In Vitro Susceptibility of Cryptosporidium parvum to Plant Antiparasitic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parasites
2.2. Plant Material
2.3. Cell Culture
2.4. Cytotoxicity Assay
2.5. Cryptosporidium parvum Growth Inhibition Assay
2.5.1. Pre-Treatment of Oocysts and Infection of Host Cells
2.5.2. Extract Screening at a Single Concentration
2.5.3. Dose–Response Analysis
2.5.4. Quantification of Inhibition
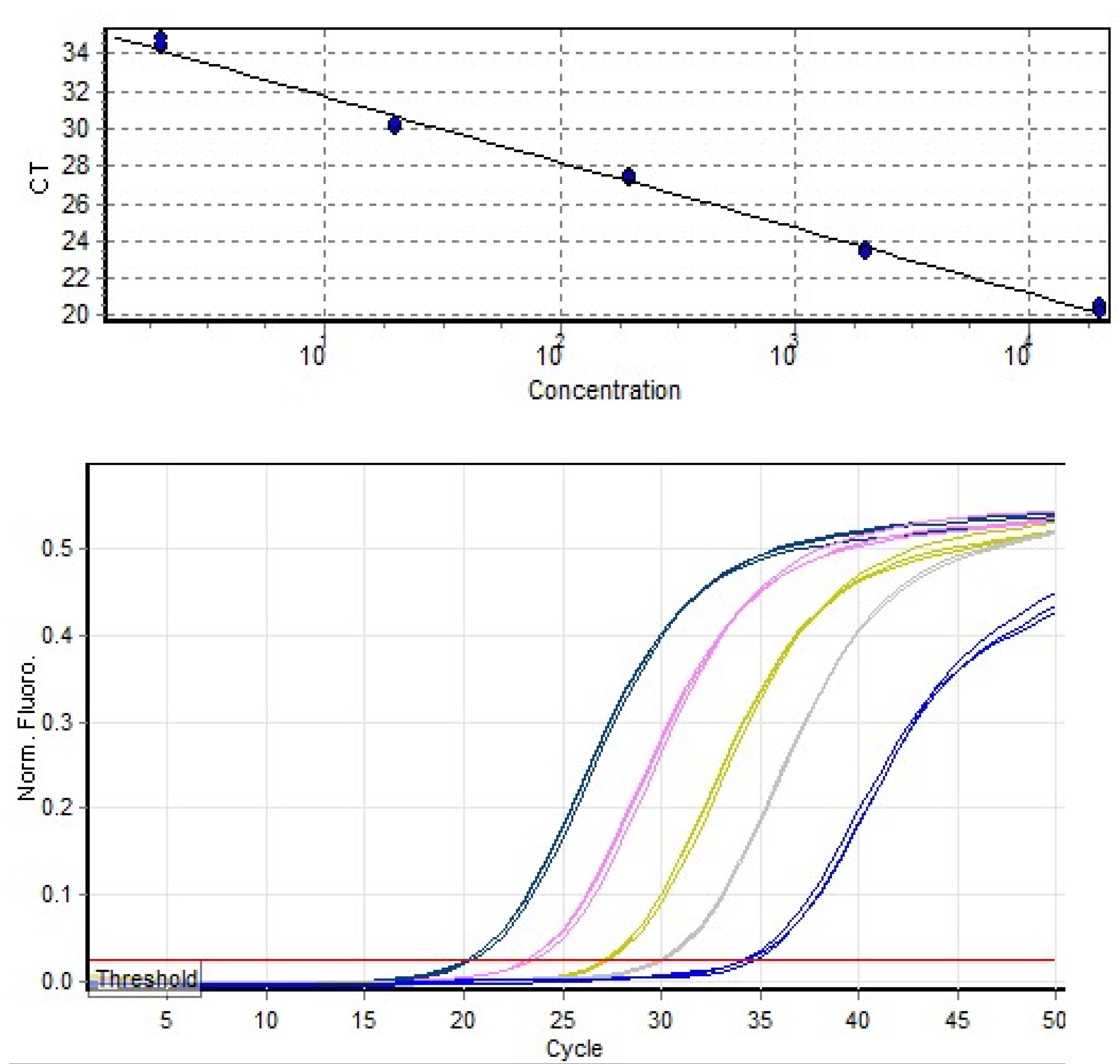
2.5.5. DNA Sequencing
2.6. Data Analysis
3. Results
3.1. Cytotoxicity Assay
3.2. Cryptosporidium Parvum Growth Inhibition Assay
3.2.1. Growth Inhibition at a Single Concentration
3.2.2. Dose–Response Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, U.; Paparini, A.; Oskam, C. New technologies for detection of enteric parasites. Trends. Parasitol. 2017, 33, 532–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva, M.T. Infectious diseases: Decrypting Cryptosporidium. Nat. Rev. Drug. Discov. 2017, 16, 527. [Google Scholar] [CrossRef]
- Tamomh, A.G.; Agena, A.M.; Elamin, E.; Suliman, M.A.; Elmadani, M.; Omara, A.B.; Musa, S.A. Prevalence of cryptosporidiosis among children with diarrhoea under five years admitted to Kosti teaching hospital, Kosti City, Sudan. BMC Infect. Dis. 2021, 21, 349. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sow, S.O.; Muhsen, K.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Sur, D.; Zaidi, A.K.; Faruque, A.S.; et al. The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of Sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS. Negl. Trop. Dis. 2016, 10, e0004729. [Google Scholar] [CrossRef] [Green Version]
- Robertson, L.J.; Björkman, C.; Axén, C.; Fayer, R. Cryptosporidiosis in farmed animals. In Cryptosporidium: Parasite and Disease; Springer: Berlin/Heidelberg, Germany, 2014; pp. 149–235. [Google Scholar]
- Thomson, S.; Hamilton, C.A.; Hope, J.C.; Katzer, F.; Mabbott, N.A.; Morrison, L.J.; Innes, E.A. Bovine cryptosporidiosis: Impact, host-parasite interaction and control strategies. Vet. Res. 2017, 48, 42. [Google Scholar] [CrossRef] [Green Version]
- Sanford, S.; Josephson, G. Bovine cryptosporidiosis: Clinical and pathological findings in forty-two scouring neonatal calves. Can. Vet. J. 1982, 23, 343. [Google Scholar] [PubMed]
- Barrera, J.P.; Carmena, D.; Rodríguez, E.; Checa, R.; López, A.M.; Fidalgo, L.E.; Gálvez, R.; Marino, V.; Fuentes, I.; Miró, G. The red fox (Vulpes vulpes) as a potential natural reservoir of human cryptosporidiosis by Cryptosporidium hominis in Northwest Spain. Transbound. Emerg. Dis. 2020, 67, 2172–2182. [Google Scholar] [CrossRef]
- Silverlås, C.; Mattsson, J.G.; Insulander, M.; Lebbad, M. Zoonotic transmission of Cryptosporidium meleagridis on an organic Swedish farm. Int. J. Parasitol. 2012, 42, 963–967. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Xiao, L.; Feng, Y. Molecular epidemiology of human cryptosporidiosis in low-and middle-income countries. Clin. Microbiol. Rev. 2021, 34, e00087-19. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.V.; Corcoran, G.D. New drugs and treatment for cryptosporidiosis. Curr. Opin. Infect. Dis. 2004, 17, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Sparks, H.; Nair, G.; Castellanos-Gonzalez, A.; White, A.C., Jr. Treatment of Cryptosporidium: What we know, gaps, and the way forward. Curr. Trop. Med. Rep. 2015, 2, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamil, M.; Aleem, M.T.; Shaukat, A.; Khan, A.; Mohsin, M.; Rehman, T.u.; Abbas, R.Z.; Saleemi, M.K.; Khatoon, A.; Babar, W.; et al. Medicinal plants as an alternative to control poultry parasitic diseases. Life 2022, 12, 449. [Google Scholar] [CrossRef]
- Hussain, K.; Abbas, R.; Abbas, A.; Samiullah, K.; Ahmed, T.; Siddique, F.; Mohsin, M.; Rehman, A.; Rahman, A.U.; Waqas, M. Anticoccidial potential of Ageratum conyzoides and its effect on Blood parameters of experimentally infected Broiler Chickens. J. Hell. Vet. Med. Soc. 2021, 72, 3085–3090. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Vargas-Villarreal, J.; Verde-Star, M.J.; Rivas-Galindo, V.M.; Torres-Hernández, Á.D. Antiprotozoal activity against Entamoeba histolytica of flavonoids isolated from Lippia graveolens Kunth. Molecules 2020, 25, 2464. [Google Scholar] [CrossRef]
- Astashkina, A.; Mann, B.; Grainger, D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro experiments to in vivo and clinical studies; pros and cons. Curr. Drug Discov. Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef]
- Armson, A.; Sargent, K.; MacDonald, L.M.; Finn, M.P.; Thompson, R.C.A.; Reynoldson, J.A. A comparison of the effects of two dinitroanilines against Cryptosporidium parvum in vitro and in vivo in neonatal mice and rats. FEMS Immunol. Med. Microbiol. 1999, 26, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Arrowood, M.J. In Vitro cultivation of Cryptosporidium Species. Clin. Microbiol. Rev. 2002, 15, 390. [Google Scholar] [CrossRef]
- Hijjawi, N.S. Successful in vitro cultivation of Cryptosporidium andersoni: Evidence for the existence of novel extracellular stages in the life cycle and implications for the classification of Cryptosporidium. Int. J. Parasitol. 2002, 32, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Borowski, H.; Thompson, R.; Armstrong, T.; Clode, P. Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system. Parasitology 2010, 137, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karanis, P.; Aldeyarbi, H. Evolution of Cryptosporidium in vitro culture. Int. J. Parasitol. 2011, 41, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Warrier, P.K. Indian Medicinal Plants: A. Compendium of 500 Species; Orient Blackswan: Hyderabad, India, 1993; Volume 5. [Google Scholar]
- Yelne, M.; Sharma, P.; Dennis, T. Database on medicinal plants used in Ayurveda. Cent. Counc. Res. Ayurveda. Siddha. New Delhi 2000, 2, 69–73. [Google Scholar]
- Hijjawi, N.; Meloni, B.; Morgan, U.; Thompson, R. Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. Int. J. Parasitol. 2001, 31, 1048–1055. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Arrowood, M.J.; Mead, J.R.; Xie, L.; You, X. In vitro anticryptosporidial activity of dinitroaniline herbicides. FEMS Microbiol. Lett. 1996, 136, 245–249. [Google Scholar] [CrossRef]
- MacDonald, L.M.; Sargent, K.; Armson, A.; Thompson, R.C.A.; Reynoldson, J.A. The development of a real-time quantitative-PCR method for characterisation of a Cryptosporidium parvum in vitro culturing system and assessment of drug efficacy. Mol. Biochem. Parasitol. 2002, 121, 279–282. [Google Scholar] [CrossRef]
- Di Giovanni, G.D.; LeChevallier, M.W. Quantitative-PCR assessment of Cryptosporidium parvum cell culture infection. Appl. Environ. Microbiol. 2005, 71, 1495–1500. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, A.; Greay, T.L.; Paparini, A.; Linge, K.L.; Joll, C.A.; Ryan, U.M. Identification of eukaryotic microorganisms with 18S rRNA next-generation sequencing in wastewater treatment plants, with a more targeted NGS approach required for Cryptosporidium detection. Water Res. 2019, 158, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Certad, G.; Zahedi, A.; Gantois, N.; Sawant, M.; Creusy, C.; Duval, E.; Benamrouz, S.; Ryan, U.; Viscogliosi, E. Molecular characterization of novel Cryptosporidium fish genotypes in edible marine fish. Microorganisms 2020, 8, 2014. [Google Scholar] [CrossRef]
- Cai, X.; Woods, K.M.; Upton, S.J.; Zhu, G. Application of quantitative real-time reverse transcription-PCR in assessing drug efficacy against the intracellular pathogen Cryptosporidium parvum in vitro. Antimicrob. Agents. Chemother. 2005, 49, 4437–4442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, U.M.; Constantine, C.C.; Forbes, D.A.; Thompson, R.C. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J. Parasitol. 1997, 83, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, B.J.; Keegan, A.R.; Monis, P.T.; Saint, C.P. Environmental temperature controls Cryptosporidium oocyst metabolic rate and associated retention of infectivity. Appl. Environ. Microbiol. 2005, 71, 3848–3857. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Paparini, A.; Monis, P.; Ryan, U. Comparison of next-generation droplet digital PCR (ddPCR) with quantitative PCR (qPCR) for enumeration of Cryptosporidium oocysts in faecal samples. Int. J. Parasitol. 2014, 44, 1105–1113. [Google Scholar] [CrossRef] [Green Version]
- Fischer, I.; Milton, C.; Wallace, H. Toxicity testing is evolving! Toxicol. Res. 2020, 9, 67–80. [Google Scholar] [CrossRef]
- Nasai, N.B.; Abba, Y.; Abdullah, F.F.J.; Marimuthu, M.; Tijjani, A.; Sadiq, M.A.; Mohammed, K.; Chung, E.L.T.; Omar, M.A.B. In vitro larvicidal effects of ethanolic extract of Curcuma longa Linn. on Haemonchus larval stage. Vet. World 2016, 9, 417. [Google Scholar] [CrossRef] [Green Version]
- Raul, S.K.; Padhy, G.K.; Charly, J.P.; Kumar, K.V. An in-vitro evaluation of the anthelmintic activity of rhizome extracts of Zingiber officinalis, Zingiber zerumbet and Curcuma longa, a comparative study. J. Pharm. Res. 2012, 5, 3813–3814. [Google Scholar]
- Atjanasuppat, K.; Wongkham, W.; Meepowpan, P.; Kittakoop, P.; Sobhon, P.; Bartlett, A.; Whitfield, P.J. In vitro screening for anthelmintic and antitumour activity of ethnomedicinal plants from Thailand. J. Ethnopharmacol. 2009, 123, 475–482. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Dyachenko, V.; Khalafalla, R.E.; Desouky, A.Y.; Daugschies, A. Effects of curcumin on Cryptosporidium parvum in vitro. Parasitol. Res. 2009, 105, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Leitch, G.J.; He, Q. Reactive nitrogen and oxygen species ameliorate experimental cryptosporidiosis in the neonatal BALB/c mouse model. Infect. Immun. 1999, 67, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Ramsewak, R.S.; DeWitt, D.L.; Nair, M.G. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine 2000, 7, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Wang, Y.; Wong, J.; Zhang, J.; McManus, B.M.; Luo, H. Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J. Virol. 2007, 81, 3142–3150. [Google Scholar] [CrossRef] [Green Version]
- Milacic, V.; Banerjee, S.; Landis-Piwowar, K.R.; Sarkar, F.H.; Majumdar, A.P.; Dou, Q.P. Curcumin inhibits the proteasome activity in human colon cancer cells In vitro and In vivo. Cancer Res. 2008, 68, 7283–7292. [Google Scholar] [CrossRef] [Green Version]
- Pollok, R.C.; McDonald, V.; Kelly, P.; Farthing, M.J. The role of Cryptosporidium parvum-derived phospholipase in intestinal epithelial cell invasion. Parasitol. Res. 2003, 90, 181–186. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, W.; Chen, W.; Wu, Q.; Liu, H.; Cui, G. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 427–433. [Google Scholar] [CrossRef]
- Rider, S.D., Jr.; Zhu, G. An apicomplexan ankyrin-repeat histone deacetylase with relatives in photosynthetic eukaryotes. Int. J. Parasitol. 2009, 39, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, G.; Islamuddin, M.; Want, M.Y.; Ozbak, H.A.; Hemeg, H.A.; Sahal, D.; Afrin, F. Leishmanicidal activity of Piper nigrum bioactive fractions is interceded via apoptosis In Vitro and substantiated by Th1 immunostimulatory potential In Vivo. Front. Microbiol. 2015, 6, 1368. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Sayed, A.A.; Abdeen, A.; Aleya, L.; Ali, D.; Alkahtane, A.A.; Alarifi, S.; Alkahtani, S. Piperine enhances the antioxidant and anti-inflammatory activities of thymoquinone against microcystin-LR-induced hepatotoxicity and neurotoxicity in mice. Oxidative Med. Cell. Longev. 2019, 2019, 1309175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sasmal, D.; Sharma, N. Mechanism of deltamethrin induced thymic and splenic toxicity in mice and its protection by piperine and curcumin: In Vivo study. Drug. Chem. Toxicol. 2018, 41, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, C.P.; Singh, D.; Khan, F.A.; Bhagwan, P.S.K. Anthelmintic potential of Embelia ribes seeds against Haemonchus contortus of sheep. Indian. J. Anim. Sci. 2009, 79, 167–170. [Google Scholar]
- Kekuda Prashith, T.; Kumar, P.; Nishanth, B.; Sandeep, M. In vitro anthelmintic activity of aqueous extract of Embelia ribes. Biotechnol. Indian J. 2009, 3, 87–89. [Google Scholar]
- Jalalpure, S.; Alagawadi, K.; Mahajanashetti, C.; Shah, B.; Singh, V.; Patil, J. In vitro anthelmintic property of various seed oils against Pheritima posthuma. Indian J. Pharm. Sci. 2007, 69, 158. [Google Scholar] [CrossRef]
- Ghugarkar, P.G.; Nupur, A.; Inamdar, N.; Tarkase, K. In vitro evaluation of anthelmintic activity of Embelin. World J. Pharm. Res. 2015, 4, 1433–1437. [Google Scholar]
- Al-Shaibani, I.; Phulan, M.; Arijo, A.; Qureshi, T.; Kumbher, A. Anthelmintic activity of Nigella sativa L., seeds on gastrointestinal nematodes of sheep. Pak. J. Nematol. 2008, 26, 207–218. [Google Scholar]
- Sen, E.; Ogut, T.; Olgun, A.; Kisa, O. Anthelmintic activity of Nigella sativa against Caenorhabditis elegans. Adv. Pharmacol. Pharm. 2021, 9, 117–126. [Google Scholar] [CrossRef]
- El-Baba, C.; Mahadevan, V.; Fahlbusch, F.B.; Rau, T.T.; Gali-Muhtasib, H.; Schneider-Stock, R. Thymoquinone-induced conformational changes of PAK1 interrupt prosurvival MEK-ERK signaling in colorectal cancer. Mol. Cancer 2014, 13, 201. [Google Scholar] [CrossRef] [Green Version]
- Ullah, R.; Rehman, A.; Zafeer, M.F.; Rehman, L.; Khan, Y.A.; Khan, M.A.H.; Khan, S.N.; Khan, A.U.; Abidi, S.M.A. Anthelmintic potential of thymoquinone and curcumin on Fasciola gigantica. PLoS ONE 2017, 12, e0171267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raval, B.P.; Shah, T.G.; Suthar, M.P.; Ganure, A.L. Screening of Nigella sativa seeds for antifungal activity. Ann. Biol. Res. 2010, 1, 164–171. [Google Scholar]
- Haq, A.; Lobo, P.I.; Al-Tufail, M.; Rama, N.R.; Al-Sedairy, S.T. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int. J. Immunopharmacol. 1999, 21, 283–295. [Google Scholar] [CrossRef] [PubMed]

| Plant Species (Common Name) | Family | Part Used | The Solvent Used for Extraction | Characterised Main Phytochemicals | Amount (%w/w) |
|---|---|---|---|---|---|
| Allium sativum L. (garlic) | Amaryllidaceae | Bulb/Cloves | 70% Methanol | Alliin | 1.1 |
| Allicin | 0.55 | ||||
| Volatile oil | 1.2 | ||||
| Boswellia serrata Roxb. (Indian frankincense) | Burseraceae | Oleo-gum resin | 70% Ethanol | Acetyl keto beta boswellic acid | 30.72 |
| Beta boswellia acid | 5.40 | ||||
| Acetyl beta boswellic acid | 2.96 | ||||
| Acetyl beta boswellic acid | 1.57 | ||||
| Centella asiatica (L.) Urban (Gotu kola) | Apiaceae | Leaf | 70% Ethanol | Total Terpenes (sum of Asiaticoside, Madecassoside, Asiatic acid, Madecassic acid) | 46.20 |
| Curcuma longa L. (turmeric) | Zingiberaceae | Rhizome | Water | Total Curcuminoids | 95.16 |
| Cucurbita pepo L. (pumpkin) | Cucurbitaceae | Seed | Water | Glycosides | 5.33 |
| Embelia ribes Burm. f. (false black pepper) | Primulaceae | Fruit | 70% Methanol | Tannins | 5.20 |
| Glycyrrhiza glabra L. (liquorice) | Fabaceae | Root | 80% Ethanol | Glycyrrhizinic acid | 50.82 |
| Moringa oleifera Lam. (drumstick) | Moringaceae | Leaf | Water | Total Protein Content | 22.08 |
| Saponins | 10.2% | ||||
| Alkaloids | 0.22% | ||||
| Nigella sativa L. (black cumin) | Ranunculaceae | Seed | Water | Total Saponins | 12.98 |
| Total Bitters | 4.00 | ||||
| Piper nigrum L. (black pepper) | Piperaceae | Fruit | Acetone/hexane | Piperine | 95.67 |
| Thymus vulgaris L. (thyme) | Lamiaceae | Seed | Water | Total Volatile Organic Compounds | 3.23% |
| Tribulus terrestris L. (caltrop) | Zygophyllaceae | Entire plant | Water | Total Saponin Content | 41.43 |
| Vitex negundo L. (Chinese chaste tree) | Lamiaceae | Leaf | Water | Total Glycoside | 26.98 |
| Compound | % Inhibition ± SEM |
|---|---|
| Curcuma longa L. (turmeric) | 79.6 ± 1.0 |
| Piper nigrum L. (black pepper) | 73.6 ± 2.7 |
| Nigella Sativa L. (black cumin) | 68.1 ± 1.0 |
| Embelia ribes Burm. f. (false black pepper) | 61.1 ± 1.6 |
| Allium sativum L. (garlic) | 50.3 ± 0.1 |
| Tribulus terrestris L. (goat’s-head/caltrop) | 50.3 ± 0.5 |
| Thymus vulgaris L. (thyme) | 50.2 ± 2.6 |
| Moringa oleifera Lam. (drumstick) | 46.3 ± 2.4 |
| Boswellia serrata Roxb. (Indian frankincense) | 39.8 ± 0.9 |
| Glycyrrhiza glabra L. (liquorice) | 38.7 ± 0.7 |
| Vitex negundo L. (Chinese chaste tree) | 35.2 ± 2.9 |
| Cucurbita pepo L. (pumpkin) | 31.9 ± 0.4 |
| Centella asiatica (L.) Urban (Gotu kola) | 22.5 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranasinghe, S.; Zahedi, A.; Armson, A.; Lymbery, A.J.; Ash, A. In Vitro Susceptibility of Cryptosporidium parvum to Plant Antiparasitic Compounds. Pathogens 2023, 12, 61. https://doi.org/10.3390/pathogens12010061
Ranasinghe S, Zahedi A, Armson A, Lymbery AJ, Ash A. In Vitro Susceptibility of Cryptosporidium parvum to Plant Antiparasitic Compounds. Pathogens. 2023; 12(1):61. https://doi.org/10.3390/pathogens12010061
Chicago/Turabian StyleRanasinghe, Sandamalie, Alireza Zahedi, Anthony Armson, Alan J. Lymbery, and Amanda Ash. 2023. "In Vitro Susceptibility of Cryptosporidium parvum to Plant Antiparasitic Compounds" Pathogens 12, no. 1: 61. https://doi.org/10.3390/pathogens12010061
APA StyleRanasinghe, S., Zahedi, A., Armson, A., Lymbery, A. J., & Ash, A. (2023). In Vitro Susceptibility of Cryptosporidium parvum to Plant Antiparasitic Compounds. Pathogens, 12(1), 61. https://doi.org/10.3390/pathogens12010061









