with Carvacrol against Food-Borne Bacteria. Food Control 2013, 34, 619–623. [Google Scholar] [CrossRef]
- Zhou, F.; Ji, B.; Zhang, H.; Jiang, H.; Yang, Z.; Li, J.; Li, J.; Yan, W. The Antibacterial Effect of Cinnamaldehyde, Thymol, Carvacrol and Their Combinations Against the Foodborne Pathogen Salmonella Typhimurium. J. Food Saf. 2007, 27, 124–133. [Google Scholar] [CrossRef]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial Activity of Cinnamaldehyde and Clove Oil: Effect on Selected Foodborne Pathogens in Model Food Systems and Watermelon Juice. J. Food Sci. Technol. 2015, 52, 5834–5841. [Google Scholar] [CrossRef] [Green Version]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium Avium Subsp. Paratuberculosis (Map). Foods 2017, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Cinici, E.; Dilekmen, N.; Kutlu, Z.; Dincer, B.; Cinici, O.; Balta, H.; Calik, I. Carvacrol Protects against Paclitaxel-Induced Retinal and Optic Nerve Cytotoxicity: A Histopathological Study. Beyoglu Eye J. 2020, 5, 219. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of Oregano, Carvacrol and Thymol on Staphylococcus Aureus and Staphylococcus Epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Aristatile, B.; Al-Numair, K.S.; Veeramani, C.; Pugalendi, K.V. Effect of Carvacrol on Hepatic Marker Enzymes and Antioxidant Status in D-Galactosamine-Induced Hepatotoxicity in Rats. Fundam. Clin. Pharmacol. 2009, 23, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Baranauskaite, J.; Kubiliene, A.; Marksa, M.; Petrikaite, V.; Vitkevičius, K.; Baranauskas, A.; Bernatoniene, J. The Influence of Different Oregano Species on the Antioxidant Activity Determined Using HPLC Postcolumn DPPH Method and Anticancer Activity of Carvacrol and Rosmarinic Acid. BioMed. Res. Int. 2017, 2017, e1681392. [Google Scholar] [CrossRef] [Green Version]
- Bukovská, A.; Cikoš, Š.; Juhás, Š.; Il’ková, G.; Rehák, P.; Koppel, J. Effects of a Combination of Thyme and Oregano Essential Oils on TNBS-Induced Colitis in Mice. Mediat. Inflamm. 2007, 2007, 23296. [Google Scholar] [CrossRef] [Green Version]
- Sadekuzzaman, M.; Mizan, M.F.R.; Kim, H.-S.; Yang, S.; Ha, S.-D. Activity of Thyme and Tea Tree Essential Oils against Selected Foodborne Pathogens in Biofilms on Abiotic Surfaces. LWT 2018, 89, 134–139. [Google Scholar] [CrossRef]
- De-Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; Guerra-Hernandez, E.J.; Jiménez-Valera, M.; Garcia-Villanova, B.; Ruiz-Bravo, A.; Verardo, V. Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology 2021, 10, 1091. [Google Scholar] [CrossRef]
- Quendera, A.P.; Barreto, A.S.; Semedo-Lemsaddek, T. Antimicrobial Activity of Essential Oils against Foodborne Multidrug-Resistant Enterococci and Aeromonads in Planktonic and Biofilm State. Food Sci. Technol. Int. 2019, 25, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, F.; Alaoui, T. Evaluation of Antibacterial Activity and Synergistic Effect between Antibiotic and the Essential Oils of Some Medicinal Plants. Asian Pac. J. Trop. Biomed. 2016, 6, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Niu, H.; Zhang, W.; Mu, H.; Sun, C.; Duan, J. Synergy among Thymol, Eugenol, Berberine, Cinnamaldehyde and Streptomycin against Planktonic and Biofilm-Associated Food-Borne Pathogens. Lett. Appl. Microbiol. 2015, 60, 421–430. [Google Scholar] [CrossRef]
- Smith, M.K.; Draper, L.A.; Hazelhoff, P.-J.; Cotter, P.D.; Ross, R.P.; Hill, C. A Bioengineered Nisin Derivative, M21A, in Combination with Food Grade Additives Eradicates Biofilms of Listeria Monocytogenes. Front. Microbiol. 2016, 7, 1939. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.A.T.; de Melo, M.R.; da Silva, C.M.R.; Jain, S.; Dolabella, S.S. Nisin Resistance in Gram-Positive Bacteria and Approaches to Circumvent Resistance for Successful Therapeutic Use. Crit. Rev. Microbiol. 2021, 47, 376–385. [Google Scholar] [CrossRef]
- Stincone, P.; Miyamoto, K.N.; Timbe, P.P.R.; Lieske, I.; Brandelli, A. Nisin Influence on the Expression of Listeria Monocytogenes Surface Proteins. J. Proteom. 2020, 226, 103906. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Synergistic Antibacterial and Antibiofilm Efficacy of Nisin in Combination with P-Coumaric Acid against Food-Borne Bacteria Bacillus Cereus and Salmonella Typhimurium. Lett. Appl. Microbiol. 2017, 65, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Purkait, S. Evaluation of Antibiofilm Efficacy of Essential Oil Components Β-caryophyllene, Cinnamaldehyde and Eugenol Alone and in Combination against Biofilm Formation and Preformed Biofilms of Listeria Monocytogenes and Salmonella Typhimurium. Lett. Appl. Microbiol. 2020, 71, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Panebianco, F.; Rubiola, S.; Chiesa, F.; Civera, T.; Di Ciccio, P.A. Effect of Gaseous Ozone Treatment on Biofilm of Dairy-Isolated Pseudomonas Spp. Strains. Ital. J. Food Saf. 2022, 11, 10350. [Google Scholar] [CrossRef]
- Marino, M.; Maifreni, M.; Baggio, A.; Innocente, N. Inactivation of Foodborne Bacteria Biofilms by Aqueous and Gaseous Ozone. Front. Microbiol. 2018, 9, 2024. [Google Scholar] [CrossRef] [Green Version]
- Panebianco, F.; Rubiola, S.; Chiesa, F.; Civera, T.; Di Ciccio, P.A. Effect of Gaseous Ozone on Listeria Monocytogenes Planktonic Cells and Biofilm: An In Vitro Study. Foods 2021, 10, 1484. [Google Scholar] [CrossRef]
- Di Ciccio, P.; Ghidini, S.; Zanardi, E.; Borrello, S.; Vergara, A.; Festino, A.; Ianieri, A. Effects of Gaseous Ozone on Food-Borne Pathogens. Ital. J. Food Sci. 2014, 26, 116. [Google Scholar]
- Piletić, K.; Kovač, B.; Planinić, M.; Vasiljev, V.; Karačonji, I.B.; Žigon, J.; Gobin, I.; Oder, M. Combined Biocidal Effect of Gaseous Ozone and Citric Acid on Acinetobacter Baumannii Biofilm Formed on Ceramic Tiles and Polystyrene as a Novel Approach for Infection Prevention and Control. Processes 2022, 10, 1788. [Google Scholar] [CrossRef]
- Vaid, R.; Linton, R.H.; Morgan, M.T. Comparison of Inactivation of Listeria Monocytogenes within a Biofilm Matrix Using Chlorine Dioxide Gas, Aqueous Chlorine Dioxide and Sodium Hypochlorite Treatments. Food Microbiol. 2010, 27, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Cheon, H.-L.; Park, K.-H.; Chung, M.-S.; Choi, S.H.; Ryu, S.; Kang, D.-H. Inactivation of Biofilm Cells of Foodborne Pathogen by Aerosolized Sanitizers. Int. J. Food Microbiol. 2012, 154, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, F.; Rubiola, S.; Di Ciccio, P.A. The Use of Ozone as an Eco-Friendly Strategy against Microbial Biofilm in Dairy Manufacturing Plants: A Review. Microorganisms 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm Formation and Control Strategies of Foodborne Pathogens: Food Safety Perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef] [Green Version]
- Bang, J.; Hong, A.; Kim, H.; Beuchat, L.R.; Rhee, M.S.; Kim, Y.; Ryu, J.-H. Inactivation of Escherichia Coli O157:H7 in Biofilm on Food-Contact Surfaces by Sequential Treatments of Aqueous Chlorine Dioxide and Drying. Int. J. Food Microbiol. 2014, 191, 129–134. [Google Scholar] [CrossRef]
- Han, Q.; Song, X.; Zhang, Z.; Fu, J.; Wang, X.; Malakar, P.K.; Liu, H.; Pan, Y.; Zhao, Y. Removal of Foodborne Pathogen Biofilms by Acidic Electrolyzed Water. Front. Microbiol. 2017, 8, 988. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Liu, Y.; Li, L.; Guo, Y.; Xie, Y.; Cheng, Y.; Yao, W. Ultrasound-Involved Emerging Strategies for Controlling Foodborne Microbial Biofilms. Trends Food Sci. Technol. 2020, 96, 91–101. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, S.-J.; Ha, S.-D. Synergistic Effects of Combined X-Ray and Aqueous Chlorine Dioxide Treatments against Salmonella Typhimurium Biofilm on Quail Egg Shells. LWT 2018, 92, 54–60. [Google Scholar] [CrossRef]
- Weng, X.; van Niekerk, J.; Neethirajan, S.; Warriner, K. Characterization of Antimicrobial Efficacy of Photocatalytic Polymers against Food-Borne Biofilms. LWT—Food Sci. Technol. 2016, 68, 1–7. [Google Scholar] [CrossRef]
- Chorianopoulos, N.G.; Tsoukleris, D.S.; Panagou, E.Z.; Falaras, P.; Nychas, G.-J.E. Use of Titanium Dioxide (TiO2) Photocatalysts as Alternative Means for Listeria Monocytogenes Biofilm Disinfection in Food Processing. Food Microbiol. 2011, 28, 164–170. [Google Scholar] [CrossRef]
- Barthomeuf, M.; Raymond, P.; Policarpo, N.; Castel, X.; Le Gendre, L.; Denis, M.; Pissavin, C. Bactericidal Efficiency of UVA-Active Titanium Dioxide Thin Layers on Bacteria from Food Industry Environments. Mater. Technol. 2017, 32, 782–791. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and Antibiofilm Efficacy of Self-Cleaning Surfaces Functionalized by TiO2 Photocatalytic Nanoparticles against Staphylococcus Aureus and Pseudomonas Putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Pablos, C.; Govaert, M.; Angarano, V.; Smet, C.; Marugán, J.; Van Impe, J.F.M. Photocatalytic Inactivation of Dual- and Mono-Species Biofilms by Immobilized TiO2. J. Photochem. Photobiol. B Biol. 2021, 221, 112253. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Durkin, D.P.; Aiello, A.; Diba, T.; Lafleur, J.; Zara, J.M.; Shen, Y.; Shuai, D. Photocatalytic Graphitic Carbon Nitride-Chitosan Composites for Pathogenic Biofilm Control under Visible Light Irradiation. J. Hazard. Mater. 2021, 408, 124890. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Mahalingam, S.; Gangadaran, P.; Ahn, Y.-H. Antibacterial and Photocatalytic Activities of 5-Nitroindole Capped Bimetal Nanoparticles against Multidrug Resistant Bacteria. Colloids Surf. B Biointerfaces 2020, 188, 110825. [Google Scholar] [CrossRef]
- Nahar, S.; Jeong, H.L.; Kim, Y.; Ha, A.J.; Roy, P.K.; Park, S.H.; Ashrafudoulla, M.; Mizan, M.F.R.; Ha, S.-D. Inhibitory Effects of Flavourzyme on Biofilm Formation, Quorum Sensing, and Virulence Genes of Foodborne Pathogens Salmonella Typhimurium and Escherichia Coli. Food Res. Int. 2021, 147, 110461. [Google Scholar] [CrossRef]
- KIM, M.-J.; LIM, E.S.; KIM, J.-S. Enzymatic Inactivation of Pathogenic and Nonpathogenic Bacteria in Biofilms in Combination with Chlorine. J. Food Prot. 2019, 82, 605–614. [Google Scholar] [CrossRef]
- Lim, E.S.; Koo, O.K.; Kim, M.-J.; Kim, J.-S. Bio-Enzymes for Inhibition and Elimination of Escherichia Coli O157:H7 Biofilm and Their Synergistic Effect with Sodium Hypochlorite. Sci. Rep. 2019, 9, 9920. [Google Scholar] [CrossRef] [Green Version]
- Mazaheri, T.; Ripolles-Avila, C.; Hascoët, A.S.; Rodríguez-Jerez, J.J. Effect of an Enzymatic Treatment on the Removal of Mature Listeria Monocytogenes Biofilms: A Quantitative and Qualitative Study. Food Control 2020, 114, 107266. [Google Scholar] [CrossRef]
- Ripolles-Avila, C.; Ríos-Castillo, A.G.; Fontecha-Umaña, F.; Rodríguez-Jerez, J.J. Removal of Salmonella Enterica Serovar Typhimurium and Cronobacter Sakazakii Biofilms from Food Contact Surfaces through Enzymatic Catalysis. J. Food Saf. 2020, 40, e12755. [Google Scholar] [CrossRef]
- Balabanova, L.; Podvolotskaya, A.; Slepchenko, L.; Eliseikina, M.; Noskova, Y.; Nedashkovskaya, O.; Son, O.; Tekutyeva, L.; Rasskazov, V. Nucleolytic Enzymes from the Marine Bacterium Cobetia Amphilecti KMM 296 with Antibiofilm Activity and Biopreservative Effect on Meat Products. Food Control 2017, 78, 270–278. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Xing, T.; Wu, N.; Xu, X.; Zhou, G. Removal of Salmonella Biofilm Formed under Meat Processing Environment by Surfactant in Combination with Bio-Enzyme. LWT—Food Sci. Technol. 2016, 66, 298–304. [Google Scholar] [CrossRef]
- Vishwakarma, V. Impact of Environmental Biofilms: Industrial Components and Its Remediation. J. Basic Microbiol. 2020, 60, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, Y. Etiological Agents Implicated in Foodborne Illness World Wide. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- USDA ERS—Economic Cost of Major Foodborne Illnesses Increased $2 Billion From 2013 to 2018. Available online: https://www.ers.usda.gov/amber-waves/2021/april/economic-cost-of-major-foodborne-illnesses-increased-2-billion-from-2013-to-2018/ (accessed on 7 November 2022).
- Meroni, G.; Soares Filipe, J.F.; Drago, L.; Martino, P.A. Investigation on Antibiotic-Resistance, Biofilm Formation and Virulence Factors in Multi Drug Resistant and Non Multi Drug Resistant Staphylococcus Pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef] [Green Version]
- Giaouris, E.; Heir, E.; Hébraud, M.; Chorianopoulos, N.; Langsrud, S.; Møretrø, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.-J. Attachment and Biofilm Formation by Foodborne Bacteria in Meat Processing Environments: Causes, Implications, Role of Bacterial Interactions and Control by Alternative Novel Methods. Meat Sci. 2014, 97, 298–309. [Google Scholar] [CrossRef]
- Shivaprasad, D.; Taneja, N.K.; Lakra, A.; Sachdev, D. In Vitro and in Situ Abrogation of Biofilm Formation in E. Coli by Vitamin C through ROS Generation, Disruption of Quorum Sensing and Exopolysaccharide Production. Food Chem. 2021, 341, 128171. [Google Scholar] [CrossRef]

| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Bacteria (Bacteriocins) |
|
| [6,7,8,9,10,11,12] |
| Bacteriophages |
|
| [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] |
| Fungi |
|
| [31,32,33,34,35,36,37] |
| Phytochemicals, Plant Extracts, and Essential Oils |
|
| [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] |
| Gaseous and Aqueous |
|
| [69,70,71,72,73,74,75,76,77,78] |
| Enzymatic |
|
| [88,89,90,91,92,93,94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, M.M.; Turku, S. The Use of Natural Methods to Control Foodborne Biofilms. Pathogens 2023, 12, 45. https://doi.org/10.3390/pathogens12010045
Esposito MM, Turku S. The Use of Natural Methods to Control Foodborne Biofilms. Pathogens. 2023; 12(1):45. https://doi.org/10.3390/pathogens12010045
Chicago/Turabian StyleEsposito, Michelle Marie, and Sara Turku. 2023. "The Use of Natural Methods to Control Foodborne Biofilms" Pathogens 12, no. 1: 45. https://doi.org/10.3390/pathogens12010045
APA StyleEsposito, M. M., & Turku, S. (2023). The Use of Natural Methods to Control Foodborne Biofilms. Pathogens, 12(1), 45. https://doi.org/10.3390/pathogens12010045








